Crop improvement through Plant Tissue Culture Dr R




























- Slides: 49

Crop improvement through Plant Tissue Culture Dr. R. RAVINDHRAN Department of Plant Biology and Biotechnology Loyola College (Autonomous) Chennai - 600 034

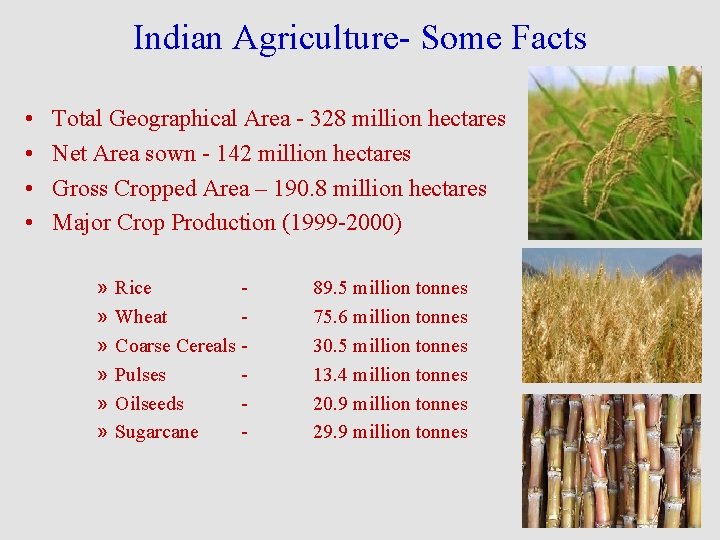
Indian Agriculture- Some Facts • • Total Geographical Area - 328 million hectares Net Area sown - 142 million hectares Gross Cropped Area – 190. 8 million hectares Major Crop Production (1999 -2000) » » » Rice Wheat Coarse Cereals Pulses Oilseeds Sugarcane - 89. 5 million tonnes 75. 6 million tonnes 30. 5 million tonnes 13. 4 million tonnes 20. 9 million tonnes 29. 9 million tonnes

Indian Agriculture- Some Facts • • • Contributes to 24% of GDP Provides food to 1 Billion people Sustains 65% of the population : helps alleviate poverty Produces 51 major Crops Provides Raw Material to Industries Contributes to 1/6 th of the export earnings • One of the 12 Bio-diversity centers in the world with over 46, 000 species of plants and 86, 000 species of animals recorded

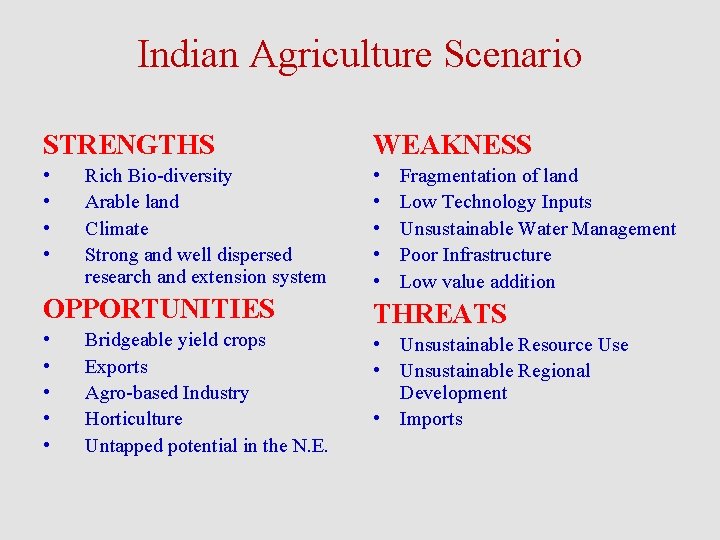
Indian Agriculture Scenario STRENGTHS WEAKNESS • • • Rich Bio-diversity Arable land Climate Strong and well dispersed research and extension system OPPORTUNITIES • • • Bridgeable yield crops Exports Agro-based Industry Horticulture Untapped potential in the N. E. Fragmentation of land Low Technology Inputs Unsustainable Water Management Poor Infrastructure Low value addition THREATS • Unsustainable Resource Use • Unsustainable Regional Development • Imports

Current Concerns ü ü ü Pressure of the Population on Land Skewed distribution of operational holdings Land Degradation Water Balance Low level of mechanization Low Fertilizer Consumption

CROP IMPROVEMENT conventional method

Hurdles

Plant tissue culture

Plant Tissue Culture: Collection of experimental methods of isolation and inoculation of organs, tissues and cell in an artificial medium under aseptic condition. Principle: • Plant cell has special feature: Totipotency • Ability (Genetic potential )of any cell that develops into entire plantlet under in vitro condition

Haberlandt Carrel Tissue culture had its origins at the beginning of the 20 th century with the work of Gottleib Haberlandt (plants) and Alexis Carrel (animals).

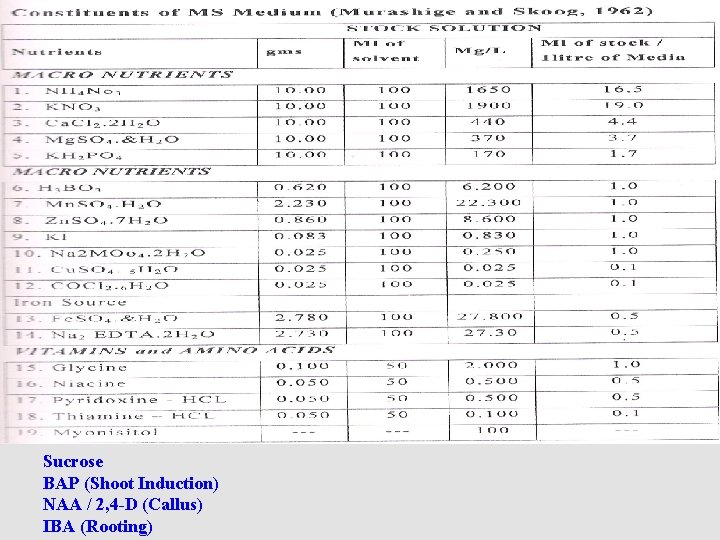
Sucrose BAP (Shoot Induction) NAA / 2, 4 -D (Callus) IBA (Rooting)

Explant: Part of the plant used for plant tissue culture. Callus: Mass of undifferentiated paranchyma cells which divide and redivide indifinetly under in vitro condition. Embryoid: structure similar to that of zygotic embryo developed from somatic explant under in vitro condition. Adventious organs: Organs (shoot and root) developed from unusual point of origin.

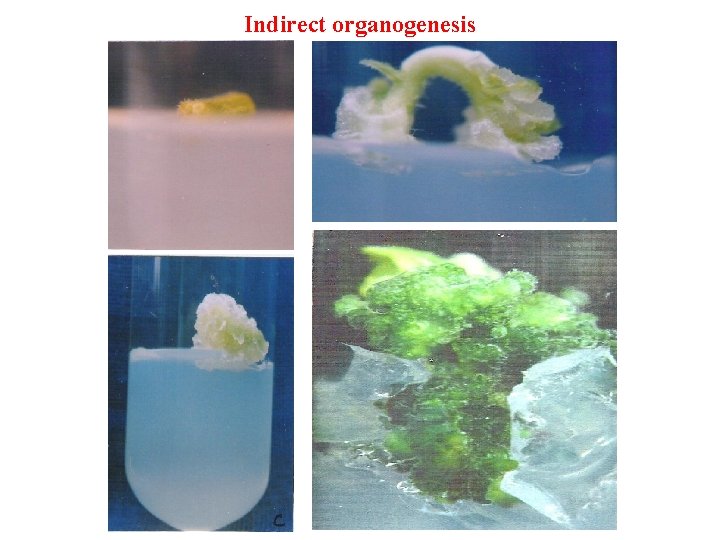
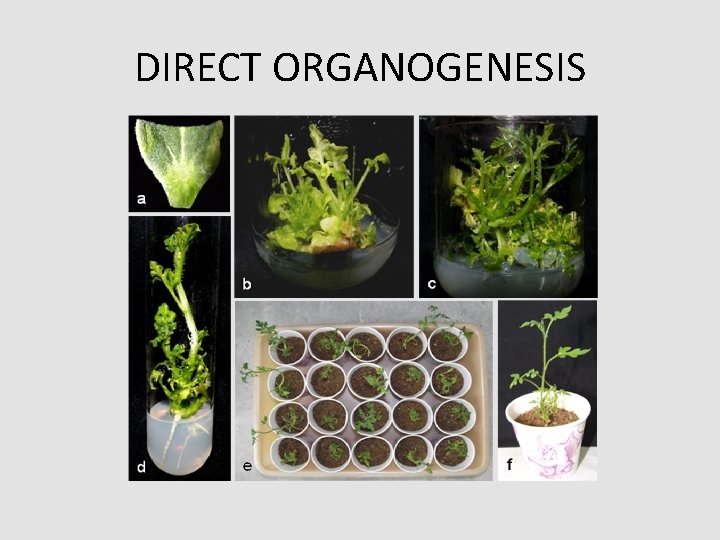
Pathways of Regeneration Organogenesis Direct Organogenesis (Micropropagation) Explant - Shoot - Root - Plantlet In. Direct Organogenesis Explant - callus – adventitious organs(shoot and root ) - Plantlet

Indirect organogenesis

INDIRECT ORGANOGENESIS A C B E H I D F G J K

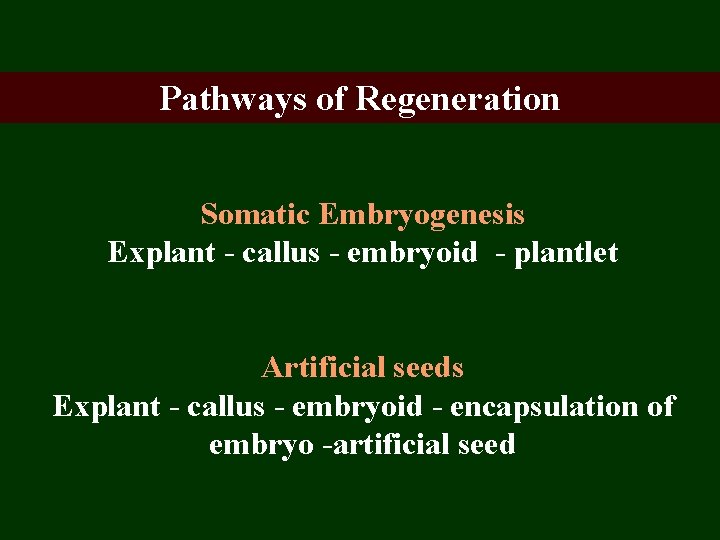
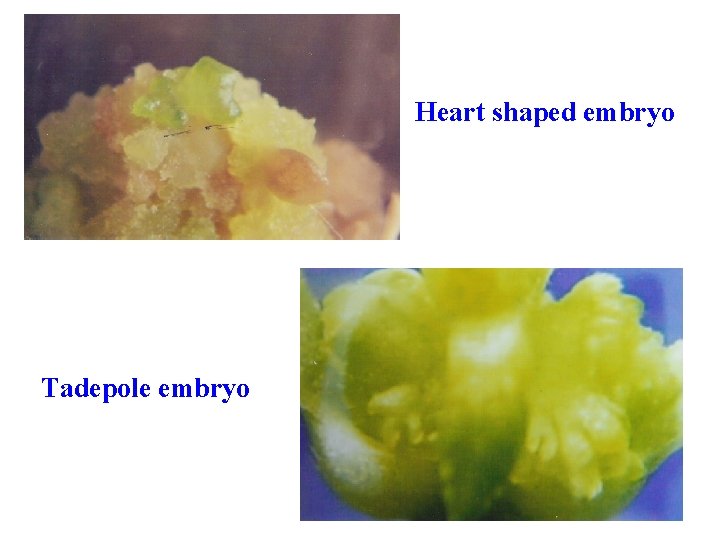
Pathways of Regeneration Somatic Embryogenesis Explant - callus - embryoid - plantlet Artificial seeds Explant - callus - embryoid - encapsulation of embryo -artificial seed

Heart shaped embryo Tadepole embryo


Applications Micropropagation Artificial seed production Androgenesis Somatic hybridization

Micropropogation : Protocol In Vivo Plant Medium Preparation Explant Sterilization Inoculation Incubation Plant let Acclimatization





DIRECT ORGANOGENESIS


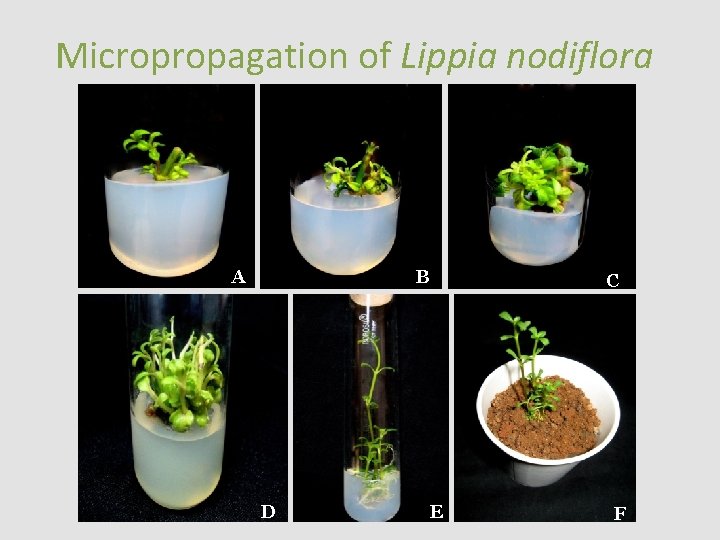
Micropropagation of Lippia nodiflora A B D C E F

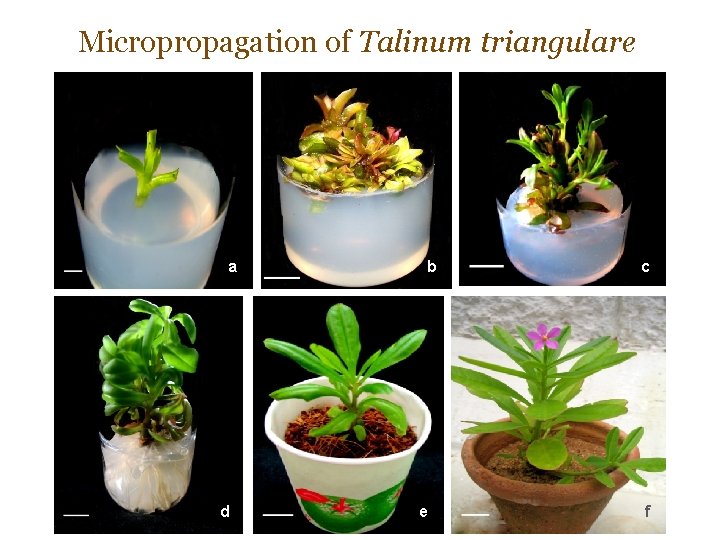
Micropropagation of Talinum triangulare a d b e c f

Micropropagation of Orthosiphon stamineus a d b c e f

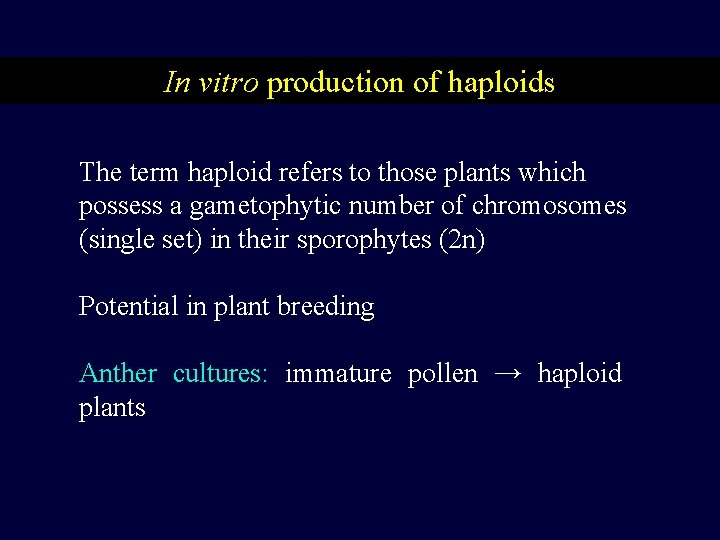
In vitro production of haploids The term haploid refers to those plants which possess a gametophytic number of chromosomes (single set) in their sporophytes (2 n) Potential in plant breeding Anther cultures: immature pollen → haploid plants





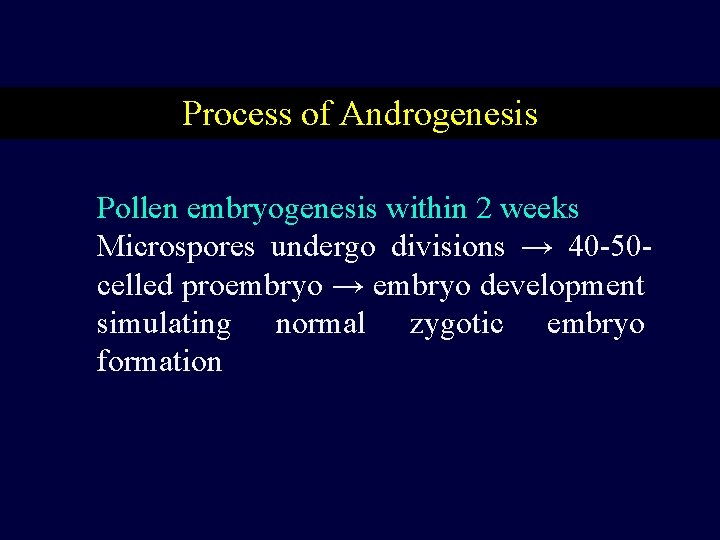
Process of Androgenesis Pollen embryogenesis within 2 weeks Microspores undergo divisions → 40 -50 celled proembryo → embryo development simulating normal zygotic embryo formation

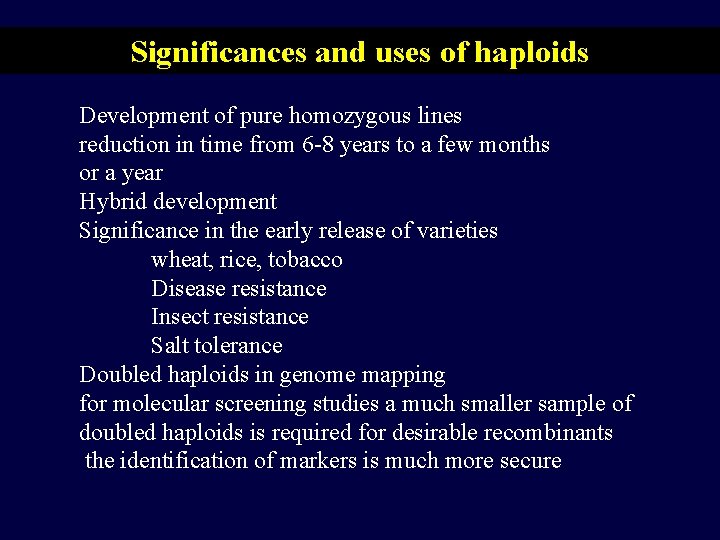
Significances and uses of haploids Development of pure homozygous lines reduction in time from 6 -8 years to a few months or a year Hybrid development Significance in the early release of varieties wheat, rice, tobacco Disease resistance Insect resistance Salt tolerance Doubled haploids in genome mapping for molecular screening studies a much smaller sample of doubled haploids is required for desirable recombinants the identification of markers is much more secure

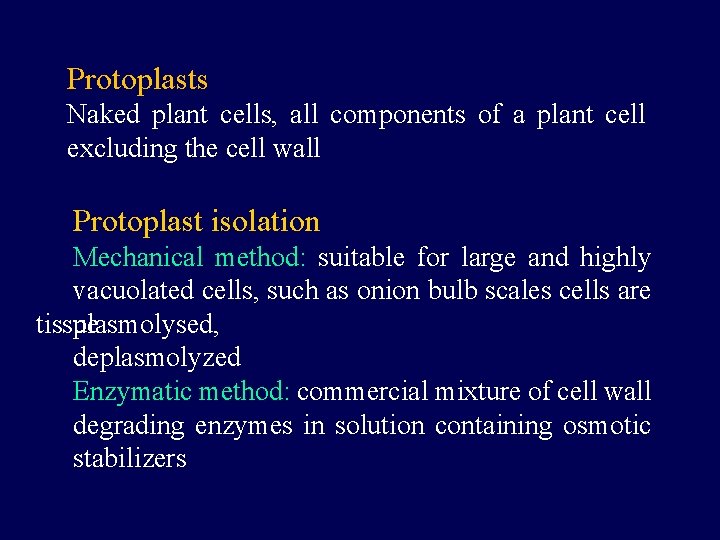
Protoplasts Naked plant cells, all components of a plant cell excluding the cell wall Protoplast isolation Mechanical method: suitable for large and highly vacuolated cells, such as onion bulb scales cells are tissue plasmolysed, deplasmolyzed Enzymatic method: commercial mixture of cell wall degrading enzymes in solution containing osmotic stabilizers



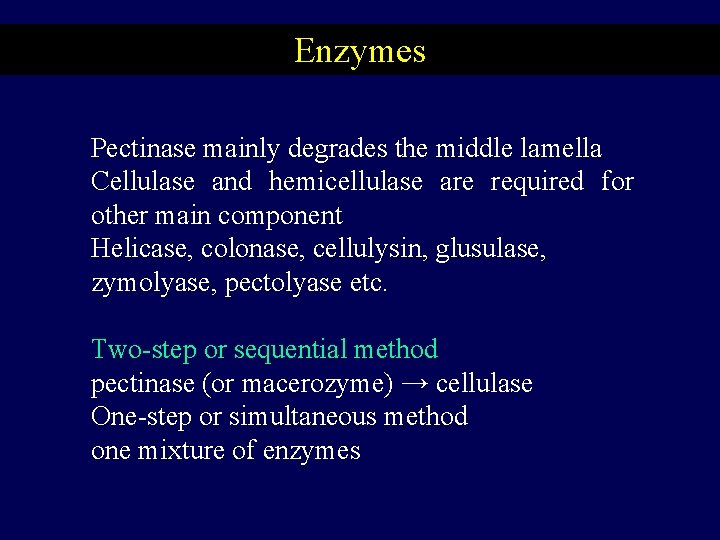
Enzymes Pectinase mainly degrades the middle lamella Cellulase and hemicellulase are required for other main component Helicase, colonase, cellulysin, glusulase, zymolyase, pectolyase etc. Two-step or sequential method pectinase (or macerozyme) → cellulase One-step or simultaneous method one mixture of enzymes

Protoplast viability and density FDA (fluorescein diacetate) staining accumulates inside the plasmalemma of viable protoplasts Phenosafranine staining, Calcofluor White (CFW), oxygen uptake, photosynthesis Maximum and minimum plating densities forgrowth

Protoplast development Regeneration of cell wall starts within a few hours after isolation → cell expansion → cell division (within 2 -7 days) → cell colonies → callus in an osmotic free medium →organogenesis/embryogenic differentation

Somatic hybridization Sexual hybridization is limited in most cases to cultivars within a species or a few wild species closely related to a cultivated crop due to incompatibility Plant propoplasts offer exciting possibilities in the somatic cell genetics and crop improvement Somatic hybridization = fusion of isolated somatic protoplasts and development of their product (heterokaryon) to a hybrid plant The nucleus and cytoplasm of both parents are fused (except in cybrids)

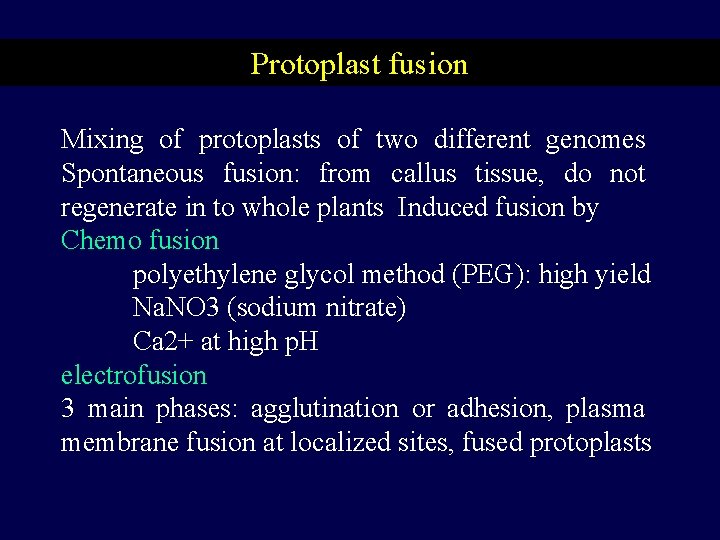
Protoplast fusion Mixing of protoplasts of two different genomes Spontaneous fusion: from callus tissue, do not regenerate in to whole plants Induced fusion by Chemo fusion polyethylene glycol method (PEG): high yield Na. NO 3 (sodium nitrate) Ca 2+ at high p. H electrofusion 3 main phases: agglutination or adhesion, plasma membrane fusion at localized sites, fused protoplasts


Verification and characterization of somatic hybrids Molecular techniques genetic constitution can be studied e. g. using specific restriction patterns of chloroplast and mitochondrial DNA, and molecular markers such as AFLP (Amplified Fragment Length Polymorphism) RFLP (Restriction Fragment Length Polymorphism) RAPD (Random Amplified Polymorphic DNA) microsatellites

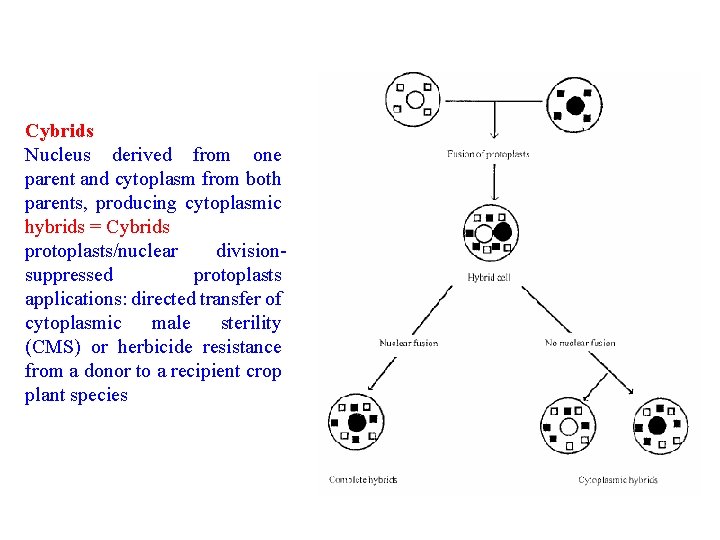
Cybrids Nucleus derived from one parent and cytoplasm from both parents, producing cytoplasmic hybrids = Cybrids protoplasts/nuclear divisionsuppressed protoplasts applications: directed transfer of cytoplasmic male sterility (CMS) or herbicide resistance from a donor to a recipient crop plant species

Biotechnology can certainly provide solutions to most problems associated with breeding for rice improvement. Anther culture technique offers great opportunities for accelerating breeding progress and generating as well as directing variation to increase genetic diversity useful for rice improvement.

Thank You
 Illinois crop improvement association
Illinois crop improvement association Biotechnology in crop improvement
Biotechnology in crop improvement Plant tissue culture terminology
Plant tissue culture terminology Uses of plant tissue culture
Uses of plant tissue culture Callus culture ppt
Callus culture ppt Tissue culture applications
Tissue culture applications Purpose of plant tissue culture
Purpose of plant tissue culture Plant tissue culture application
Plant tissue culture application Plant tissue culture
Plant tissue culture Applications of plant tissue culture
Applications of plant tissue culture Plant tissue
Plant tissue Define pharmacognosy
Define pharmacognosy Perforation plates
Perforation plates Techniques of tissue culture
Techniques of tissue culture Ms media preparation
Ms media preparation Macronutrients and micronutrients in plants
Macronutrients and micronutrients in plants Cultured pearl process
Cultured pearl process Seed culture ppt
Seed culture ppt Peta konsep struktur dan fungsi jaringan hewan
Peta konsep struktur dan fungsi jaringan hewan Ground tissue
Ground tissue Vascular tissue
Vascular tissue Which tissue transports water around a plant?
Which tissue transports water around a plant? Plant tissue and organs
Plant tissue and organs Plant tissue and organs
Plant tissue and organs Parenchyma
Parenchyma Plants are multicellular eukaryotes
Plants are multicellular eukaryotes Plant tissue
Plant tissue Agranulocytes
Agranulocytes Plant ground tissue
Plant ground tissue Protoderm
Protoderm Sclerenchyma function
Sclerenchyma function Plant ground tissue
Plant ground tissue Difference between simple and compound tissue
Difference between simple and compound tissue Relativism
Relativism Individual culture traits combine to form culture patterns.
Individual culture traits combine to form culture patterns. Batch culture vs continuous culture
Batch culture vs continuous culture Continuous culture and batch culture
Continuous culture and batch culture Individualistic culture definition
Individualistic culture definition Indian vs american culture
Indian vs american culture Stab and stroke culture
Stab and stroke culture Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram Sub culture vs counter culture
Sub culture vs counter culture What is folk culture
What is folk culture Urease test
Urease test Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram Inert organizational culture
Inert organizational culture Carpet culture method
Carpet culture method Lawn or carpet culture
Lawn or carpet culture Quality culture
Quality culture Surface culture deep culture and esol
Surface culture deep culture and esol