Reaction Engineering Continuous culture Fin Fout 0 V





















- Slides: 51

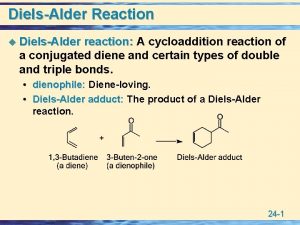
Reaction Engineering

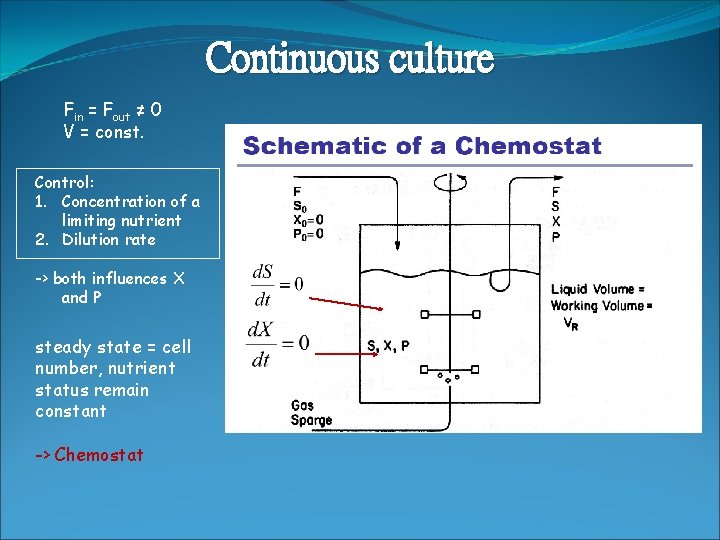
Continuous culture Fin = Fout ≠ 0 V = const. Control: 1. Concentration of a limiting nutrient 2. Dilution rate -> both influences X and P steady state = cell number, nutrient status remain constant -> Chemostat


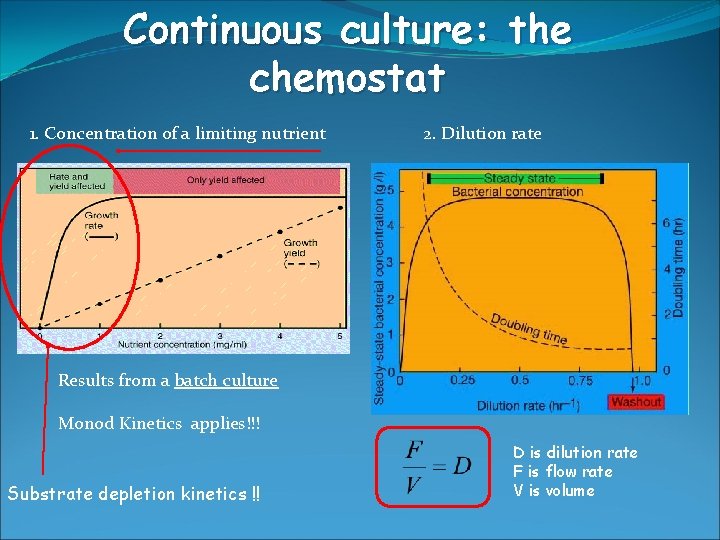
Continuous culture: the chemostat 1. Concentration of a limiting nutrient 2. Dilution rate Results from a batch culture Monod Kinetics applies!!! Substrate depletion kinetics !! D is dilution rate F is flow rate V is volume

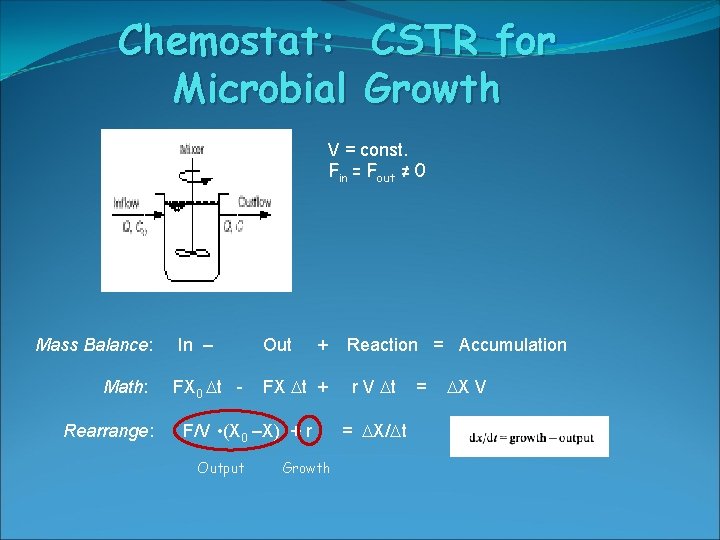
Chemostat: Microbial V = const. Fin = Fout ≠ 0 CV Mass Balance: Math: Rearrange: CSTR for Growth In – Out + FX 0 t - FX t + r V t F/V • (X 0 –X) + r Output Growth Reaction = Accumulation = X/ t = X V

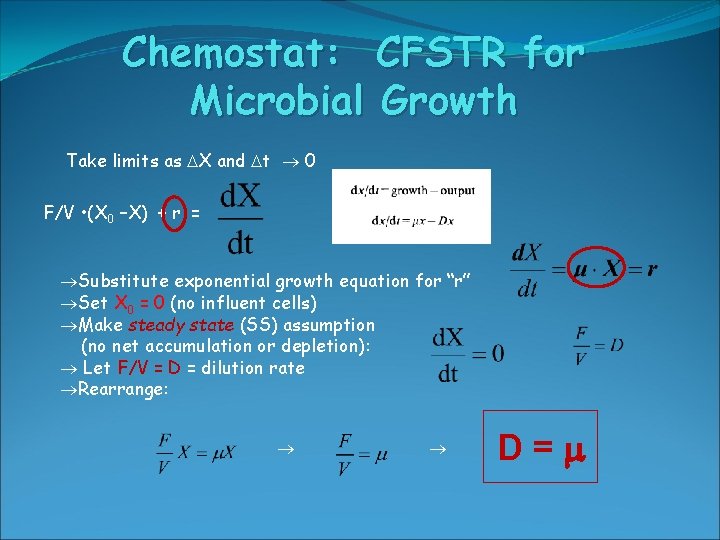
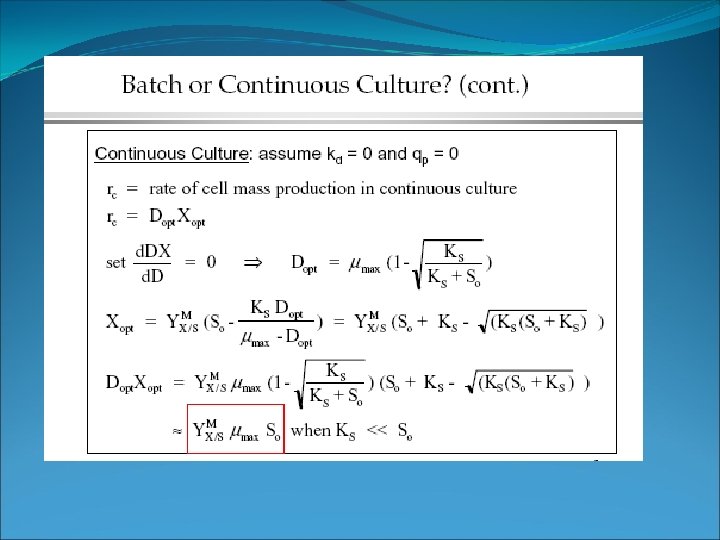
Chemostat: CFSTR for Microbial Growth Take limits as X and t 0 F/V • (X 0 –X) + r = Substitute exponential growth equation for “r” Set X 0 = 0 (no influent cells) Make steady state (SS) assumption (no net accumulation or depletion): Let F/V = D = dilution rate Rearrange: D=m


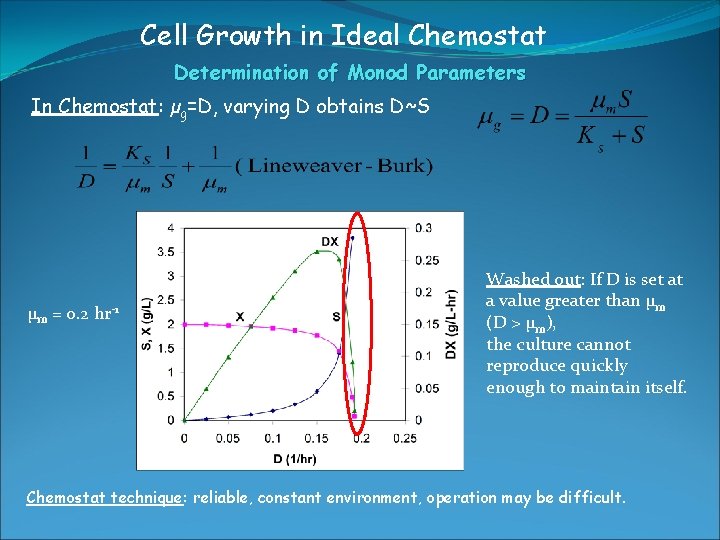
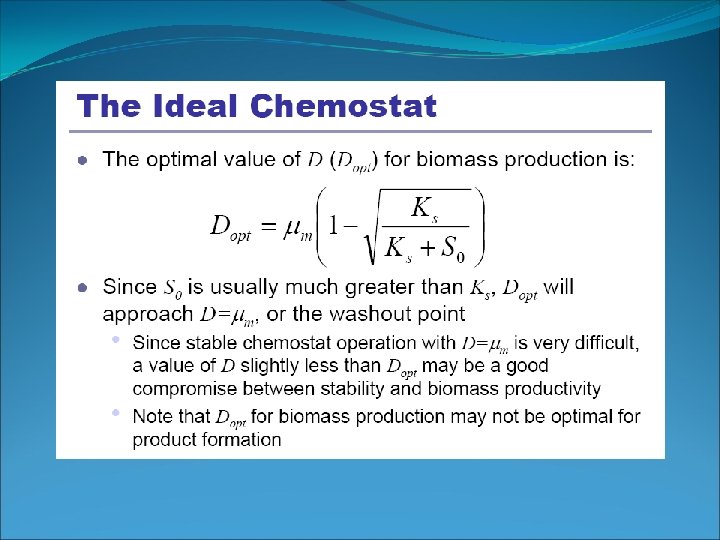
Cell Growth in Ideal Chemostat Determination of Monod Parameters In Chemostat: µg=D, varying D obtains D~S µm = 0. 2 hr-1 Washed out: If D is set at a value greater than µm (D > µm), the culture cannot reproduce quickly enough to maintain itself. Chemostat technique: reliable, constant environment, operation may be difficult.









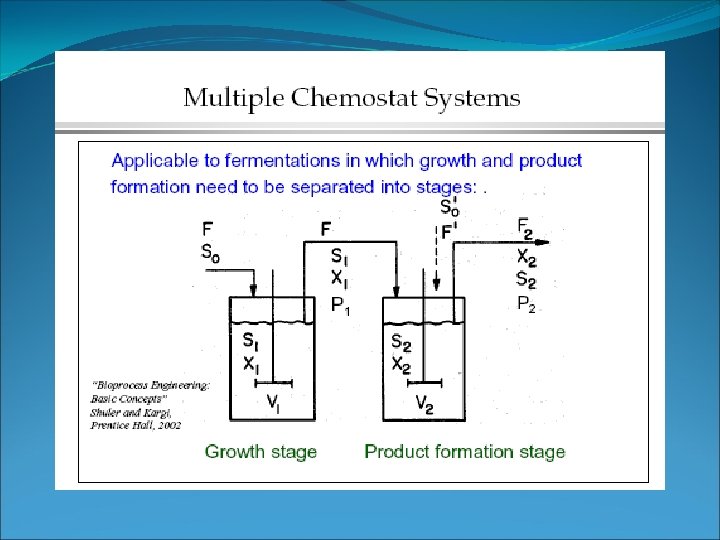
Fed batch fermentation -> In batch reactor, S and X are high. No transport of S or X and no control on µ. -> In chemostat, S and X are low. Transport of S or X and control on µ. -> In fed batch reactor. Substrate transport in, not out. No biomass transport. Why fed batch? 1. Low S no toxicity / osmotic problem 2. High X high P easier downstream processing 3. Control of µ?

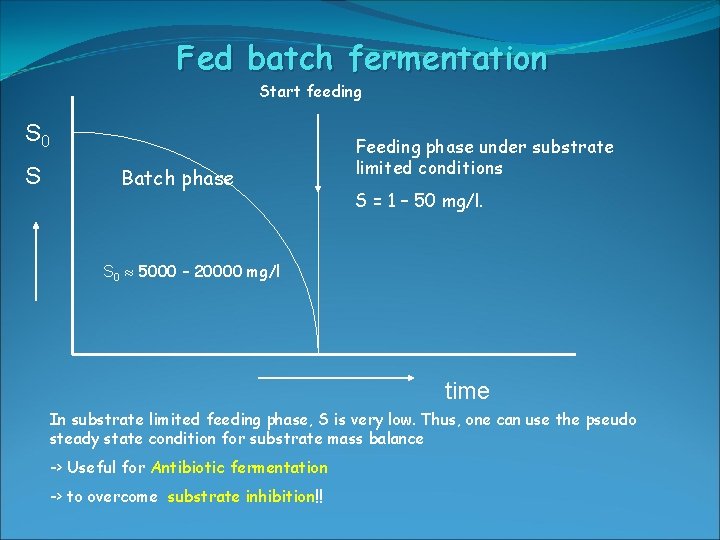
Fed batch fermentation Start feeding S 0 S Batch phase Feeding phase under substrate limited conditions S = 1 – 50 mg/l. S 0 5000 – 20000 mg/l time In substrate limited feeding phase, S is very low. Thus, one can use the pseudo steady state condition for substrate mass balance -> Useful for Antibiotic fermentation -> to overcome substrate inhibition!!



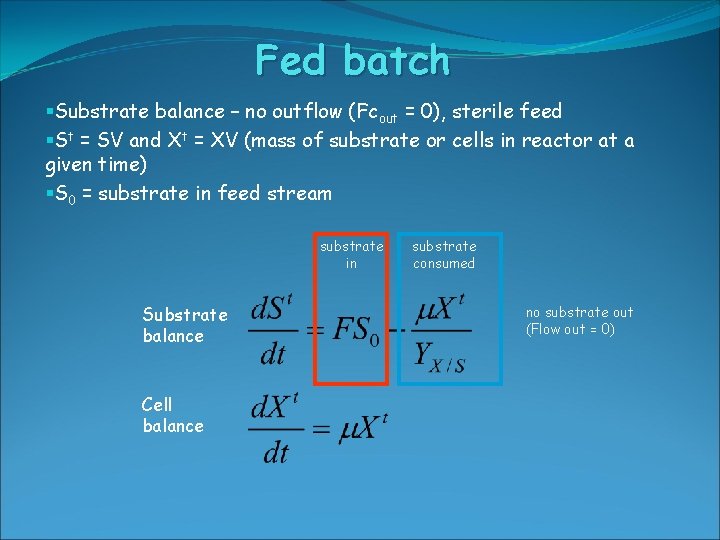
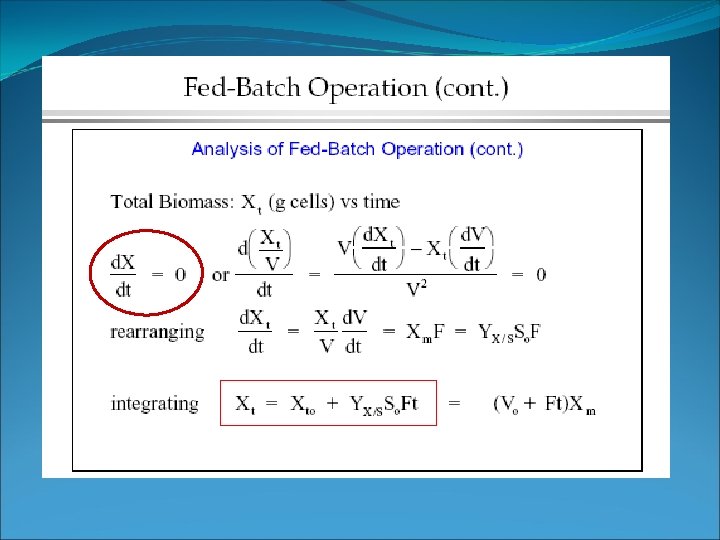
Fed batch §Substrate balance – no outflow (Fcout = 0), sterile feed §St = SV and Xt = XV (mass of substrate or cells in reactor at a given time) §S 0 = substrate in feed stream substrate in Substrate balance Cell balance substrate consumed no substrate out (Flow out = 0)

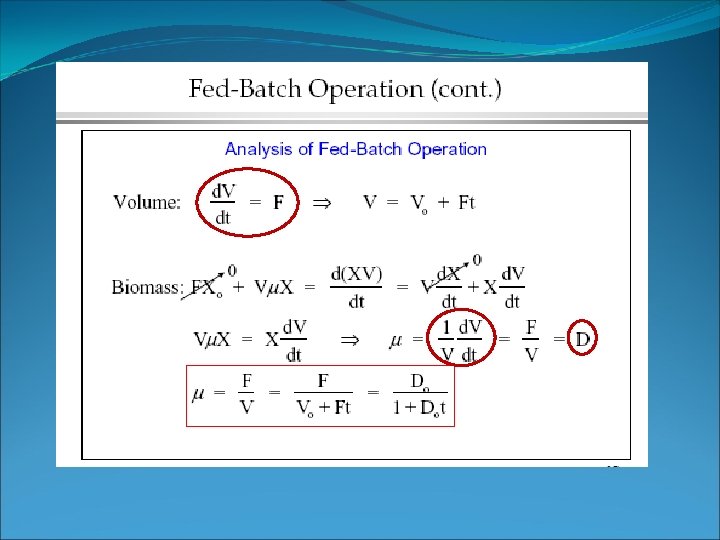
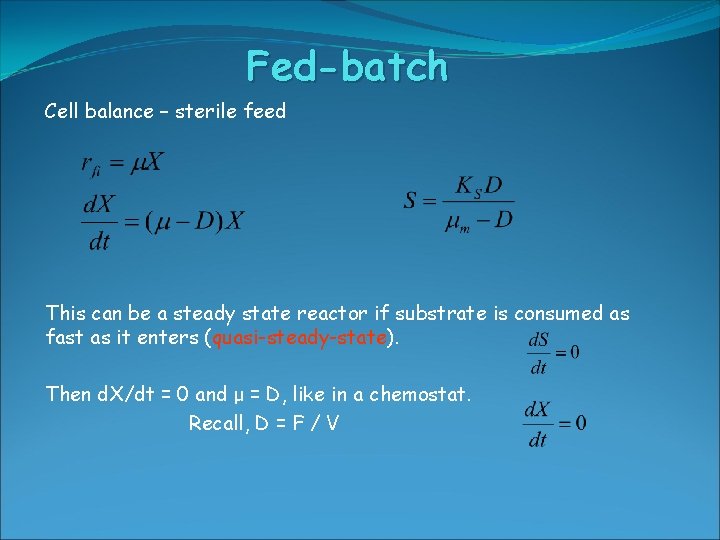
Fed-batch Cell balance – sterile feed This can be a steady state reactor if substrate is consumed as fast as it enters (quasi-steady-state). Then d. X/dt = 0 and μ = D, like in a chemostat. Recall, D = F / V


Fed batch • What this means • the total amount of cells in the reactor increases with time -> with increasing V • dilution rate and μ decrease with time in fed batch culture • Since μ = D, the growth rate is controlled by the dilution rate.


Minibioreactors -> Volumes below 100 ml Characterized by: -> area of application -> mass transfer -> mixing characteristics

Minibioreactors Why do we want to scale down ? - Parallelization (optimization, screening) - automatization - cost reduction What can you optimize? - Biocatalyst (organism) design - medium (growth conditions) design - process design

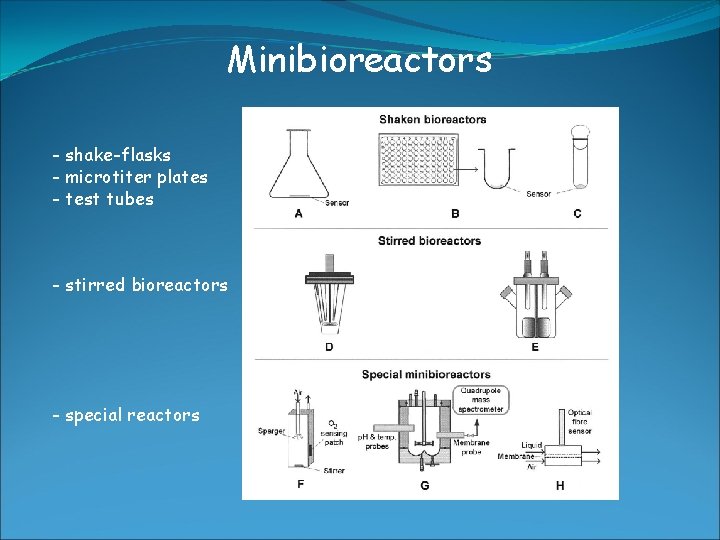
Minibioreactors - shake-flasks - microtiter plates - test tubes - stirred bioreactors - special reactors

Minibioreactors Shaking flasks: -> easy to handle -> low price -> volumne 25 ml – 5 L (filled with medium 20% of volumne) -> available with integrated sensors (O 2, p. H) -> limitation: O 2 limitation (aeration) -> during growth improved by 1. baffled flasks 2. membranes instead of cotton -> during sampling

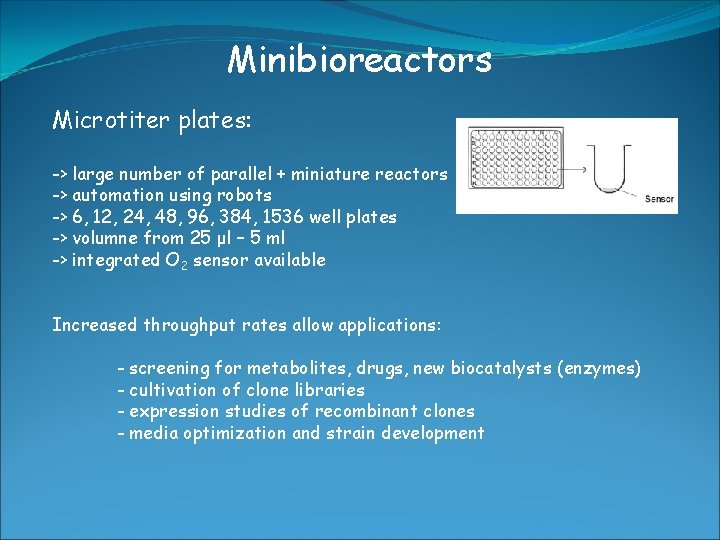
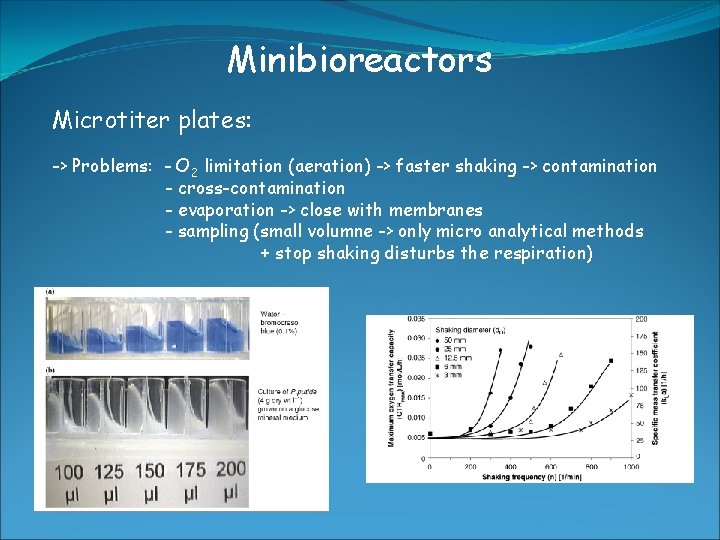
Minibioreactors Microtiter plates: -> large number of parallel + miniature reactors -> automation using robots -> 6, 12, 24, 48, 96, 384, 1536 well plates -> volumne from 25 μl – 5 ml -> integrated O 2 sensor available Increased throughput rates allow applications: - screening for metabolites, drugs, new biocatalysts (enzymes) - cultivation of clone libraries - expression studies of recombinant clones - media optimization and strain development

Minibioreactors Microtiter plates: -> Problems: - O 2 limitation (aeration) -> faster shaking -> contamination - cross-contamination - evaporation -> close with membranes - sampling (small volumne -> only micro analytical methods + stop shaking disturbs the respiration)

Minibioreactors Test tubes: -> useful for developing inoculums -> screening -> volumne 2 -25 ml (20% filled with medium) -> simple and low costs -> O 2 transfer rate low -> usually no online monitoring (p. H and O 2) -> interruption of shaking during sampling

Minibioreactors Stirred Systems: -> homogeneous environment -> sampling, online monitoring, control possible without disturbance of culture -> increased mixing (stirring) + mass transfer (gassing rate)

Minibioreactors Stirred Systems – Stirred Minibioreactor -> T, p. H, dissolved O 2 can be controlled -> Volumne from 50 ml – 300 ml -> small medium requirenments -> low costs (isotope labeling) -> good for research -> good for continous cultivation -> Limitation: - system expensive due to minimization (control elements) - not good for high-throughput applications

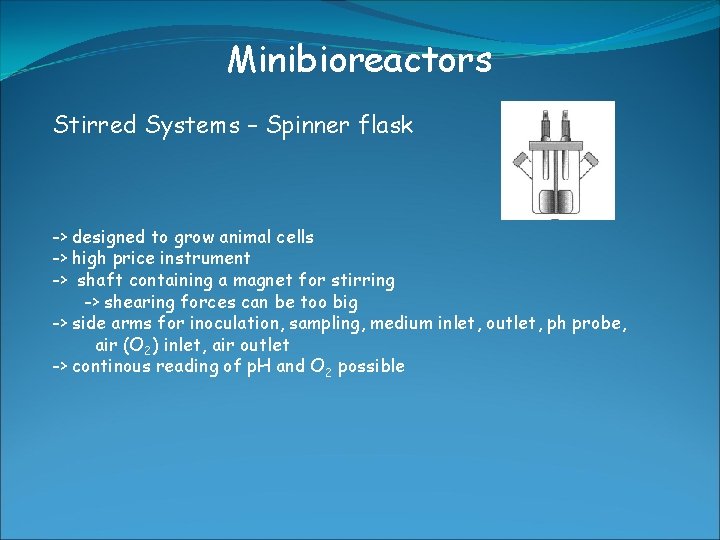
Minibioreactors Stirred Systems – Spinner flask -> designed to grow animal cells -> high price instrument -> shaft containing a magnet for stirring -> shearing forces can be too big -> side arms for inoculation, sampling, medium inlet, outlet, ph probe, air (O 2) inlet, air outlet -> continous reading of p. H and O 2 possible

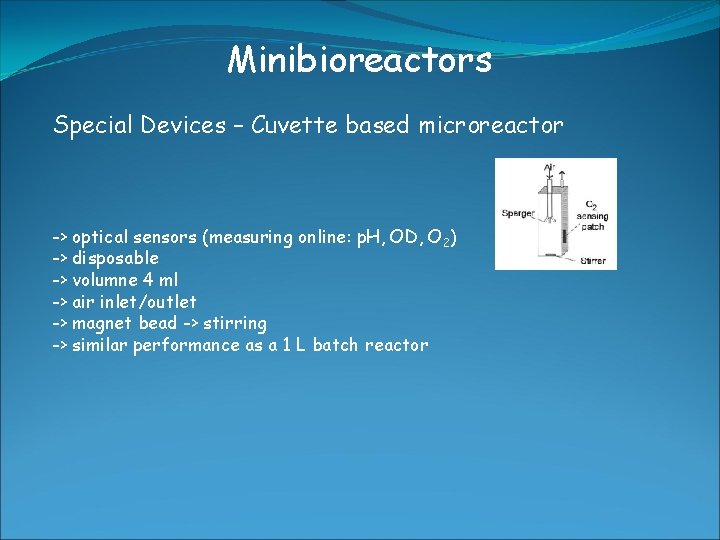
Minibioreactors Special Devices – Cuvette based microreactor -> optical sensors (measuring online: p. H, OD, O 2) -> disposable -> volumne 4 ml -> air inlet/outlet -> magnet bead -> stirring -> similar performance as a 1 L batch reactor

Minibioreactors Special Devices – Miniature bioreactor with integrated membrane for MS measurement: -> custom made -> expensive -> a few ml -> online analysis of H 2, CH 4, O 2, N 2, CO 2, and many other products, substrate, . . . -> used to follow respiratory dynamics of culture (isotope labeling) -> stirred vessel with control of T, O 2, p. H -> MS measurements within a few seconds to minutes -> continous detection -> fast kinetic measurements, metabolic studies

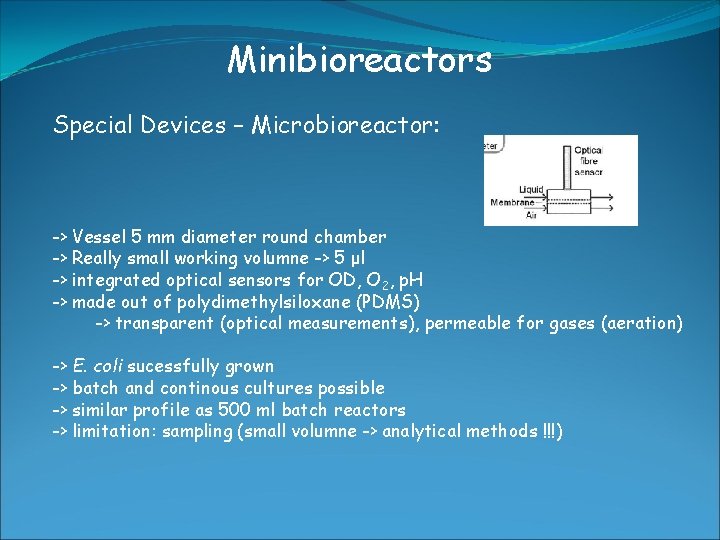
Minibioreactors Special Devices – Microbioreactor: -> Vessel 5 mm diameter round chamber -> Really small working volumne -> 5 μl -> integrated optical sensors for OD, O 2, p. H -> made out of polydimethylsiloxane (PDMS) -> transparent (optical measurements), permeable for gases (aeration) -> E. coli sucessfully grown -> batch and continous cultures possible -> similar profile as 500 ml batch reactors -> limitation: sampling (small volumne -> analytical methods !!!)

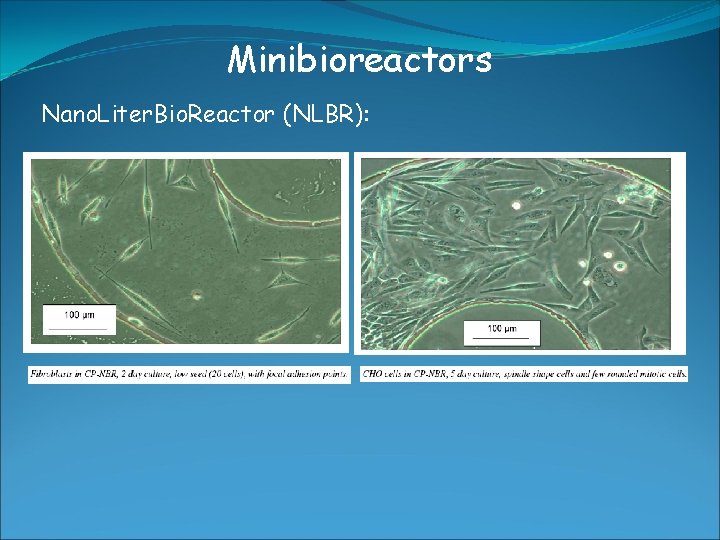
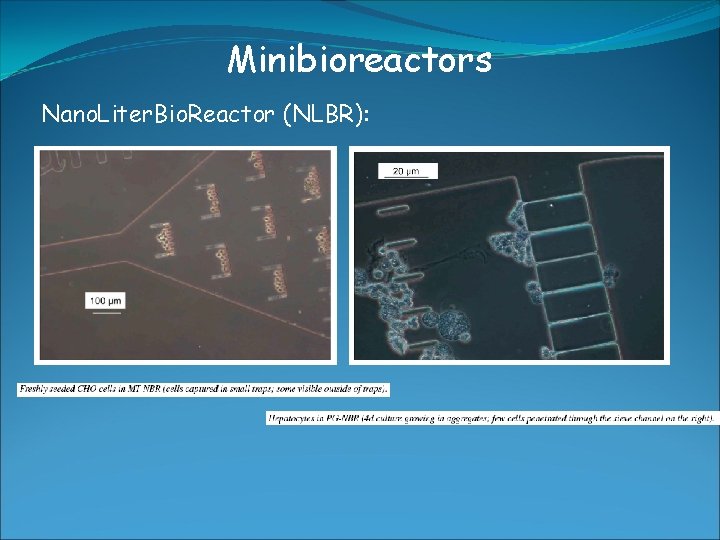
Minibioreactors Nano. Liter. Bio. Reactor (NLBR): -> used for growing up to several 100 mammalien cells -> culture volumne around 20 μl -> online control of O 2, p. H, T -> culture chamber with inlet/outlet ports (microfluidic systems) -> manufactured by soft-lithography techniques -> made out of polydimethylsiloxane (PDMS) -> transparent (optical measurements), permeable for gases (aeration) -> direct monitoring of culture condition -> PDMS is transparent -> flourescence microscope -> limitation: batch culture very difficult-> too small volumne -> suffers from nutrient limitation -> But in principle system allows -> batch, fed-batch, continous

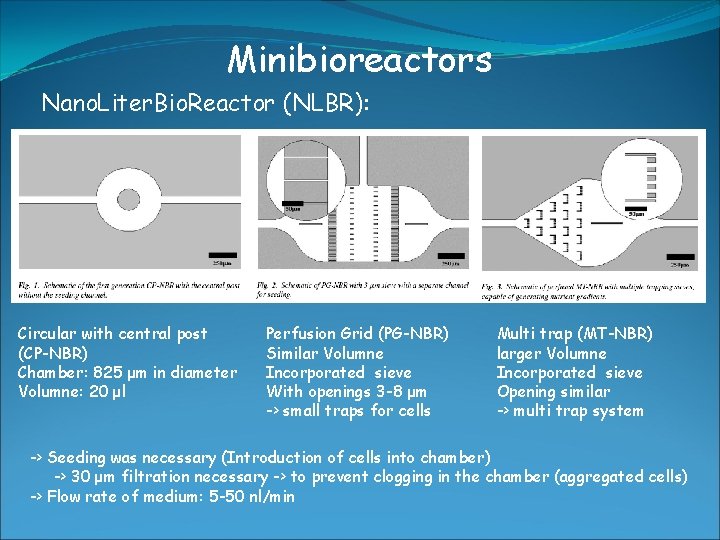
Minibioreactors Nano. Liter. Bio. Reactor (NLBR): Circular with central post (CP-NBR) Chamber: 825 μm in diameter Volumne: 20 μl Perfusion Grid (PG-NBR) Similar Volumne Incorporated sieve With openings 3 -8 μm -> small traps for cells Multi trap (MT-NBR) larger Volumne Incorporated sieve Opening similar -> multi trap system -> Seeding was necessary (Introduction of cells into chamber) -> 30 μm filtration necessary -> to prevent clogging in the chamber (aggregated cells) -> Flow rate of medium: 5 -50 nl/min

Minibioreactors Nano. Liter. Bio. Reactor (NLBR):

Minibioreactors Nano. Liter. Bio. Reactor (NLBR):


Minibioreactors Why do we want micro-and nano reactors? Applications in: - Molecular biology - Biochemistry - Cell biology - Medical devices - Biosensors -> with the aim to look at single cells !!!

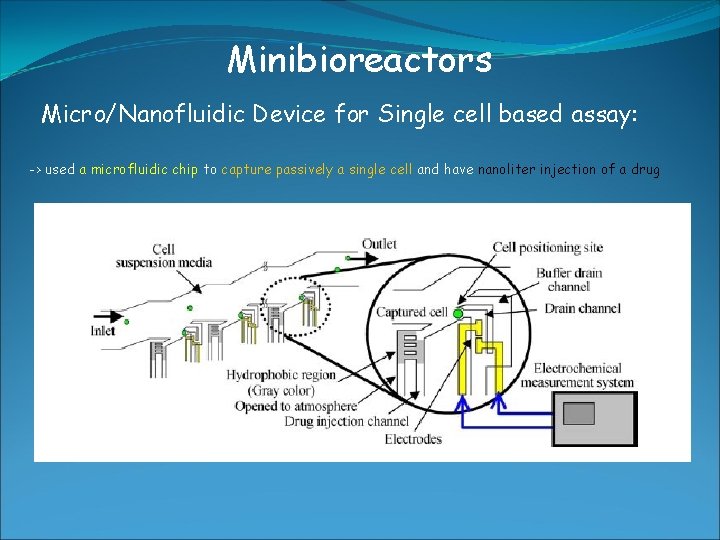
Minibioreactors Micro/Nanofluidic Device for Single cell based assay: -> used a microfluidic chip to capture passively a single cell and have nanoliter injection of a drug

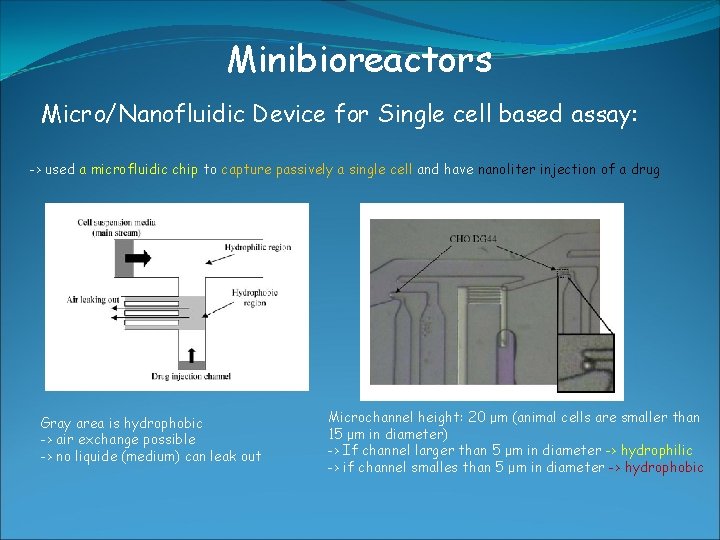
Minibioreactors Micro/Nanofluidic Device for Single cell based assay: -> used a microfluidic chip to capture passively a single cell and have nanoliter injection of a drug Gray area is hydrophobic -> air exchange possible -> no liquide (medium) can leak out Microchannel height: 20 μm (animal cells are smaller than 15 μm in diameter) -> If channel larger than 5 μm in diameter -> hydrophilic -> if channel smalles than 5 μm in diameter -> hydrophobic


Class Exercise Problem 6. 17 E. coli is cultivated in continuous culture under aerobic conditions with glucose limitation. When the system is operated at D= 0. 2 hr-1, determine the effluent glucose and biomass concentrations assuming Monod kinetics (S 0 = 5 g/l, mm= 0. 25 hr-1 , KS = 100 mg/L, Y x/s = 0. 4 g/g)



Class Exercise – 9. 4 Penicillin is produced in a fed-batch culture with the intermittent addition of glucose solution to the culture medium. The initial culture volume at quasisteady state is V 0= 500 L, and the glucose containing nutrient solution is added with a flow rate of F = 50 L/h. X 0 = 20 g/L, S 0 = 300 g/L, mm = 0. 2 h-1, Ks = 0. 5 g/L and Y x/s= 0. 3 g/g Determine culture volume at t = 10 h Determine concentration of glucose at t = 10 h Determine the concentration and total amount of cells at t = 10 h If qp = 0. 05 g product. g cells h and P 0 = 0. 1 g/L, determine the product concentration at t = 10 h
 Type 1 fout
Type 1 fout Type 1 fout
Type 1 fout Type 1 fout
Type 1 fout Welk woord is fout gespeld groep 8
Welk woord is fout gespeld groep 8 Pleonasme betekenis
Pleonasme betekenis Spf fin fod fin
Spf fin fod fin Faces superposables
Faces superposables Batch culture vs continuous culture
Batch culture vs continuous culture Continuous culture and batch culture
Continuous culture and batch culture Parallel action in the future
Parallel action in the future Without information
Without information Order of reaction
Order of reaction Addition reaction and substitution reaction
Addition reaction and substitution reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Proton capture equation
Proton capture equation Material culture and nonmaterial culture
Material culture and nonmaterial culture Sociologists define a symbol as
Sociologists define a symbol as Individualistic culture vs. collectivist culture
Individualistic culture vs. collectivist culture Indian vs american culture
Indian vs american culture Stroke culture method
Stroke culture method Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram A sub-culture group
A sub-culture group Folk cultures are spread primarily by
Folk cultures are spread primarily by Tsi
Tsi Homework due today
Homework due today In an inert organizational culture,
In an inert organizational culture, Stroke culture method
Stroke culture method Describe lawn culture and surface plating
Describe lawn culture and surface plating Characteristics of quality culture
Characteristics of quality culture Surface culture deep culture and esol
Surface culture deep culture and esol Chemical reaction engineering
Chemical reaction engineering Chemical reaction engineering
Chemical reaction engineering Chemical reaction engineering
Chemical reaction engineering Chemical reaction engineering
Chemical reaction engineering Series reaction example
Series reaction example Chemical reaction engineering
Chemical reaction engineering System procurement process in software engineering
System procurement process in software engineering Forward engineering and reverse engineering
Forward engineering and reverse engineering Dicapine
Dicapine Engineering elegant systems: theory of systems engineering
Engineering elegant systems: theory of systems engineering Reverse engineering vs forward engineering
Reverse engineering vs forward engineering Agile ibm continuous engineering
Agile ibm continuous engineering Present and past continuous tense
Present and past continuous tense Present and past progressive tense
Present and past progressive tense Past simple present perfect past continuous
Past simple present perfect past continuous Future continuous razlaga
Future continuous razlaga Come past simple
Come past simple Future perfect by the time
Future perfect by the time Continuous monitoring vs continuous auditing
Continuous monitoring vs continuous auditing Continuous control monitoring tools
Continuous control monitoring tools Past simple past continuous past perfect simple
Past simple past continuous past perfect simple Future perfect continuous and simple
Future perfect continuous and simple