Diagnosis of HIV1 Infection in Phase I II









- Slides: 17

Diagnosis of HIV-1 Infection in Phase I & II HIV Vaccine Trials RW Coombs 1, J Dragavon 1, B Metch 2, CJ Cooper 2 and the NIAID HIV Vaccine Trials Network (HVTN) 1 University of Washington & 2 Fred Hutchinson Cancer Research Center, Seattle, Washington 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 1

Objective n n n To evaluate the UNAIDS & WHO HIV Testing Strategy III for diagnosis of HIV infection in low-risk, low-seroprevalence phase I/II HIV vaccine trials conducted in the United States Hypothesis: The UNAIDS/WHO HIV testing strategy III would be unreliable for diagnosis HIV infection post-vaccination in this study population Significance: This information will be useful for developing HIV diagnostic criteria for both domestic & international HIV vaccine trials 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 2

Background n UNAIDS/WHO recommends that resource limited countries consider HIV testing strategies, which use a combination of ELISAs and/or simple/rapid assays rather than ELISA/Western blot for HIV antibody detection n 12/6/07 v. 3 WHO/HIV Assays: Operational Characteristics, Report 14/Simple/Rapid tests (2004); page 3 CDC 2007 HIV Diagnostic Conference 3

Background cont’d n UNAIDS & WHO recommend three testing strategies, which depend on the testing objective and the prevalence of HIV in the population WHO/HIV Assays: Operational Characteristics, Report 14/Simple/Rapid tests (2004); page 3 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 4

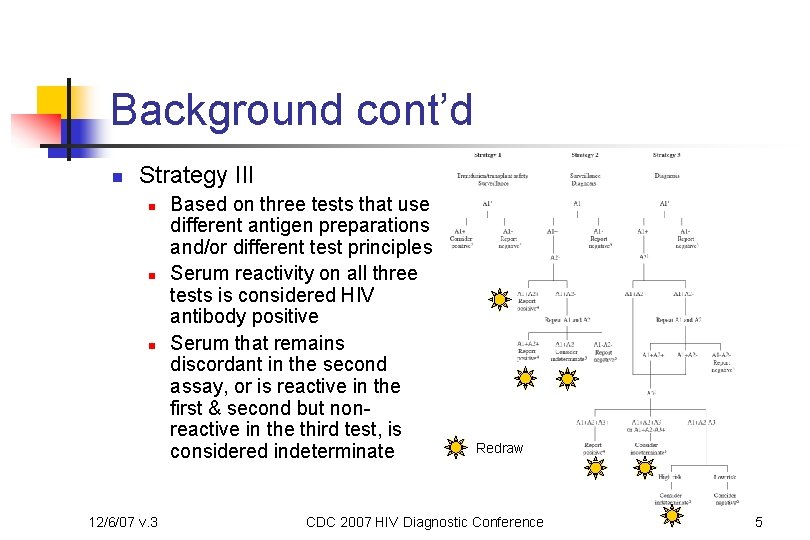
Background cont’d n Strategy III n n n 12/6/07 v. 3 Based on three tests that use different antigen preparations and/or different test principles Serum reactivity on all three tests is considered HIV antibody positive Serum that remains discordant in the second assay, or is reactive in the first & second but nonreactive in the third test, is considered indeterminate Redraw CDC 2007 HIV Diagnostic Conference 5

Methods n Study design n HVTN initiated 15 phase-I and one phase-II vaccine trials in the United States since January 2004 at the following 13 study sites in 12 cities: US HIV Vaccine Trial Network (HVTN) Sites 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 6

Methods cont’d n n A routine diagnostic algorithm that distinguished between vaccine-induced seropositivity and true HIV infection was used for participants who completed these clinical trials after March 2006 Testing was performed by the HVTN HIV diagnostic laboratory in Seattle, Washington 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 7

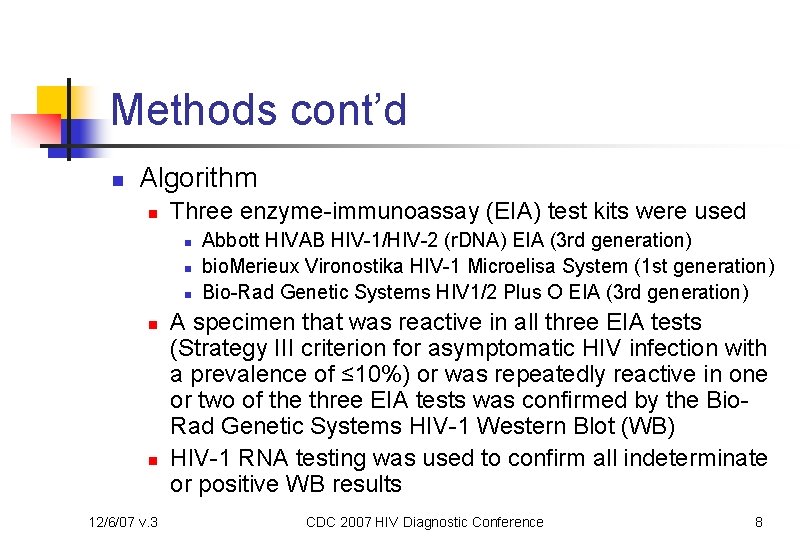
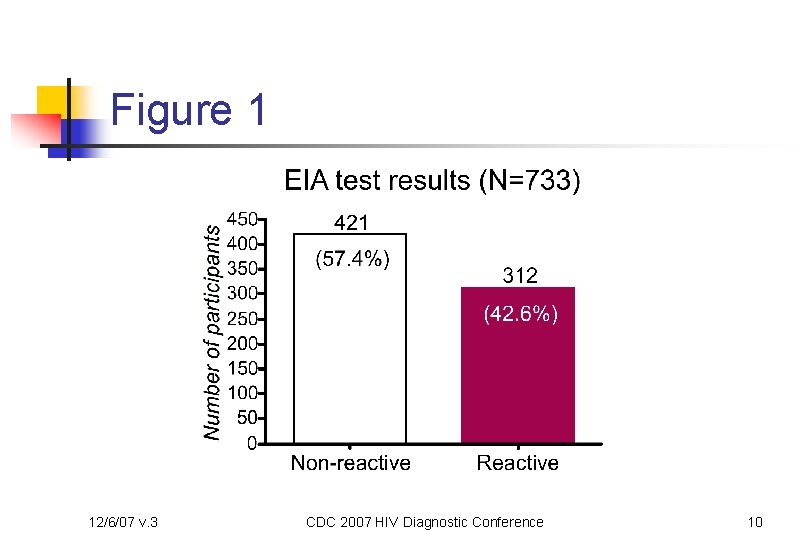
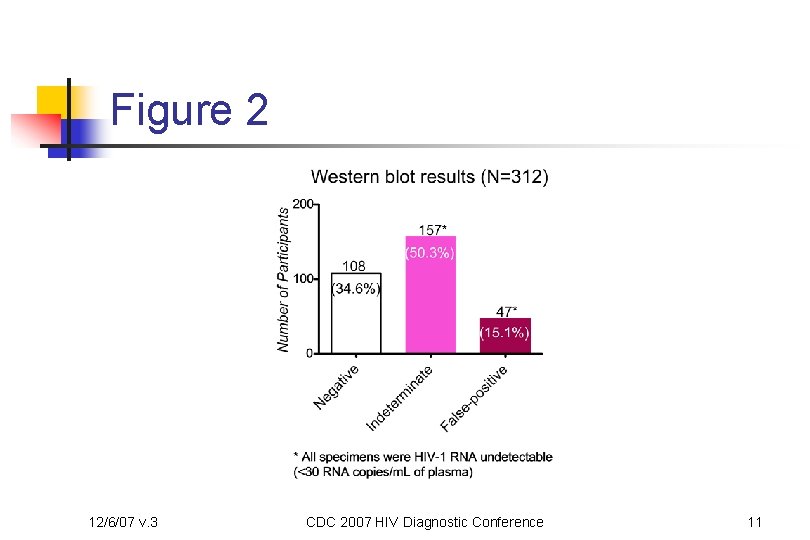
Methods cont’d n Algorithm n Three enzyme-immunoassay (EIA) test kits were used n n n 12/6/07 v. 3 Abbott HIVAB HIV-1/HIV-2 (r. DNA) EIA (3 rd generation) bio. Merieux Vironostika HIV-1 Microelisa System (1 st generation) Bio-Rad Genetic Systems HIV 1/2 Plus O EIA (3 rd generation) A specimen that was reactive in all three EIA tests (Strategy III criterion for asymptomatic HIV infection with a prevalence of ≤ 10%) or was repeatedly reactive in one or two of the three EIA tests was confirmed by the Bio. Rad Genetic Systems HIV-1 Western Blot (WB) HIV-1 RNA testing was used to confirm all indeterminate or positive WB results CDC 2007 HIV Diagnostic Conference 8

12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 9

Figure 1 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 10

Figure 2 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 11

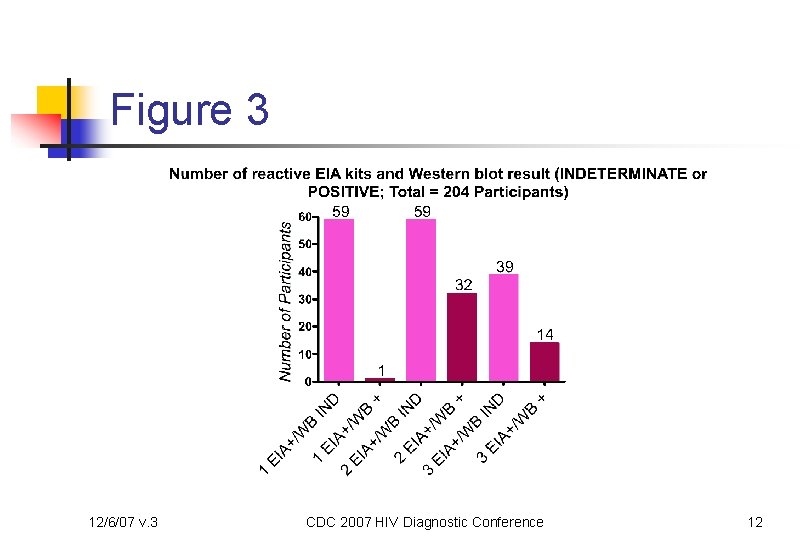
Figure 3 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 12

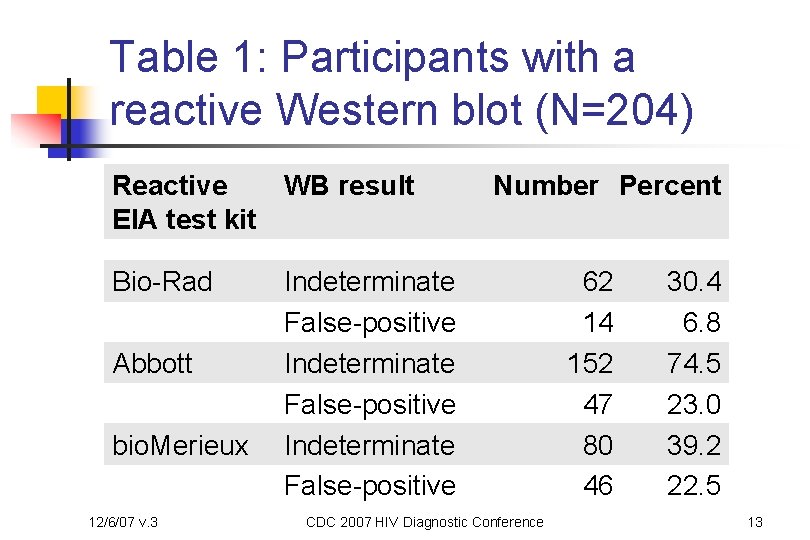
Table 1: Participants with a reactive Western blot (N=204) Reactive WB result EIA test kit Bio-Rad Abbott bio. Merieux 12/6/07 v. 3 Number Percent Indeterminate False-positive CDC 2007 HIV Diagnostic Conference 62 14 152 47 80 46 30. 4 6. 8 74. 5 23. 0 39. 2 22. 5 13

Summary of HIV-1 infection status n n The UNAIDS/WHO Testing Strategy III assigned 53/733 (7. 2%) of these uninfected vaccine participants as HIV-1 infected using EIA criteria Fourteen (26%) of these 53 participants had a positive (confirmed) Western blot using CDC criteria and would have been assigned as HIV-1 infected An additional 33 participants were infected using CDC criteria, for a total of 47/733 (6. 4%) Importantly, all 86 (11. 7%) participants (EIA + or WB + or both) were HIV-1 uninfected using HIV RNA criteria 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 14

Conclusions n n In the United States, current HIV-1 diagnostic algorithms based solely on serologic criteria for infection are inadequate for diagnosing HIV-1 infection in HIV vaccine trial participants because of vaccine-induced false-positive confirmatory Western blot results Similar concerns may be associated with EIA-based algorithms specified by UNAIDS/WHO for resourcelimited countries with a seroprevalence of ≤ 10% 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 15

Conclusions cont’d n n As such, future diagnostic algorithms should incorporate HIV nucleic acid testing Finally, to reduce potential harm for vaccine trial participants, HIV testing outside of the study protocol should be approached with caution until health care providers are educated about HIV vaccines and the need for diagnostic algorithms that incorporate HIV nucleic acid testing (see poster by CJ Cooper et al: Implications for HIV testing outside of the study during preventative HIV vaccine trials in the United States) 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 16

Acknowledgements n n n NIAID/DAIDS funding for the HVTN HIV Diagnostic Laboratory staff University of Washington CFAR 12/6/07 v. 3 CDC 2007 HIV Diagnostic Conference 17
 Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Nursing diagnosis three parts
Nursing diagnosis three parts Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Phase to phase voltage
Phase to phase voltage Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Csce 441
Csce 441 Hplc reverse phase vs normal phase
Hplc reverse phase vs normal phase Line current and phase current
Line current and phase current Mobile phase and stationary phase
Mobile phase and stationary phase Adsorption chromatography
Adsorption chromatography Column chromatography images
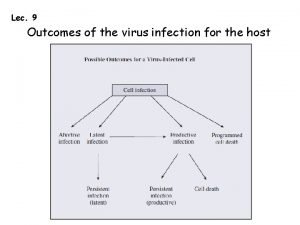
Column chromatography images Virus infection
Virus infection Infection control
Infection control Mobilivirus
Mobilivirus Chapter 16 infection control and standard precautions
Chapter 16 infection control and standard precautions