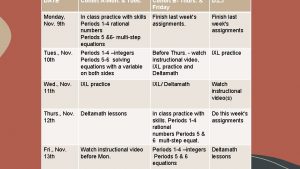
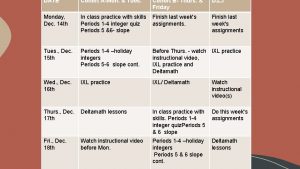
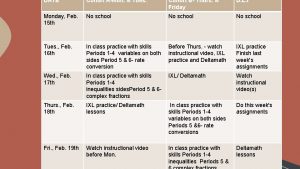
Creating a Cohort of Cases ICTR Workshop on


















- Slides: 28

Creating a Cohort of Cases – ICTR Workshop on Clinical Registries Josef Coresh, MD, Ph. D Professor of Epidemiology, Biostatistics & Medicine Johns Hopkins University Director, George W. Comstock Center for Public Health Research and Prevention Director, Cardiovascular Epidemiology Training Program

Outline • Cohort definition (see Gordis “Epidemiology” text for overview) – Membership criteria (“Case” Definition in a clinical cohort of cases – but remember that case series is a weak design) - Considering Referral Pathway - Considering Precohort Factors • Data collection – Exposures, Treatments & outcomes (mostly covered by other lectures) • Examples of different cohorts to illustrate ideas: – ARIC – CHOICE – CLUE • Discussion of planned cohorts by participants

Taxonomy of Designs • Randomized Controlled Trial • Prospective Cohort Study – Variations exist – non-concurrent (going back to old records etc. ) • Case-Control Study • Cross-Sectional Study • Other Designs – Quasi-Experimental – Ecologic – Case Report

The basic fighting unit was a cohort, composed of six centuries (480 men plus 6 centurions). The legion itself was composed of ten cohorts, and the first cohort had many extra men—the clerks, engineers, and other specialists who did not usually fight—and the senior centurion of the legion, the primipilus, or “number one javelin. ”

pro·spec·tive Pronunciation: pr&-'spek-tiv also 'prä-", pr. O-', prä-' Function: adjective Date: circa 1699 1 : relating to or effective in the future 2 a : likely to come about : EXPECTED <the prospective benefits of this law> b : likely to be or become <a prospective mother>

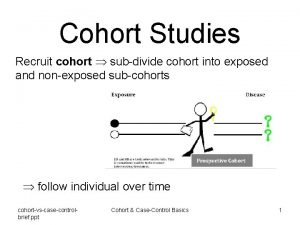
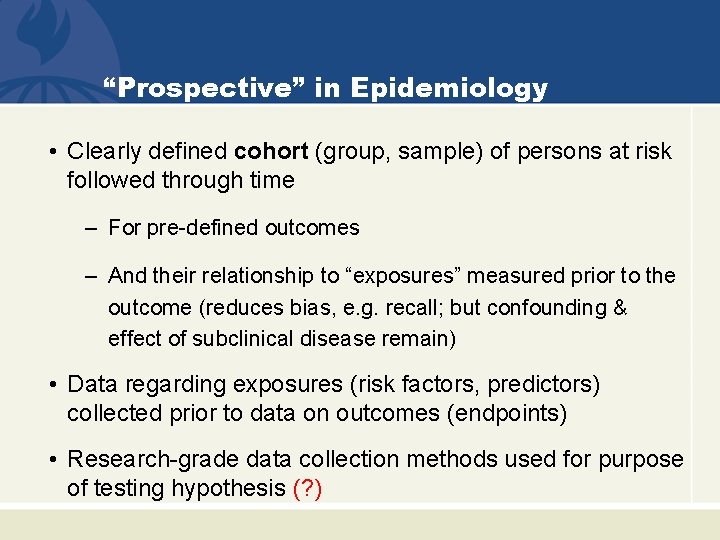
“Prospective” in Epidemiology • Clearly defined cohort (group, sample) of persons at risk followed through time – For pre-defined outcomes – And their relationship to “exposures” measured prior to the outcome (reduces bias, e. g. recall; but confounding & effect of subclinical disease remain) • Data regarding exposures (risk factors, predictors) collected prior to data on outcomes (endpoints) • Research-grade data collection methods used for purpose of testing hypothesis (? )

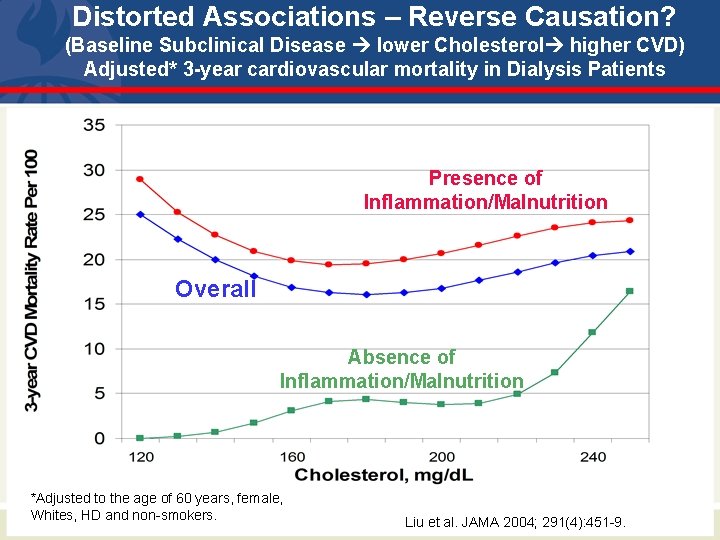
Distorted Associations – Reverse Causation? (Baseline Subclinical Disease lower Cholesterol higher CVD) Adjusted* 3 -year cardiovascular mortality in Dialysis Patients Presence of Inflammation/Malnutrition Overall Absence of Inflammation/Malnutrition *Adjusted to the age of 60 years, female, Whites, HD and non-smokers. Liu et al. JAMA 2004; 291(4): 451 -9.

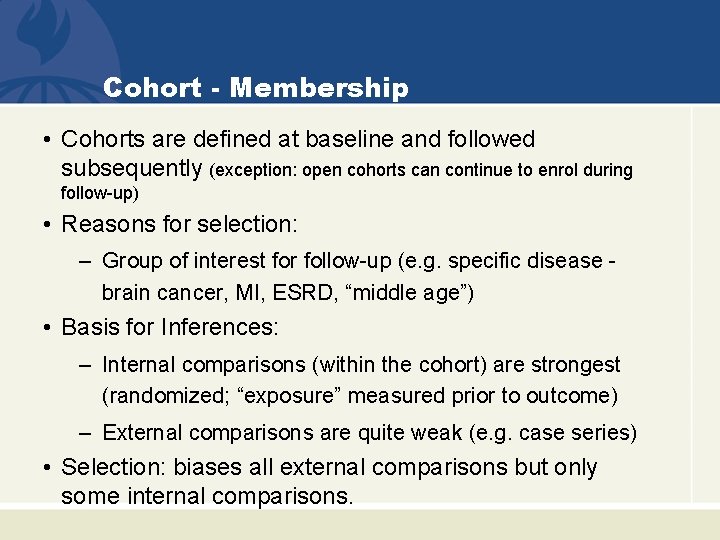
Cohort - Membership • Cohorts are defined at baseline and followed subsequently (exception: open cohorts can continue to enrol during follow-up) • Reasons for selection: – Group of interest for follow-up (e. g. specific disease brain cancer, MI, ESRD, “middle age”) • Basis for Inferences: – Internal comparisons (within the cohort) are strongest (randomized; “exposure” measured prior to outcome) – External comparisons are quite weak (e. g. case series) • Selection: biases all external comparisons but only some internal comparisons.

Why Do A Cohort Study? • • • Get incidence data Study a range of possible risk factors Establish temporal sequence (risk factor before outcome) Get representative data (of some population) Prepare for randomized controlled trial – Effect size estimates – Population of eligible participants (“registry”) • Establish a research empire (not a good primary goal)

Types of Cohorts • • Occupational (e. g. Asbestos workers) Convenience (e. g. Precursors, Nurses) Geographic (e. g. Framingham, ARIC) Disease or Procedure – Natural History (e. g. Syncope, Lupus) – Outcomes Research (e. g. Dialysis, Cataracts)

Sources of Cohort Data • Clinic Visits – Laboratory Assays • Medical Records • Administrative Data – Interview – Medicare – Physical Examination – Medicaid – Imaging – Managed Care – Physiologic tests – Veterans Admin • Home visits • Mailed materials • Telephone Interview • Birth Records • Death Certificates • Specimen Bank

Challenges in Cohort Studies • • • Possibly long duration Possibly large sample size Need to recruit people “at risk” Drop outs, Deaths, Other losses Concern about residual confounding Multiple comparisons Type I error

How to Exploit Cohort Design When Time is Short & Money is Scarce • • Analyze existing data from another study Piggy-back onto on-going study Choose hospital-based cohort Choose short-term outcome Consider administrative data Consider public-use data Consider non-concurrent design

Examples – Food for Thought

Results Drift – Even in a “good” lab Serum Creatinine Compared to the Mean of All Labs: College of American Pathologists (CAP) Data Coresh J et al. Am J Kid Dis 2002; 39: 920 -929

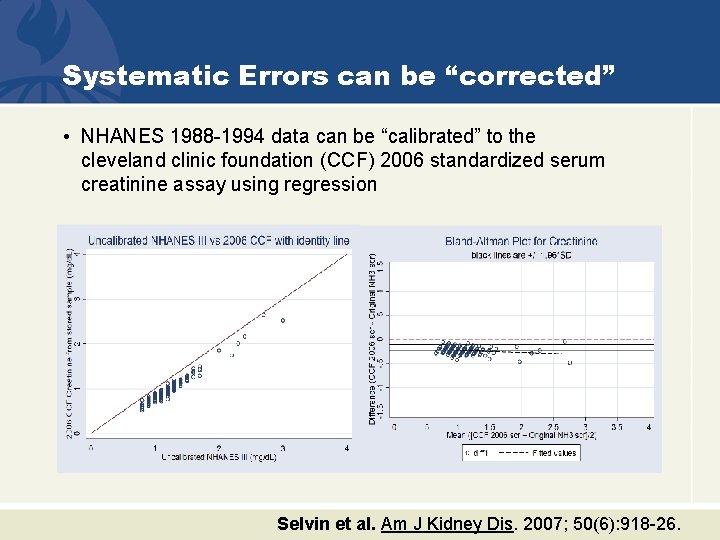
Systematic Errors can be “corrected” • NHANES 1988 -1994 data can be “calibrated” to the cleveland clinic foundation (CCF) 2006 standardized serum creatinine assay using regression Selvin et al. Am J Kidney Dis. 2007; 50(6): 918 -26.

ARIC – Atherosclerosis Risk in Communities • NHLBI cohort to study atherosclerosis – Community based sample ages 45 -64 – ~5 hour examination: interview, exam, phlebotomy, carotid ultrasound (all standardized) • Baseline, 3, 6, 9 years … 25 years – Annual telephone calls – Chart abstraction of all hospitalizations – Morbidity and Mortality Classification Committee review of CHD outcomes

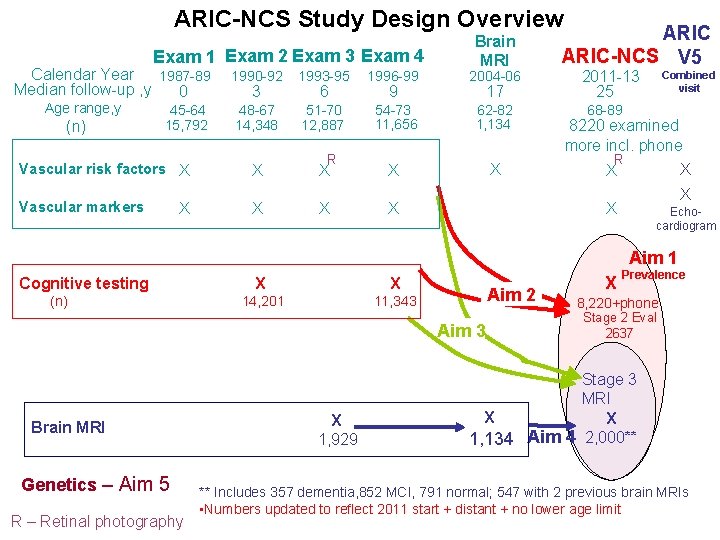
ARIC-NCS Study Design Overview Exam 1 Exam 2 Exam 3 Exam 4 Calendar Year 1987 -89 Median follow-up , y 0 Brain MRI 1990 -92 1993 -95 1996 -99 2004 -06 48 -67 14, 348 51 -70 12, 887 54 -73 11, 656 62 -82 1, 134 Vascular risk factors X X X Vascular markers X X X Age range, y (n) 45 -64 15, 792 X 3 6 R 9 17 ARIC-NCS V 5 2011 -13 25 Combined visit 68 -89 8220 examined more incl. phone R X X X Echocardiogram X Aim 1 Cognitive testing (n) X X 14, 201 11, 343 Aim 2 Aim 3 Brain MRI Genetics – Aim 5 R – Retinal photography X 1, 929 X Prevalence 8, 220+phone Stage 2 Eval 2637 Stage 3 MRI X X 1, 134 Aim 4 2, 000** ** Includes 357 dementia, 852 MCI, 791 normal; 547 with 2 previous brain MRIs • Numbers updated to reflect 2011 start + distant + no lower age limit

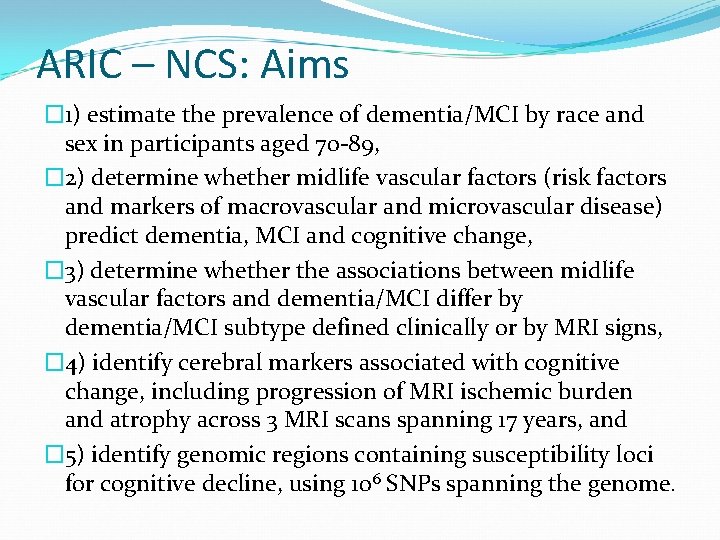
ARIC – NCS: Aims � 1) estimate the prevalence of dementia/MCI by race and sex in participants aged 70 -89, � 2) determine whether midlife vascular factors (risk factors and markers of macrovascular and microvascular disease) predict dementia, MCI and cognitive change, � 3) determine whether the associations between midlife vascular factors and dementia/MCI differ by dementia/MCI subtype defined clinically or by MRI signs, � 4) identify cerebral markers associated with cognitive change, including progression of MRI ischemic burden and atrophy across 3 MRI scans spanning 17 years, and � 5) identify genomic regions containing susceptibility loci for cognitive decline, using 106 SNPs spanning the genome.

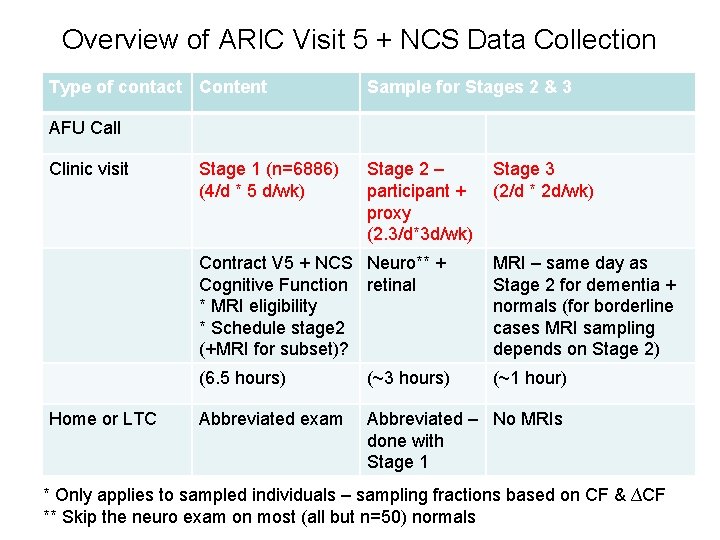
Overview of ARIC Visit 5 + NCS Data Collection Type of contact Content Sample for Stages 2 & 3 AFU Call Clinic visit Home or LTC Stage 1 (n=6886) (4/d * 5 d/wk) Stage 2 – participant + proxy (2. 3/d*3 d/wk) Stage 3 (2/d * 2 d/wk) Contract V 5 + NCS Neuro** + Cognitive Function retinal * MRI eligibility * Schedule stage 2 (+MRI for subset)? MRI – same day as Stage 2 for dementia + normals (for borderline cases MRI sampling depends on Stage 2) (6. 5 hours) (~3 hours) (~1 hour) Abbreviated exam Abbreviated – No MRIs done with Stage 1 * Only applies to sampled individuals – sampling fractions based on CF & ∆CF ** Skip the neuro exam on most (all but n=50) normals

CHOICE Cohort Choices for Healthy Outcomes in Caring for ESRD • Study Design: national prospective cohort study (CHOICE; PI: Powe & Klag & specimen bank Coresh) • Study Population: – 1026 incident outpatient dialysis patients – Enrolled between 10/95 and 06/98 (DCI + St. Raph) – Recruited within a median of 45 days from 1 st dialysis (98% within 4 months) – From 81 dialysis clinics in 19 States – Age 18 years or older, English or Spanish speaker – Provided informed consent • Main research topics: Dose & Modality Outcomes 2 1

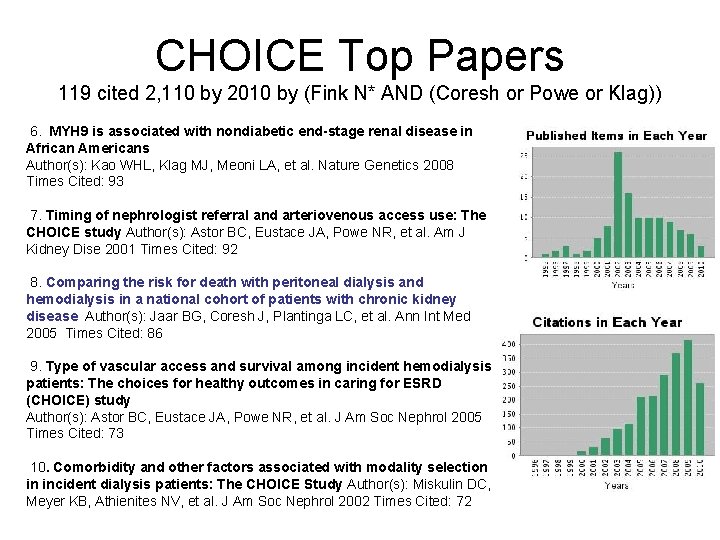
CHOICE Top Papers 119 cited 2, 110 by 2010 by (Fink N* AND (Coresh or Powe or Klag)) 1. Association between cholesterol level and mortality in - Role of inflammation dialysis patients and malnutrition. Author(s): Liu YM, Coresh J, Eustace JA, et al. JAMA 2004 Times Cited: 209 2. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE study. Author(s): Longenecker JC, Coresh J, Powe NR, et al. JASN 2002 Times Cited: 180 3. The timing of specialist evaluation in chronic kidney disease and mortality Author(s): Kinchen KS, Sadler J, Fink N, et al. Ann Int Med 2002 Times Cited: 176 4. Validation of comorbid conditions on the end-stage renal disease medical evidence report: The CHOICE study. Author(s): Longenecker JC, Coresh J, Klag MJ, et al. JASN 2000 Times Cited: 141 5. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Author(s): Melamed ML, Eustace JA, Plantinga L, et al. Kidney Int 2006 Times Cited: 96

CHOICE Top Papers 119 cited 2, 110 by 2010 by (Fink N* AND (Coresh or Powe or Klag)) 6. MYH 9 is associated with nondiabetic end-stage renal disease in African Americans Author(s): Kao WHL, Klag MJ, Meoni LA, et al. Nature Genetics 2008 Times Cited: 93 7. Timing of nephrologist referral and arteriovenous access use: The CHOICE study Author(s): Astor BC, Eustace JA, Powe NR, et al. Am J Kidney Dise 2001 Times Cited: 92 8. Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease Author(s): Jaar BG, Coresh J, Plantinga LC, et al. Ann Int Med 2005 Times Cited: 86 9. Type of vascular access and survival among incident hemodialysis patients: The choices for healthy outcomes in caring for ESRD (CHOICE) study Author(s): Astor BC, Eustace JA, Powe NR, et al. J Am Soc Nephrol 2005 Times Cited: 73 10. Comorbidity and other factors associated with modality selection in incident dialysis patients: The CHOICE Study Author(s): Miskulin DC, Meyer KB, Athienites NV, et al. J Am Soc Nephrol 2002 Times Cited: 72

Washington County, MD Johns Hopkins University Research Opportunities in Washington County: From shoeleather epidemiology to genomics Josef Coresh, MD, Ph. D Professor of Epidemiology, Biostatistics & Medicine Johns Hopkins University Director, George W. Comstock Center for Public Health Research and Prevention Ana Navas-Acien, MD, Ph. D Assistant Professor, Environmental Health Sciences & Epidemiology Sleep Heart Health

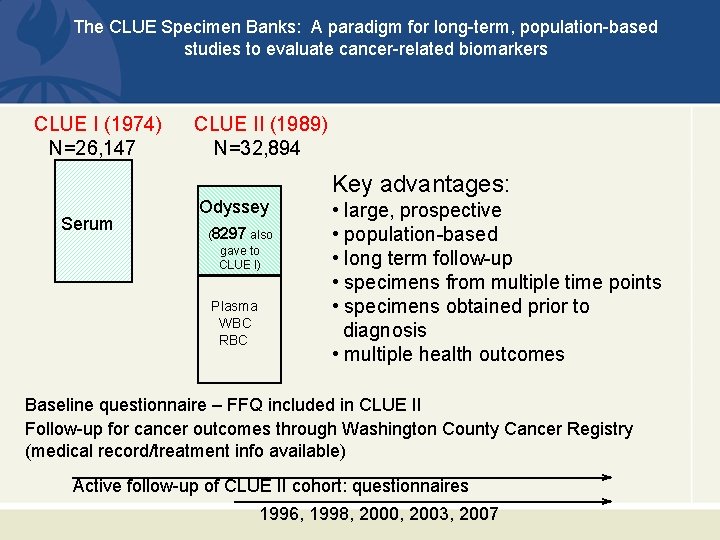
CLUE I & CLUE II Studies CLUE I (1974) N=26, 147 • Serum stored at -70 o • Baseline questionnaire CLUE II (1989) N=32, 894 • Plasma , RBC, DNA -70 o • Toenail sample • Baseline questionnaire • Food freq. questionnaire

The CLUE Specimen Banks: A paradigm for long-term, population-based studies to evaluate cancer-related biomarkers CLUE I (1974) N=26, 147 Serum CLUE II (1989) N=32, 894 Odyssey (8297 also gave to CLUE I) Plasma WBC RBC Key advantages: • large, prospective • population-based • long term follow-up • specimens from multiple time points • specimens obtained prior to diagnosis • multiple health outcomes Baseline questionnaire – FFQ included in CLUE II Follow-up for cancer outcomes through Washington County Cancer Registry (medical record/treatment info available) Active follow-up of CLUE II cohort: questionnaires 1996, 1998, 2000, 2003, 2007

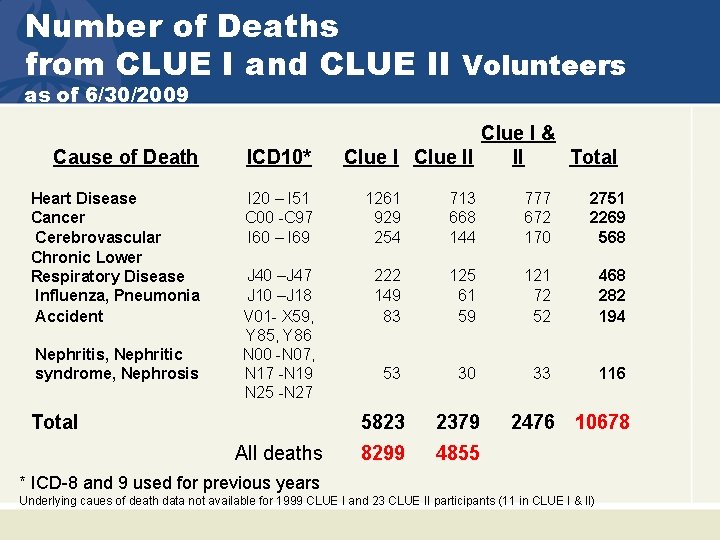
Number of Deaths from CLUE I and CLUE II Volunteers as of 6/30/2009 Clue I & Clue II II Total Cause of Death ICD 10* Heart Disease Cancer Cerebrovascular Chronic Lower Respiratory Disease Influenza, Pneumonia Accident I 20 – I 51 C 00 -C 97 I 60 – I 69 1261 929 254 713 668 144 777 672 170 2751 2269 568 J 40 –J 47 J 10 –J 18 V 01 - X 59, Y 85, Y 86 N 00 -N 07, N 17 -N 19 N 25 -N 27 222 149 83 125 61 59 121 72 52 468 282 194 53 30 33 116 5823 2379 8299 4855 Nephritis, Nephritic syndrome, Nephrosis Total All deaths 2476 10678 * ICD-8 and 9 used for previous years Underlying caues of death data not available for 1999 CLUE I and 23 CLUE II participants (11 in CLUE I & II)

Thank you! CKD-Epi CHOICE Study CVD-Epi (it takes a team ) Stein Hallan ARIC Staff
 Retrospective cohort study vs prospective cohort study
Retrospective cohort study vs prospective cohort study Qualtrics johns hopkins
Qualtrics johns hopkins Criminal cases vs civil cases
Criminal cases vs civil cases Cohort model
Cohort model Cohort adalah
Cohort adalah Retrospective cohort study vs case control
Retrospective cohort study vs case control Cohort effects definition
Cohort effects definition Icarus first cohort
Icarus first cohort Cohort model
Cohort model Students cohort
Students cohort Ecological study vs cohort study
Ecological study vs cohort study Pregnancy and infant cohort monitoring and evaluation
Pregnancy and infant cohort monitoring and evaluation Cohort study definition
Cohort study definition Case control vs retrospective cohort
Case control vs retrospective cohort Cohort effects definition
Cohort effects definition Cohort study example
Cohort study example Kritik jurnal
Kritik jurnal Luxury consumer demographics
Luxury consumer demographics Cohort analysis lean startup
Cohort analysis lean startup Cohort model psycholinguistics
Cohort model psycholinguistics Difference between case control and cohort study
Difference between case control and cohort study Cohort study community medicine
Cohort study community medicine Types of cohort studies
Types of cohort studies Golden cohort
Golden cohort Types of cohort studies
Types of cohort studies Sequential research design
Sequential research design Patient chart
Patient chart Difference between case control and cohort study
Difference between case control and cohort study Cohort study bias
Cohort study bias