Plant Tissue Culture Topics covered in this presentationHistory


















- Slides: 45

Plant Tissue Culture Topics covered in this presentation-History and scope, preparation and sterilization, terms used in tissue culture, plant tissue culture media. Types of culture, basic technique of plant tissue culture. Ppt By- Nitin Swamy Asst. Professor, Department of Biotecnology, St. Aloysius College (Autonomous), Jabalpur

What is plant tissue culture? Plant tissue culture is a technique of growing plant cells, tissues, organs, seeds or other plant parts in a sterile environment on a nutrient medium. Tissue culture had its origins at the beginning of the 20 th century with the work of Gottleib Haberlandt (plants).

The Background, The first commercial use of plant clonal propagation on artificial media was in the germination and growth of orchid plants, in the 1920’s In the 1950’s and 60’s there was a great deal of research, but it was only after the development of a reliable artificial medium (Murashige & Skoog, 1962) that plant tissue culture really ‘took off’ commercially Young cymbidium orchids

WHY? The production of clones of plants that produce particularly good flowers, fruits, or have other desirable traits. To quickly produce mature plants. The production of multiples of plants in the absence of seeds or necessary pollinators to produce seeds. The regeneration of whole plants from plant cells that have been genetically modified. The production of plants in sterile containers reduces disease transmission Allows production of plants from seeds that otherwise have very low chances of germinating and growing, i. e. : orchids and Nepenthes. To clean particular plants of viral and other infections and to quickly multiply these plants as 'cleaned stock' for horticulture and agriculture.

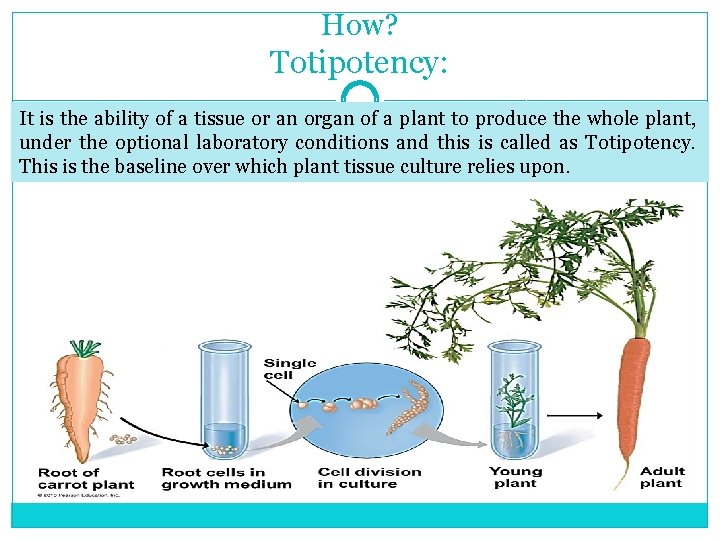
How? Totipotency: It is the ability of a tissue or an organ of a plant to produce the whole plant, under the optional laboratory conditions and this is called as Totipotency. This is the baseline over which plant tissue culture relies upon.

Plant Tissue Culture Terminology Adventitious Developing from unusual points of origin, such as shoot or root tissues, from callus or embryos, from sources other than zygotes. Agar a polysaccharide powder derived from algae used to gel a medium. Agar is generally used at a concentration of 6 12 g/liter. Aseptic Free of microorganisms. Aseptic Technique- Procedures used to prevent the introduction of fungi, bacteria, viruses, mycoplasma or other microorganisms into cultures. Callus An unorganized, proliferate mass of differentiated plant cells, a wound response. Chemically Defined Medium- A nutritive solution for culturing cells in which each component is specifiable and ideally of known chemical structure. Contamination Being infested with unwanted microorganisms such as bacteria or fungi. Culture—A plant growing in vitro. Differentiated Cells that maintain, in culture, all or much of the specialized structure and function typical of the cell type in vivo. Modifications of new cells to form tissues or organs with a specific function.

Plant Tissue Culture Terminology Explant Tissue taken from its original site and transferred to an artificial medium for growth or maintenance. Hormones Growth regulators, generally synthetic in occurrence, that strongly affects growth (i. e. cytokinins, auxins, and gibberellins). Internode The space between two nodes on a stem Media Plural of medium Medium A nutritive solution, solid or liquid, for culturing cells. Micropropagation In vitro Clonal propagation of plants from shoot tips or nodal explants, usually with an accelerated proliferation of shoots during subcultures. Node—A part of the plant stem from which a leaf, shoot or flower originates. Plant Tissue Culture The growth or maintenance of plant cells, tissues, organs or whole plants in vitro. Regeneration In plant cultures, a morphogenetic response to a stimulus that results in the products of organs, embryos, or whole plants. Somaclones Plants derived from any form of cell culture involving the use of somatic plant cells.

Plant Tissue Culture Terminology Stage I A step in in vitro propagation characterized by the establishment of an aseptic tissue culture of a plant. Stage II A step in in vitro propagation characterized by the rapid numerical increase of organs or other structures. Stage III A step in in vitro propagation characterized by preparation of propagules for successful transfer to soil, a process involving rooting of shoot cuttings, hardening of plants, and initiating the change from the heterotrophic to the autotropic state. Stage IV A step in in vitro plant propagation characterized by the establishment in soil of a tissue culture derived plant, either after undergoing a Stage III pretransplant treatment, or in certain species, after the direct transfer of plants from Stage II into soil. Sterile (A) Without life. (B) Inability of an organism to produce functional gametes. (C) A culture that is free of viable microorganisms. Sterile Techniques The practice of working with cultures in an environment free from microorganisms.

Plant Tissue Culture Terminology Subculture See “Passage”. With plant cultures, this is the process by which the tissue or explant is first subdivide, then transferred into fresh culture medium. Tissue Culture The maintenance or growth of tissue, in vitro, in a way that may allow differentiation and preservation of their function. Totipotency A cell characteristic in which the potential forming all the cell types in the adult organism are retained. Undifferentiated With plant cells, existing in a state of cell development characterized by isodiametric cell shape, very little or no vacuole, a large nucleus, and exemplified by cells comprising an apical meristem or embryo.

Plant tissue Culture Basics Modern plant tissue culture is performed under aseptic conditions. Living plant materials from the environment are naturally contaminated on their surfaces (and sometimes interiors) with microorganisms, so surface sterilization of starting material (explants) in chemical solutions (usually alcohol and sodium or calcium hypochlorite is required).

Plant tissue Culture Basics Explants are then usually placed on the surface of a solid culture medium, but are sometimes placed directly into a liquid medium, when cell suspension cultures are desired. Culture media are generally composed of inorganic salts plus a few organic nutrients, vitamins and plant hormones.

Plant Tissue Culture Laboratory A plant tissue culture laboratory, whether for research or for commercial purpose, should provide certain basic facilities (i) washing and storage of glassware, plasticware and other labwares, (ii) preparation, sterilization and storage of nutrient media, (iii) aseptic manipulation of plant material, (iv) maintenance of cultures under controlled conditions of temperature, light and humidity, (v) observation of cultures and (vi) hardening of in vitro developed plants. The extent of sophistication in terms of equipment and facilities depends on the need and the funds available. Therefore, establishment of a new tissue culture facility requiring ingenuity and careful planning.

Plant Tissue Culture Media WHAT’S REALLY IMPORTANT?

Plant Tissue Culture owes its origin to the ideas of the German Scientist, Haberlandt, in the beginning of the 20 th century. This was just the beginning of the tissue culture; thereafter in 70's began the commercialization of the technology. Synthetic and natural media: When a medium is composed of chemically defined components, it is referred to as a synthetic medium. On the other hand, if a medium contains chemically undefined compounds (e. g. , vegetable extract, fruit juice, plant extract), it is regarded as a natural medium. Synthetic media have almost replaced the natural media for tissue culture. Expression of concentrations in media: The concentrations of inorganic and organic constituents in culture media are usually expressed as mass values (mg/l or ppm or mg I 1). However, as per the recommendations of the International Association of Plant Physiology, the concentrations of macronutrients should be expressed as mmol/l– and micronutrients as µmol/l–.

Major Types of Media: Ø Ø Ø White’s medium: This is one of the earliest plant tissue culture media developed for root culture. MS medium: Murashige and Skoog (MS) originally formulated a medium to induce organogenesis, and regeneration of plants in cultured tissues. These days, MS medium is widely used for many types of culture systems. B 5 medium: Developed by Gamborg, B 5 medium was originally designed for cell suspension and callus cultures. At present with certain modifications, this medium is used for protoplast culture. N 6 medium: Chu formulated this medium and it is used for cereal anther culture, besides other tissue cultures. Nitsch’s medium: This medium was developed by Nitsch and frequently used for anther cultures. Among the media referred above, MS medium is most frequently used in plant tissue culture work due to its success with several plant species and culture systems.

Major Constituents �Salt Mixtures �Organic Substances �Natural Complexes �Inert Supportive Materials �Growth Regulators

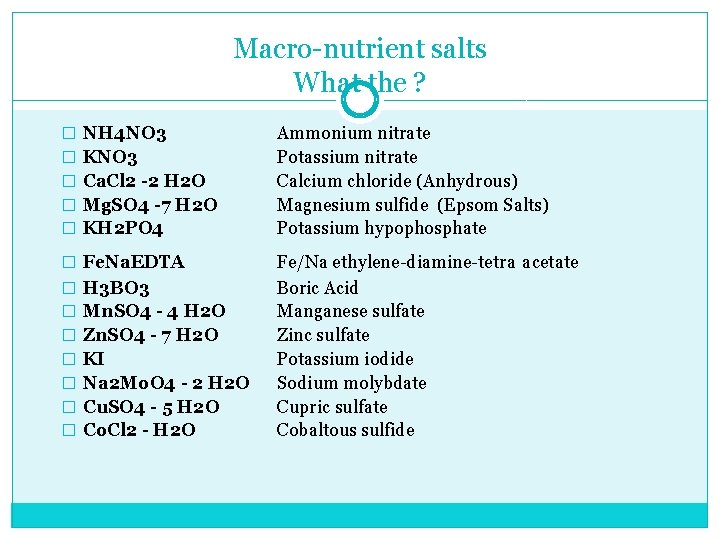
Macro nutrient salts What the ? � NH 4 NO 3 � KNO 3 � Ca. Cl 2 -2 H 2 O � Mg. SO 4 -7 H 2 O � KH 2 PO 4 � Fe. Na. EDTA � H 3 BO 3 � Mn. SO 4 - 4 H 2 O � Zn. SO 4 - 7 H 2 O � KI � Na 2 Mo. O 4 - 2 H 2 O � Cu. SO 4 - 5 H 2 O � Co. Cl 2 - H 2 O Ammonium nitrate Potassium nitrate Calcium chloride (Anhydrous) Magnesium sulfide (Epsom Salts) Potassium hypophosphate Fe/Na ethylene diamine tetra acetate Boric Acid Manganese sulfate Zinc sulfate Potassium iodide Sodium molybdate Cupric sulfate Cobaltous sulfide

Macronutrient salts � Nitrogen – Influences plant growth rate, essential in plant nucleic acids (DNA), proteins, chlorophyll, amino acids, and hormones. � Phosphorus – Abundant in meristematic and fast growing tissue, essential in photosynthesis, respiration. � Potassium – Necessary for cell division, meristematic tissue, helps in the pathways for carbohydrate, protein and chlorophyll synthesis. � Calcium Involved in formation of cell walls and root and leaf development. Participates in translocation of sugars, amino acids, and ties up oxalic acid (toxin). � Iron Involved in respiration , chlorophyll synthesis and photosynthesis. � Fe. Na. EDTA = sodium salt of EDTA sequesters iron, making it available to plants.

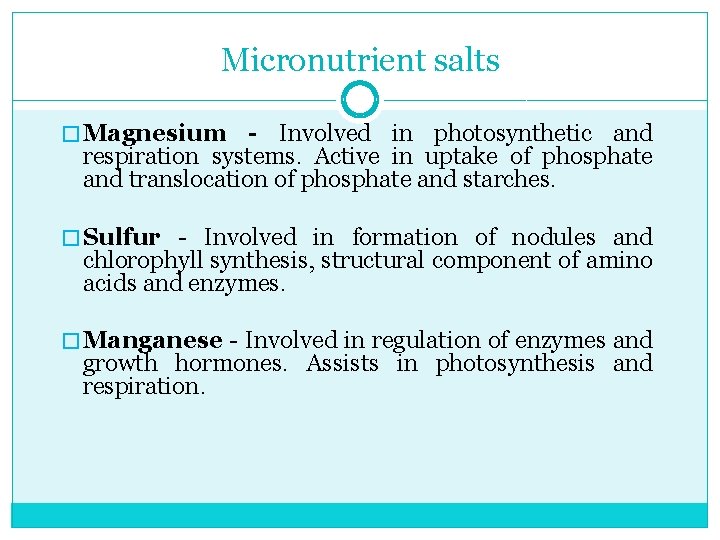
Micronutrient salts � Magnesium - Involved in photosynthetic and respiration systems. Active in uptake of phosphate and translocation of phosphate and starches. � Sulfur Involved in formation of nodules and chlorophyll synthesis, structural component of amino acids and enzymes. � Manganese Involved in regulation of enzymes and growth hormones. Assists in photosynthesis and respiration.

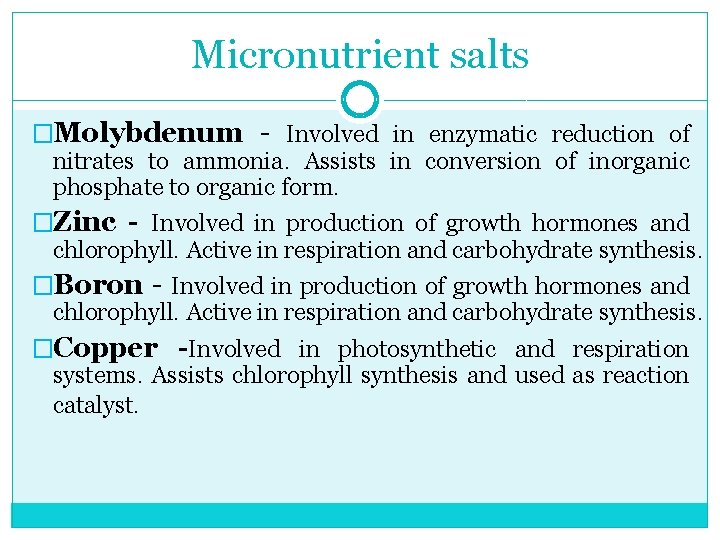
Micronutrient salts �Molybdenum Involved in enzymatic reduction of nitrates to ammonia. Assists in conversion of inorganic phosphate to organic form. �Zinc - Involved in production of growth hormones and chlorophyll. Active in respiration and carbohydrate synthesis. �Boron Involved in production of growth hormones and chlorophyll. Active in respiration and carbohydrate synthesis. �Copper -Involved in photosynthetic and respiration systems. Assists chlorophyll synthesis and used as reaction catalyst.

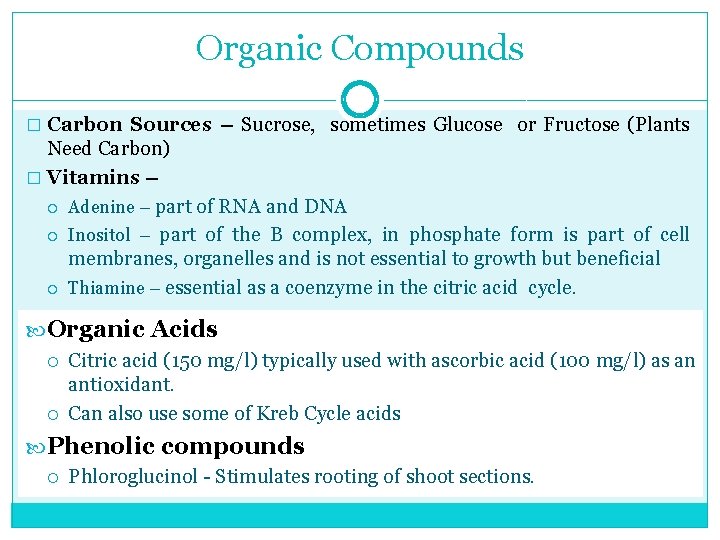
Organic Compounds � Carbon Sources – Sucrose, sometimes Glucose or Fructose (Plants Need Carbon) � Vitamins – Adenine – part of RNA and DNA Inositol – part of the B complex, in phosphate form is part of cell membranes, organelles and is not essential to growth but beneficial Thiamine – essential as a coenzyme in the citric acid cycle. Organic Acids Citric acid (150 mg/l) typically used with ascorbic acid (100 mg/l) as an antioxidant. Can also use some of Kreb Cycle acids Phenolic compounds Phloroglucinol Stimulates rooting of shoot sections.

Natural Complexes � Coconut endosperm � Protein hydrolysates � Tomato juice � Yeast extracts � Malt extract � Potato agar Activated charcoal is used as a detoxifying agent. Detoxifies wastes from plant tissues, impurities Impurities and absorption quality vary Concentration normally used is 0. 3 % or lower Charcoal for tissue culture acid washed and neutralized never reuse

Growth regulators What is a Growth Regulator? Plant Cell Growth regulators (e. g. Auxins, Cytokinins and Gibberellins) Plant hormones play an important role in growth and differentiation of cultured cells and tissues. There are many classes of plant growth regulators used in culture media involves namely: Auxins, Cytokinins, Gibberellins, Abscisic acid, and Ethylene. �Auxin Roots �Cytokinin Shoots �Gibberellin – Cell Enlargement �Abscisic acid – Plant stress hormone �Ethylene – BAD!

Auxins �The Auxins facilitate cell division and root differentiation. Auxins induce cell division, cell elongation, and formation of callus in cultures. Indole 3 acetic acid =IAA Naphthalene acetic acid NAA Indole 3 butyric acid 2, 4 D 2, 4, 5 T Picloram For example, 2, 4 -dichlorophenoxy acetic acid is one of the most commonly added auxins in plant cell cultures.

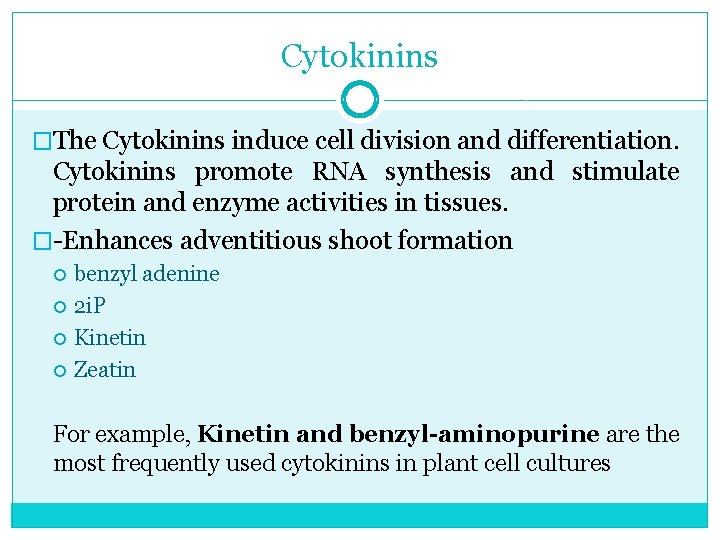
Cytokinins �The Cytokinins induce cell division and differentiation. Cytokinins promote RNA synthesis and stimulate protein and enzyme activities in tissues. � Enhances adventitious shoot formation benzyl adenine 2 i. P Kinetin Zeatin For example, Kinetin and benzyl-aminopurine are the most frequently used cytokinins in plant cell cultures

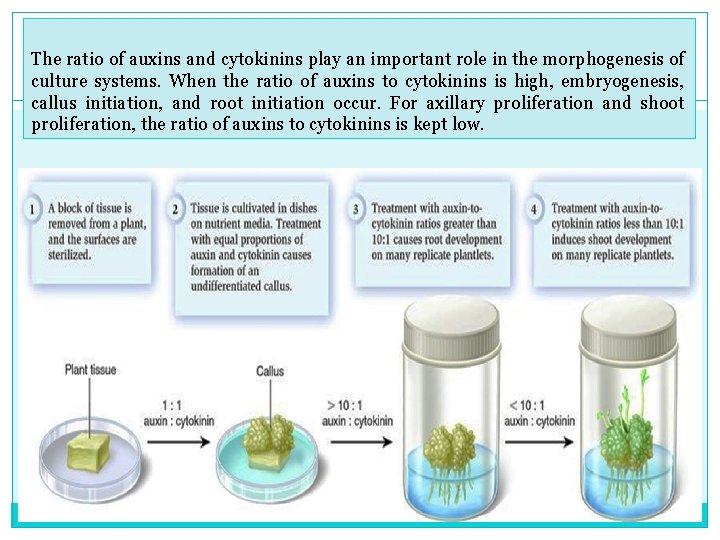
The ratio of auxins and cytokinins play an important role in the morphogenesis of culture systems. When the ratio of auxins to cytokinins is high, embryogenesis, callus initiation, and root initiation occur. For axillary proliferation and shoot proliferation, the ratio of auxins to cytokinins is kept low.

Gibberellin Abscisic Acid � Not generally used in tissue Primarily a growth inhibitor culture � Tends to suppress root formation and adventitious embryo formation. but enables more normal development of embryos, both zygotic and adventitious. Ethylene Question is not how much to add but how to get rid of it in-vitro Natural substance produced by tissue cultures at fairly high levels especially when cells are under stress Enhances senescense Supresses embryogenesis and development in general.

Hormone Combinations �Callus development �Adventitious embryogenesis �Rooting of shoot cuttings �Adventitious shoot and root formation

Culturing (Micropropagating) Plant Tissue The Steps, I • Selection of the plant tissue (explant) from a healthy vigorous ‘mother plant’ this is often the apical bud, but can be other tissue. • This tissue must be sterilized to remove microbial contaminants.

The Steps, II Establishment of the explant in a culture medium. The medium sustains the plant cells and encourages cell division. It can be solid or liquid Each plant species (and sometimes the variety within a species) has particular medium requirements that must be established by trial and error.

The Steps, III Multiplication- Dividing shoots The explant gives rise to a callus (a mass of loosely arranged cells) which is manipulated by varying sugar concentrations and the auxin (low): cytokinin (high) ratios to form multiple shoots. The Warmth and good light are essential callus may be subdivided a number of times.

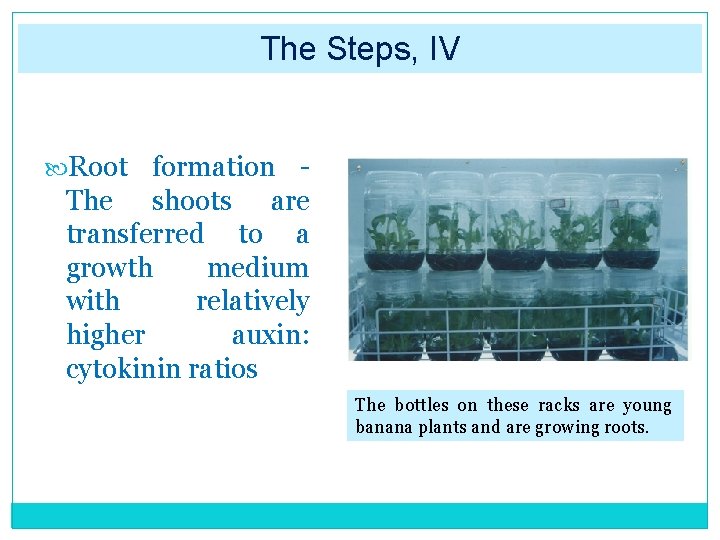
The Steps, IV Root formation The shoots are transferred to a growth medium with relatively higher auxin: cytokinin ratios The bottles on these racks are young banana plants and are growing roots.

The Steps, V The rooted shoots are potted up (deflasked) and ‘hardened off’ by gradually decreasing the humidity This is necessary as many young tissue culture plants have no waxy cuticle to prevent water loss Tissue culture plants sold to a nursery & then potted up.

Transfer to soil

Plant Tissue Culture Applications The commercial production of plants used as potting, landscape, and florist subjects To conserve rare or endangered plant species. To screen cells rather than plants for advantageous characters, e. g. herbicide resistance/tolerance. Large scale growth of plant cells in liquid culture in bioreactors for production of valuable compounds, like plant derived secondary metabolites and recombinant proteins used as biopharmaceuticals. To cross distantly related species by protoplast fusion and regeneration of the novel hybrid. To produce clean plant material from stock infected by viruses or other pathogens. Production of identical sterile hybrid species can be obtained.

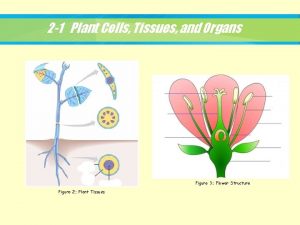
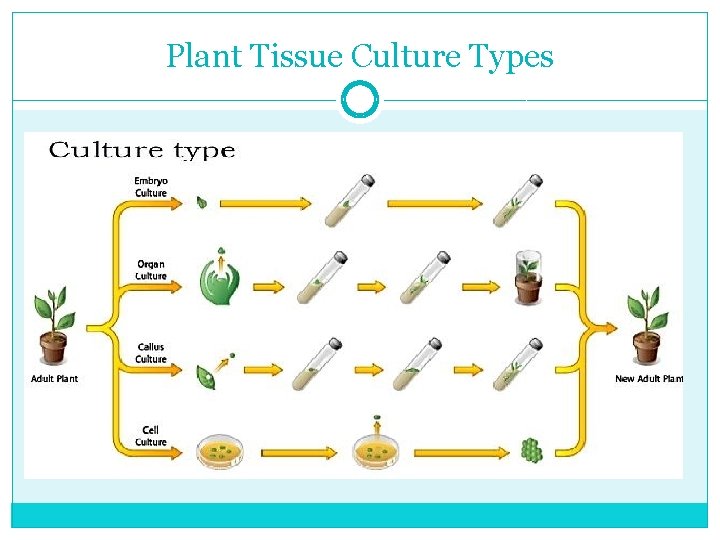
Plant Tissue Culture Types

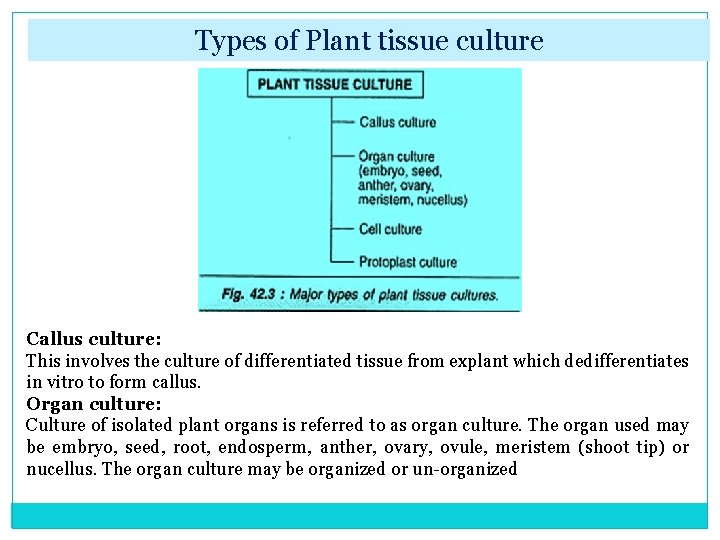
Types of Plant tissue culture Callus culture: This involves the culture of differentiated tissue from explant which dedifferentiates in vitro to form callus. Organ culture: Culture of isolated plant organs is referred to as organ culture. The organ used may be embryo, seed, root, endosperm, anther, ovary, ovule, meristem (shoot tip) or nucellus. The organ culture may be organized or un organized.

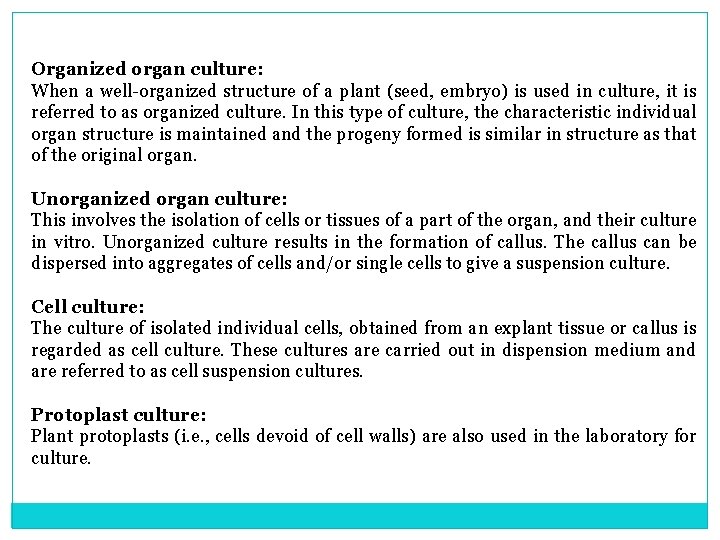
Organized organ culture: When a well organized structure of a plant (seed, embryo) is used in culture, it is referred to as organized culture. In this type of culture, the characteristic individual organ structure is maintained and the progeny formed is similar in structure as that of the original organ. Unorganized organ culture: This involves the isolation of cells or tissues of a part of the organ, and their culture in vitro. Unorganized culture results in the formation of callus. The callus can be dispersed into aggregates of cells and/or single cells to give a suspension culture. Cell culture: The culture of isolated individual cells, obtained from an explant tissue or callus is regarded as cell culture. These cultures are carried out in dispension medium and are referred to as cell suspension cultures. Protoplast culture: Plant protoplasts (i. e. , cells devoid of cell walls) are also used in the laboratory for culture.

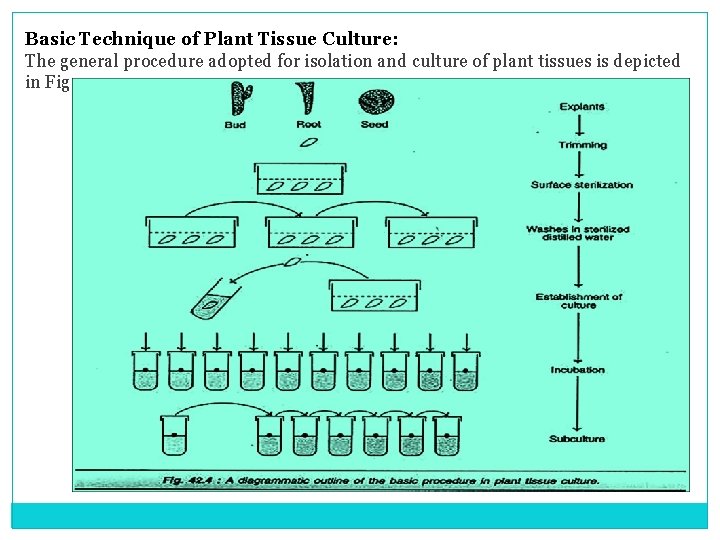
Basic Technique of Plant Tissue Culture: The general procedure adopted for isolation and culture of plant tissues is depicted in Fig

• The requisite explants (buds, stem, seeds) are trimmed and then subjected to sterilization in a detergent solution. • After washing in sterile distilled water, the explants are placed in a suitable culture medium (liquid or semisolid form) and incubated. This results in the establishment of culture. • The mother cultures can be subdivided, as frequently as needed, to give daughter cultures. • The most important aspect of in vitro culture technique is to carry out all the operations under aseptic conditions. • Bacteria and fungi are the most common contaminants in plant tissue culture. They grow much faster in culture and often kill the plant tissue. • Further, the contaminants also produce certain compounds which are toxic to the plant tissue. • Therefore, it is absolutely essential that aseptic conditions are maintained throughout the tissue culture operations. Some of the culture techniques are described here while a few others are discussed at appropriate places.

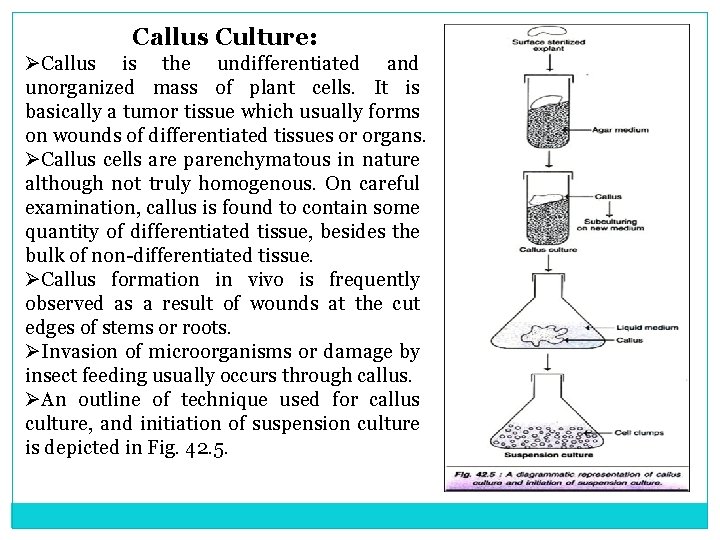
Callus Culture: ØCallus is the undifferentiated and unorganized mass of plant cells. It is basically a tumor tissue which usually forms on wounds of differentiated tissues or organs. ØCallus cells are parenchymatous in nature although not truly homogenous. On careful examination, callus is found to contain some quantity of differentiated tissue, besides the bulk of non differentiated tissue. ØCallus formation in vivo is frequently observed as a result of wounds at the cut edges of stems or roots. ØInvasion of microorganisms or damage by insect feeding usually occurs through callus. ØAn outline of technique used for callus culture, and initiation of suspension culture is depicted in Fig. 42. 5.

Explants for callus culture: The starting materials (explates) for callus culture may be the differentiated tissue from any part of the plant (root, stem, leaf, anther, flower etc. ). The selected explant tissues may be at different stages of cell division, cell proliferation and organization into different distinct specialized structures. If the explant used possesses meristematic cells, then the cell division and multiplication will be rapid. Factors Affecting Callus Culture: Many factors are known to influence callus formation in vitro culture. These include the source of the explant and its genotype, composition of the medium (MS medium most commonly used), physical factors (temperature, light etc. ) and growth factors. Other important factors affecting callus culture are — age of the plant, location of explant, physiology and growth conditions of the plant.

Physical factors: A temperature in the range of 22 28°C is suitable for adequate callus formation. As regards the effect of light on callus, it is largely dependent on the plant species light may be essential for some plants while darkness is required by others. Growth regulators: The growth regulators to the medium strongly influence callus formation. Based on the nature of the explant and its genotype, and the endogenous content of the hormone, the requirements of growth regulators may be categorized into 3 groups 1. Auxin alone 2. Cytokinin alone 3. Both auxin and cytokinin.

Suspension culture from callus: Suspension cultures can be initiated by transferring friable callus to liquid nutrient medium (Fig. 42. 5). As the medium is liquid in nature, the pieces of callus remain submerged. This creates anaerobic condition and ultimately the cells may die. For this reason, suspension cultures have to be agitated by a rotary shaker. Due to agitation, the cells gets dispersed, besides their exposure to aeration. Applications of Callus Cultures: Callus cultures are slow growth plant culture systems in static medium. This enables to conduct several studies related to many aspects of plants (growth, differentiation and metabolism) as listed below. i. Nutritional requirements of plants. ii. Cell and organ differentiation. iii. Development of suspension and protoplast cultures. iv. Somaclonal variations. v. Genetic transformations. vi. Production of secondary metabolites and their regulation.

Cell Culture: The first attempt to culture single cells (obtained from leaves of flowering plants) was made in as early as 1902 by Haberlandt. Although he was unsuccessful to achieve cell division in vitro, his work gave a stimulus to several researchers. In later years, good success was achieved not only for cell division but also to raise complete plants from single cell cultures. Applications of Cell Cultures: Cultured cells have a wide range of applications in biology. 1. Elucidation of the pathways of cellular metabolism. 2. Serve as good targets for mutation and selection of desirable mutants. 3. Production of secondary metabolites of commercial interest. 4. Good potential for crop improvement.
 Callus culture diagram
Callus culture diagram Plant tissue culture terminology
Plant tissue culture terminology Definition of plant tissue culture
Definition of plant tissue culture Applications of plant tissue culture
Applications of plant tissue culture Tissue culture conclusion
Tissue culture conclusion Tissue culture applications
Tissue culture applications Plant tissue culture
Plant tissue culture Applications of plant tissue culture
Applications of plant tissue culture Phloem function in plants
Phloem function in plants Pharmacognosy history and scope
Pharmacognosy history and scope What topics will be covered in this unit
What topics will be covered in this unit Perforation plates
Perforation plates Techniques of tissue culture
Techniques of tissue culture Ms media preparation
Ms media preparation Macronutrients and micronutrients in plants
Macronutrients and micronutrients in plants What is pearl culture definition
What is pearl culture definition Protoplast fusion
Protoplast fusion Mind map plant tissue
Mind map plant tissue Plant tissue and organs
Plant tissue and organs Which tissue transports water around a plant?
Which tissue transports water around a plant? Which tissue transports water around a plant?
Which tissue transports water around a plant? Plant tissue and organs
Plant tissue and organs Colonchyma
Colonchyma Plant tissue
Plant tissue Plant tissue
Plant tissue Plant tissue
Plant tissue Agranulocytes
Agranulocytes Collenchyma
Collenchyma Lateral growth in plants
Lateral growth in plants Tissue
Tissue Plant ground tissue
Plant ground tissue Ground tissue plant
Ground tissue plant Example of pop culture
Example of pop culture Sociologists define a symbol as
Sociologists define a symbol as Batch culture vs continuous culture
Batch culture vs continuous culture Fed-batch
Fed-batch Characteristics of collectivism
Characteristics of collectivism Indian vs american culture
Indian vs american culture Stab and stroke culture
Stab and stroke culture Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram Subculture group
Subculture group Chapter 4 folk and popular culture
Chapter 4 folk and popular culture Stab and stroke culture
Stab and stroke culture Homework due today
Homework due today Inert organizational culture
Inert organizational culture Carpet culture method
Carpet culture method