Computer Aided Drug Design and Hits identification La


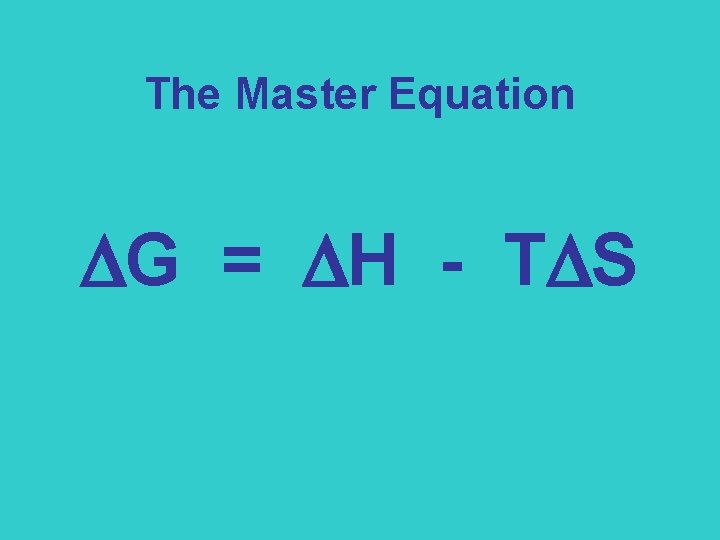



![Log PA = Log[Aoct/Awater] water Log Po/w = G° / 2. 303 RT octanole Log PA = Log[Aoct/Awater] water Log Po/w = G° / 2. 303 RT octanole](https://slidetodoc.com/presentation_image_h2/9a1fa7e789d615c2dff15e33cf793f9c/image-13.jpg)
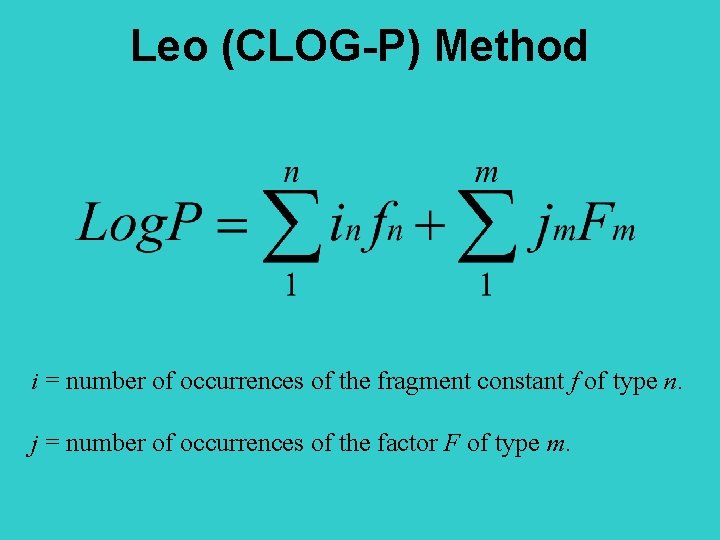





- Slides: 46

Computer Aided Drug Design and Hits identification La struttura tridimensionale della proteina è stata determinata mediante cristallografia a raggi X o NMR oppure è stata ottenuta per homology modelling

De novo drug design Building Ricerca con gruppi funzionali partendo da un sito ed espandendosi nel sito attivo. Linking Ricerca con gruppi funzionali partendo da diversi punti del sito attivo e collegandoli successivamente con uno scaffold. In silico screening Docking da librerie virtuali o reali Scoring function

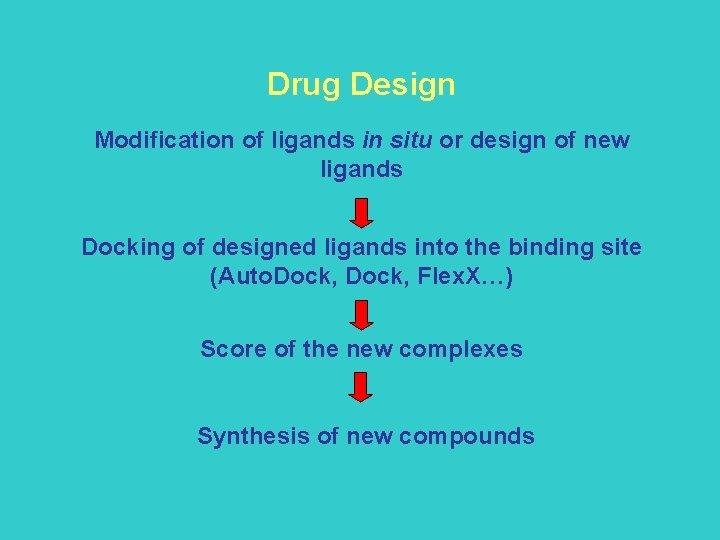
Drug Design Modification of ligands in situ or design of new ligands Docking of designed ligands into the binding site (Auto. Dock, Flex. X…) Score of the new complexes Synthesis of new compounds
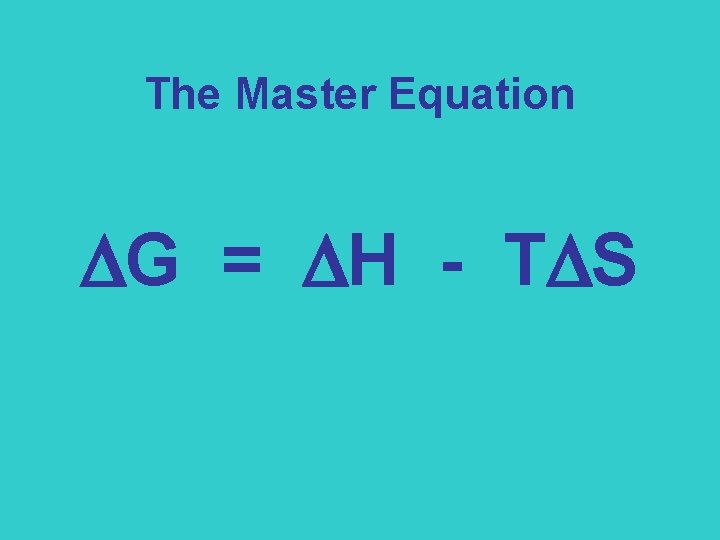
The Master Equation G = H - T S




H is relatively easy to calculate • Enthalpy (DH) is derived from static models of molecular or bimolecular structure. • Molecular mechanics force field methods deconvolve DH into intramolecular and intermolecular terms from bond stretches, angle bends, torsions, etc. , electrostatic interactions and van der Waals (London) forces. • Many academic and commercial force field programs are available, using similar approaches with essentially comparable results.

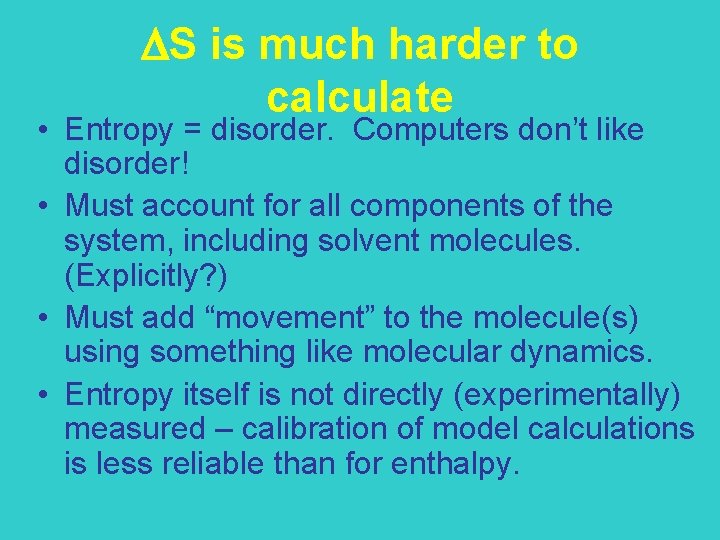
S is much harder to calculate • Entropy = disorder. Computers don’t like disorder! • Must account for all components of the system, including solvent molecules. (Explicitly? ) • Must add “movement” to the molecule(s) using something like molecular dynamics. • Entropy itself is not directly (experimentally) measured – calibration of model calculations is less reliable than for enthalpy.

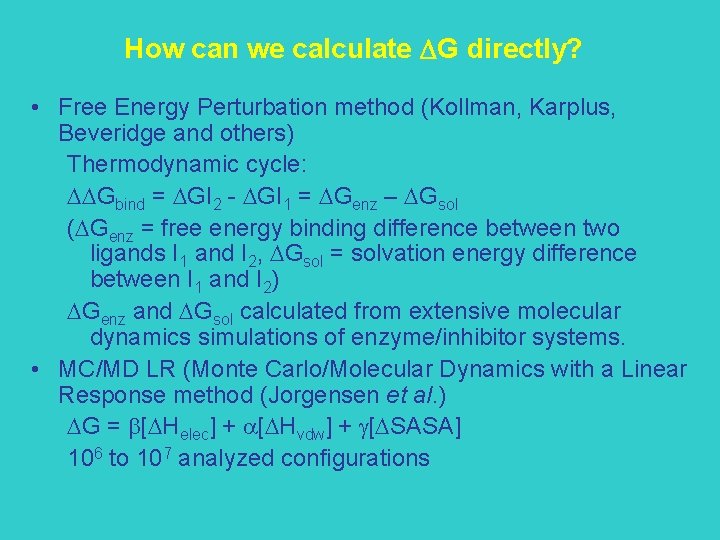
How can we calculate G directly? • Free Energy Perturbation method (Kollman, Karplus, Beveridge and others) Thermodynamic cycle: DDGbind = DGI 2 - DGI 1 = DGenz – DGsol (DGenz = free energy binding difference between two ligands I 1 and I 2, DGsol = solvation energy difference between I 1 and I 2) DGenz and DGsol calculated from extensive molecular dynamics simulations of enzyme/inhibitor systems. • MC/MD LR (Monte Carlo/Molecular Dynamics with a Linear Response method (Jorgensen et al. ) DG = b[DHelec] + a[DHvdw] + g[DSASA] 106 to 107 analyzed configurations

Metodi di predizione • Decomposizione del G° (la teoria dice che è una procedura scorretta) • Metodi FEP (Free Energy Perturbation) [Kollman, Karplus, Beveridge…. ] • MC/MD LR (Monte Carlo/Molecular Dynamics con un metodo Linear Response) [Jorgensen et al. ] • Metodi empirici

A “natural” force field • No preservatives and 9944/100% Hamiltonian and wave function free! • Biological binding events are not a neat set of terms specific to hydrogen bonding, acid -base, Coulombic and/or hydrophobic interactions – binding is a concerted process! • Design a Free Energy force field derived from an experiment that measures the free energy of molecular interactions.
![Log PA LogAoctAwater water Log Pow G 2 303 RT octanole Log PA = Log[Aoct/Awater] water Log Po/w = G° / 2. 303 RT octanole](https://slidetodoc.com/presentation_image_h2/9a1fa7e789d615c2dff15e33cf793f9c/image-13.jpg)
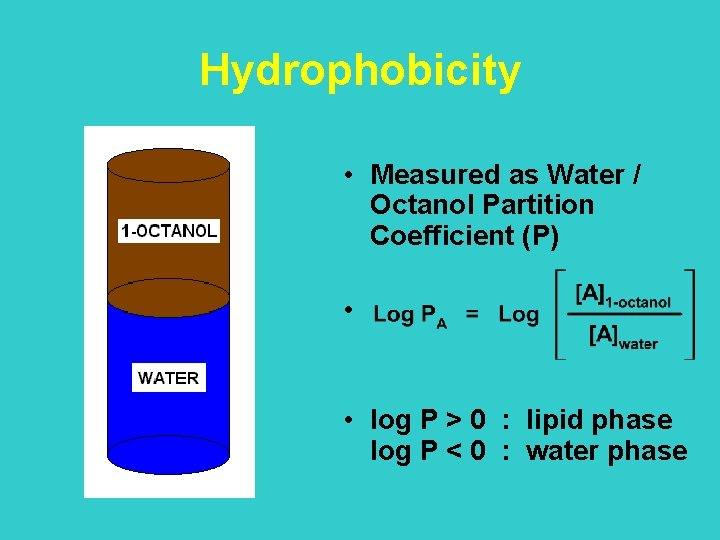
Log PA = Log[Aoct/Awater] water Log Po/w = G° / 2. 303 RT octanole The partition of a compound between water and octanole is a process driven by intermolecular interactions between the solvent and the compound, and by hydrophobic interactions, involving solvation-desolvation. These events are the same that take place in the formation of a ligand-protein complex.

Hydrophobicity • Measured as Water / Octanol Partition Coefficient (P) • • log P > 0 : lipid phase log P < 0 : water phase
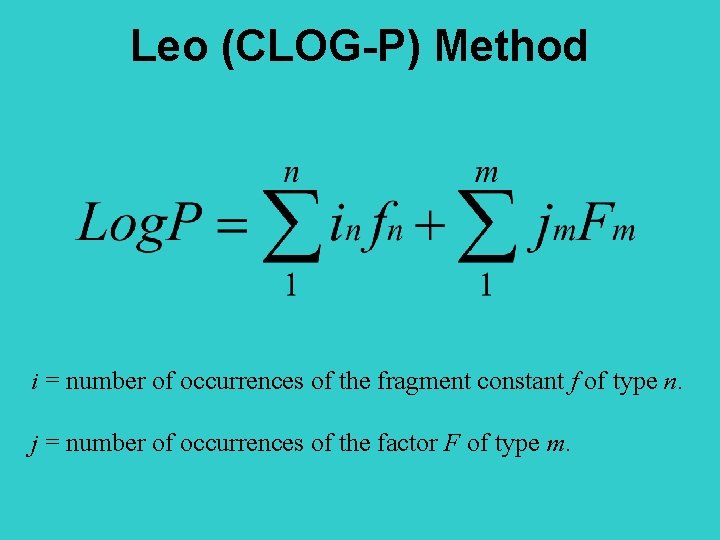
Leo (CLOG-P) Method i = number of occurrences of the fragment constant f of type n. j = number of occurrences of the factor F of type m.

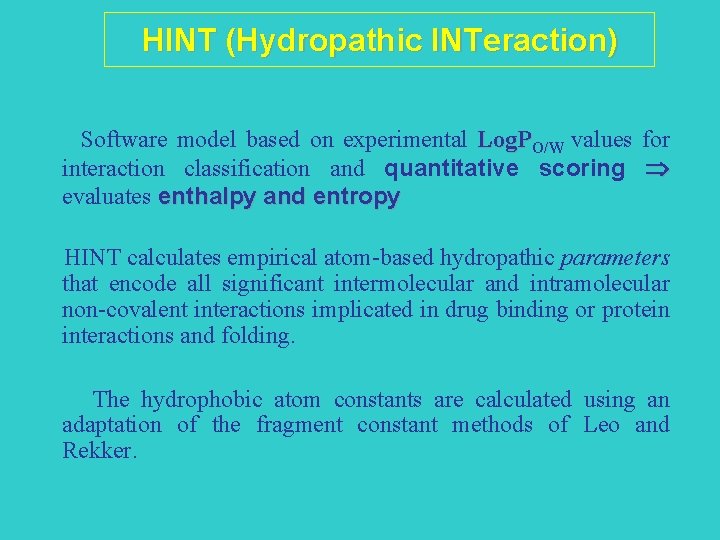
What is HINT?

HINT (Hydropathic INTeraction) Software model based on experimental Log. PO/W values for interaction classification and quantitative scoring evaluates enthalpy and entropy HINT calculates empirical atom-based hydropathic parameters that encode all significant intermolecular and intramolecular non-covalent interactions implicated in drug binding or protein interactions and folding. The hydrophobic atom constants are calculated using an adaptation of the fragment constant methods of Leo and Rekker.

The “HINT equation” HINT SCORE = SS bij = SS ai. Si aj. Sj Rij Tij + rij a S Rij Tij rij = = = hydrophobic atom constant solvent accessible surface area exponential (e-r) discriminant function for polar-polar interactions van der Waals term

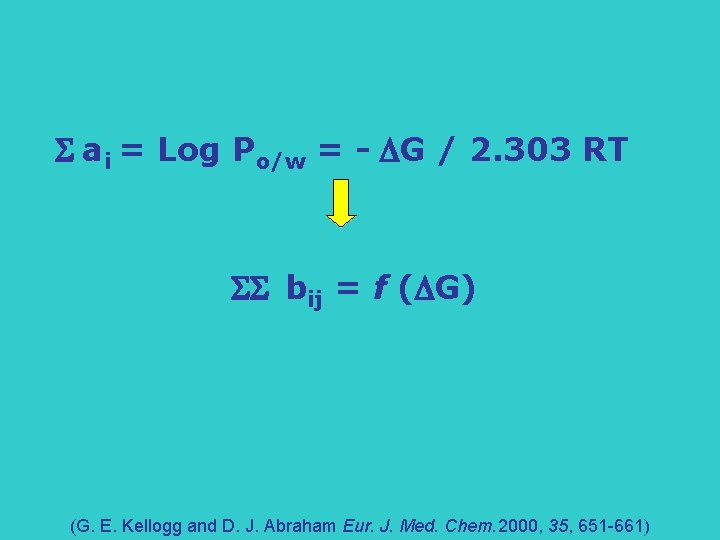
S ai = Log Po/w = - G / 2. 303 RT SS bij = f ( G) (G. E. Kellogg and D. J. Abraham Eur. J. Med. Chem. 2000, 35, 651 -661)

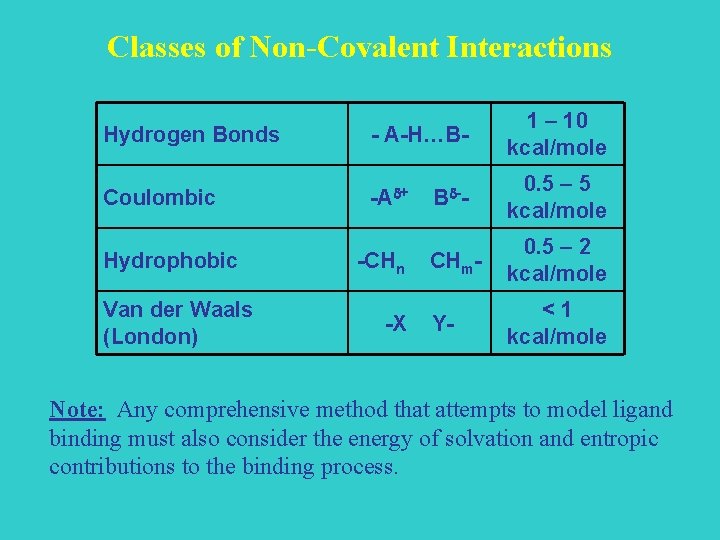
Classes of Non-Covalent Interactions Hydrogen Bonds - A-H…B- 1 – 10 kcal/mole Coulombic -Ad+ Bd-- 0. 5 – 5 kcal/mole CHm- 0. 5 – 2 kcal/mole Y- <1 kcal/mole Hydrophobic Van der Waals (London) -CHn -X Note: Any comprehensive method that attempts to model ligand binding must also consider the energy of solvation and entropic contributions to the binding process.

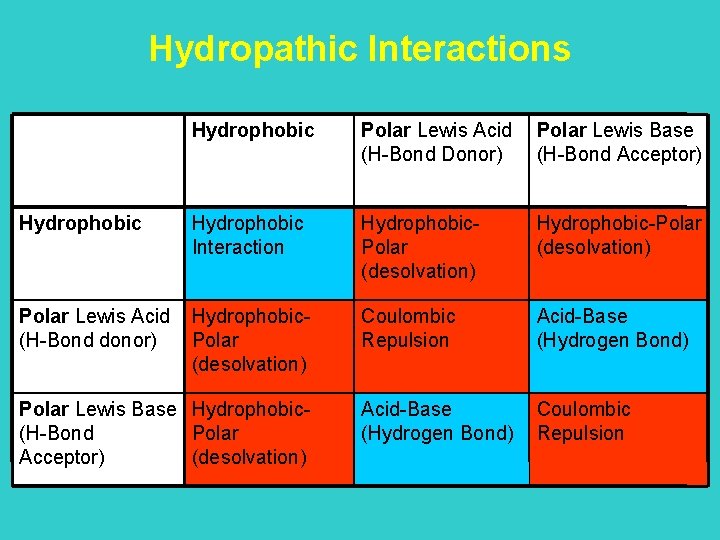
Hydropathic Interactions Hydrophobic Polar Lewis Acid (H-Bond Donor) Polar Lewis Base (H-Bond Acceptor) Hydrophobic Interaction Hydrophobic. Polar (desolvation) Hydrophobic-Polar (desolvation) Polar Lewis Acid (H-Bond donor) Hydrophobic. Polar (desolvation) Coulombic Repulsion Acid-Base (Hydrogen Bond) Coulombic Repulsion Polar Lewis Base Hydrophobic(H-Bond Polar Acceptor) (desolvation)

Negative interactions

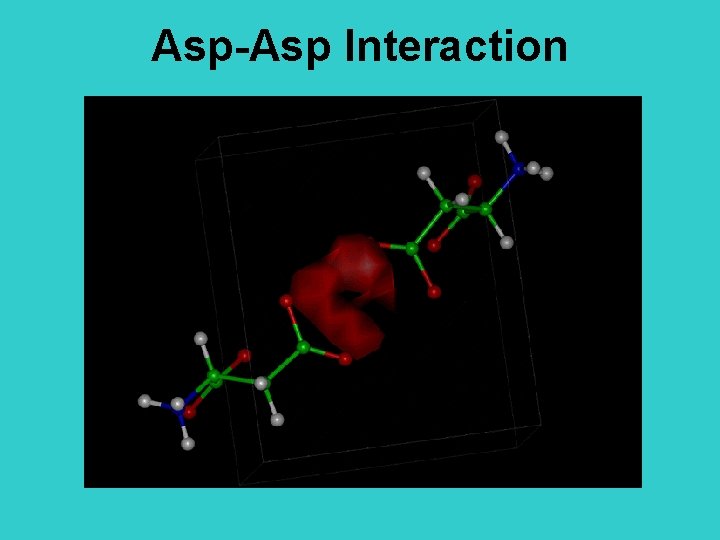
Asp-Asp Interaction

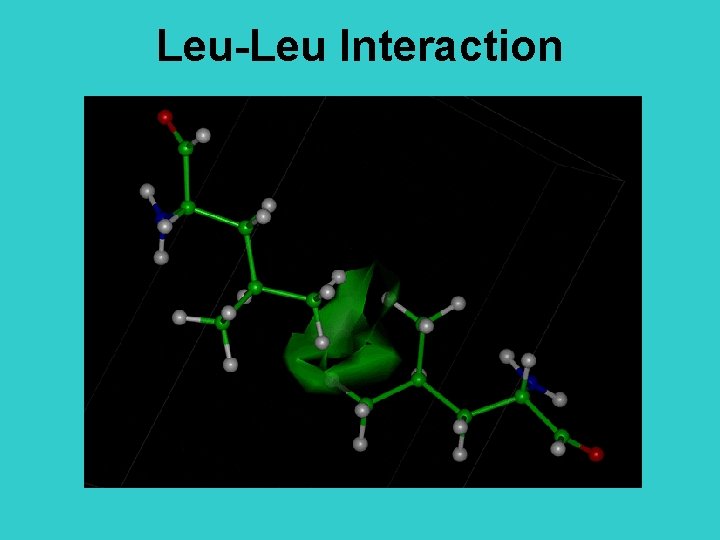
Leu-Leu Interaction

Correlazione tra l’energia libera di legame tra proteina e ligandi determinata mediante HINT e l’energia libera determinata mediante metodi sperimentali

Working Procedure Model Building • Starting point: protein-ligand complexes for which 3 D structure (PDB files) and experimental binding affinity are determined • Hydrogen atoms added and minimized, hydrogen bound to polar atoms examined and optimized • Evaluation of the protonation state of ionizable groups on protein and ligand (Computational Titration Fornabaio et al. J. Med. Chem. 2003, 46, 4487 -4500) • Optimization of water molecules bridging protein and ligand Hydropathic Analysis for the evaluation of all contributions to the ligand-protein complex formation

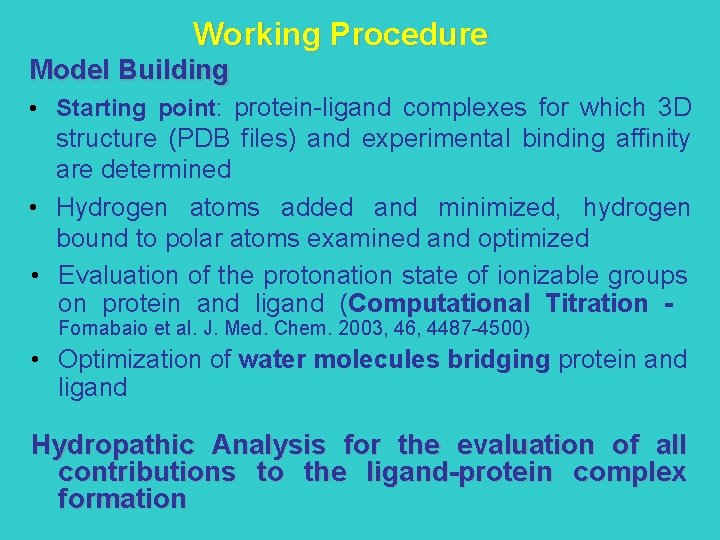
General Relationship between Hint Score and G° 93 complexes formed by 18 different proteins of know 3 D structure, R < 3. 2 Å G° = -0. 0018 HINT score – 3. 9041 R 2 = 0. 47 R = 0. 68 Cozzini et al. , J. Med. Chem. 2002, 45, 2469 -2483 se = 2. 33 Kcal/mol

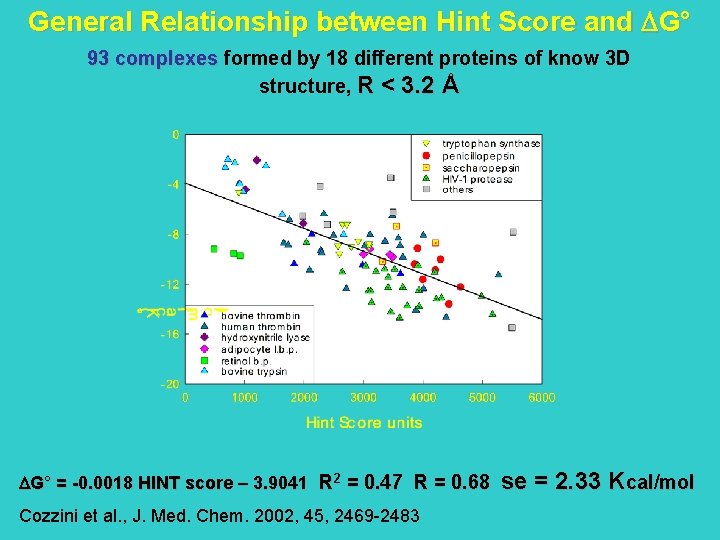
General Relationship between Hint Score and G° 73 complexes R < 2. 5 Å and at least 3 ligands for each protein G° = -0. 0024 HINT score – 2. 2187 R 2 = 0. 65 R = 0. 81 Kellogg et al. J. Mol. Graph. Model. 2004, in press se = 1. 89 Kcal/mol

Which is the contribution of water molecules bridging ligand protein to the free energy of binding?

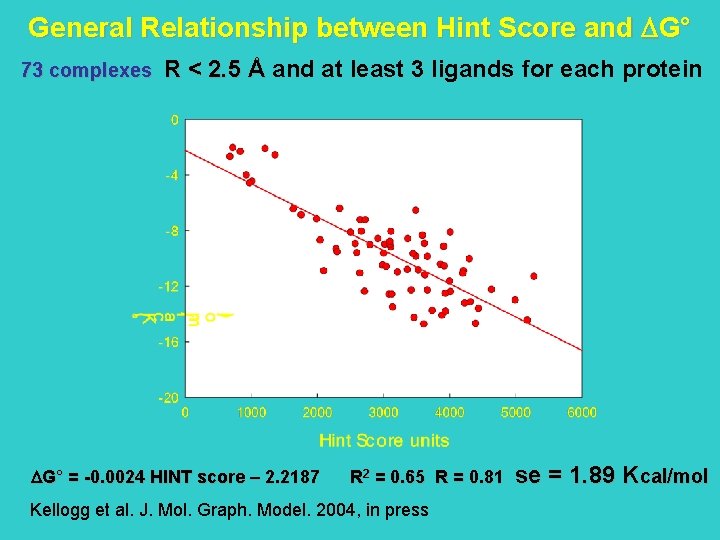
Active site of HIV-1 Protease in the absence and presence of a ligand

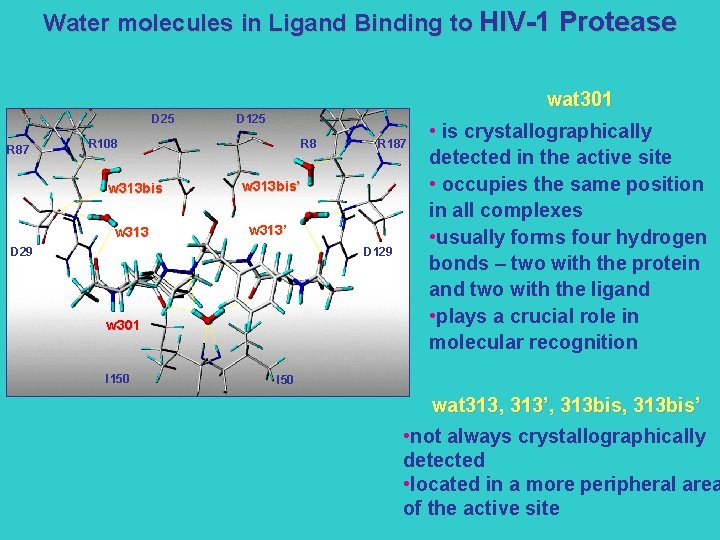
Water molecules in Ligand Binding to HIV-1 Protease wat 301 D 25 R 87 D 125 R 108 R 8 w 313 bis’ w 313’ D 29 R 187 D 129 w 301 I 150 • is crystallographically detected in the active site • occupies the same position in all complexes • usually forms four hydrogen bonds – two with the protein and two with the ligand • plays a crucial role in molecular recognition I 50 wat 313, 313’, 313 bis’ • not always crystallographically detected • located in a more peripheral area of the active site

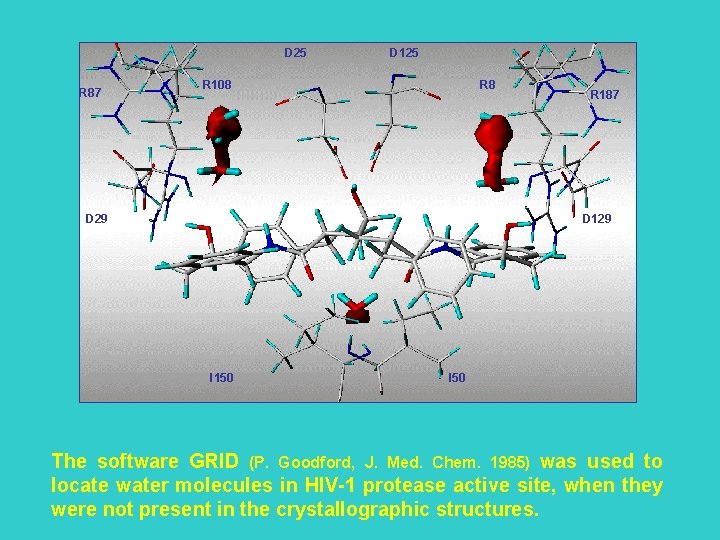
D 25 R 87 D 125 R 108 R 8 D 29 R 187 D 129 I 150 I 50 The software GRID (P. Goodford, J. Med. Chem. 1985) was used to locate water molecules in HIV-1 protease active site, when they were not present in the crystallographic structures.

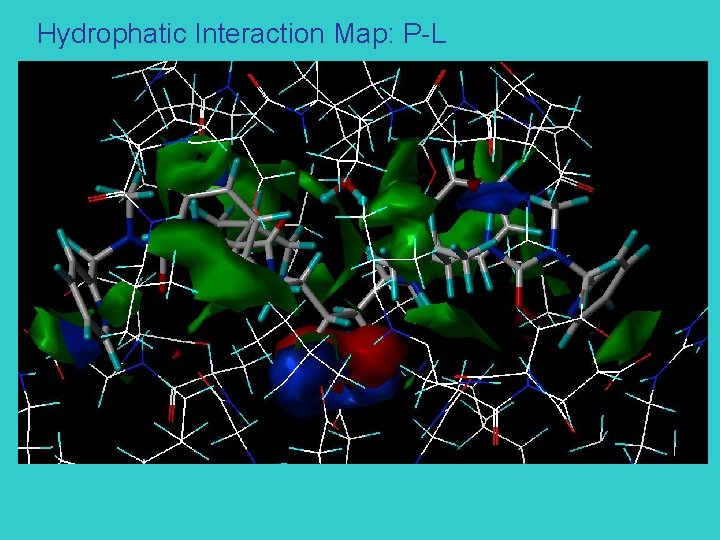
Hydrophatic Interaction Map: P-L

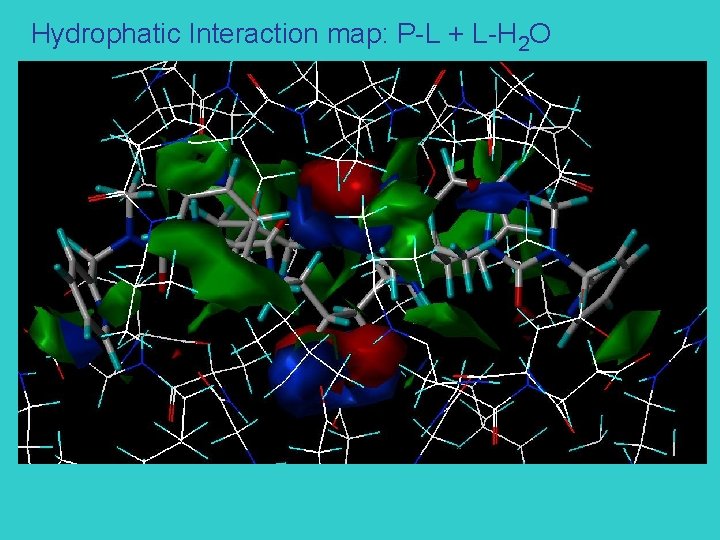
Hydrophatic Interaction map: P-L + L-H 2 O

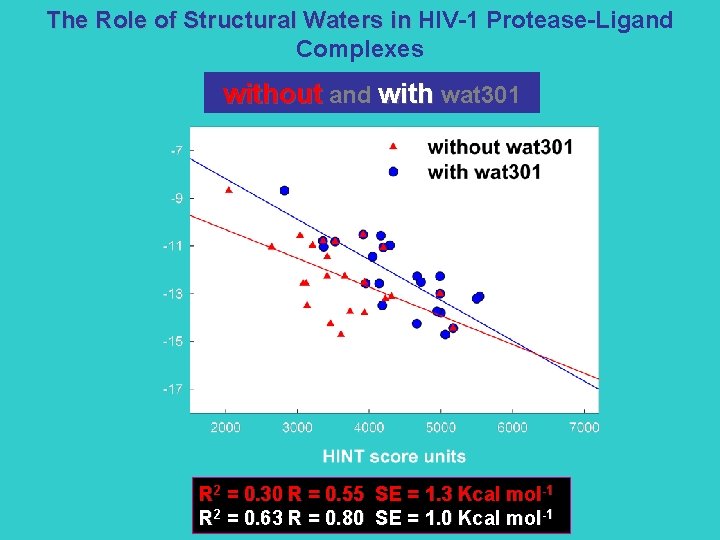
The Role of Structural Waters in HIV-1 Protease-Ligand Complexes without and with wat 301 R 2 = 0. 30 R = 0. 55 SE = 1. 3 Kcal mol-1 R 2 = 0. 63 R = 0. 80 SE = 1. 0 Kcal mol-1

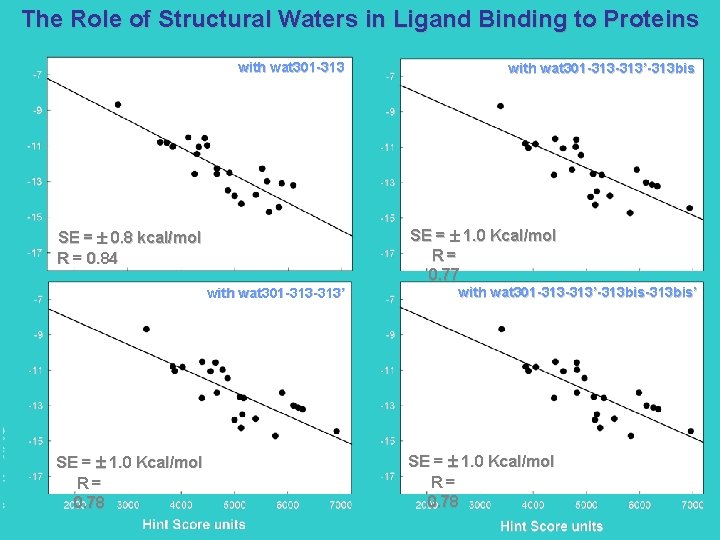
The Role of Structural Waters in Ligand Binding to Proteins with wat 301 -313 SE = 1. 0 Kcal/mol R= 0. 77 SE = 0. 8 kcal/mol R = 0. 84 with wat 301 -313’ SE = 1. 0 Kcal/mol R= 0. 78 with wat 301 -313 -313’-313 bis-313 bis’ SE = 1. 0 Kcal/mol R= 0. 78

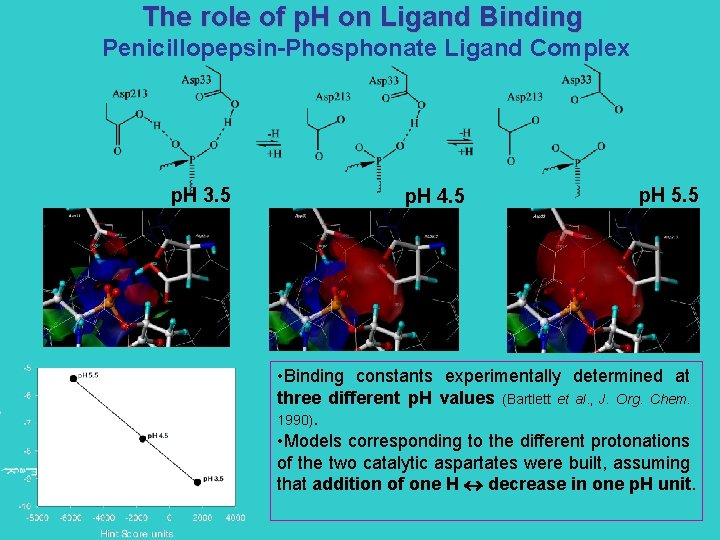
The role of p. H on Ligand Binding Penicillopepsin-Phosphonate Ligand Complex p. H 3. 5 p. H 4. 5 p. H 5. 5 • Binding constants experimentally determined at three different p. H values (Bartlett et al. , J. Org. Chem. 1990). • Models corresponding to the different protonations of the two catalytic aspartates were built, assuming that addition of one H decrease in one p. H unit.

The role of p. H on Ligand Binding Computational Titration v Drop protons into the molecular models, one hydrogen at a time acidification in silico of the environment of the protein-ligand complexes at the binding site v Model ALL possible cases for each “p. H” level (corresponding to a defined number of protons into the model) v Calculate the HINT score for each model, averaging the values that correspond to each “p. H” level v Plot the mean HINT score values as a function of the number of added protons

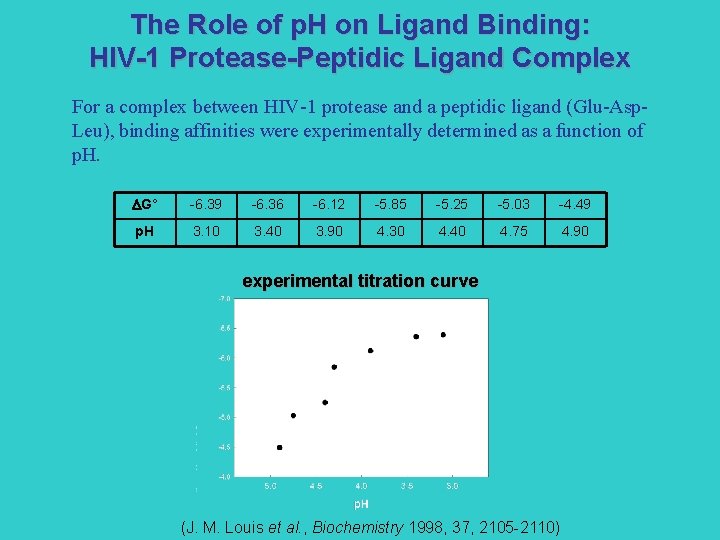
The Role of p. H on Ligand Binding: HIV-1 Protease-Peptidic Ligand Complex For a complex between HIV-1 protease and a peptidic ligand (Glu-Asp. Leu), binding affinities were experimentally determined as a function of p. H. G° -6. 39 -6. 36 -6. 12 -5. 85 -5. 25 -5. 03 -4. 49 p. H 3. 10 3. 40 3. 90 4. 30 4. 40 4. 75 4. 90 experimental titration curve (J. M. Louis et al. , Biochemistry 1998, 37, 2105 -2110)

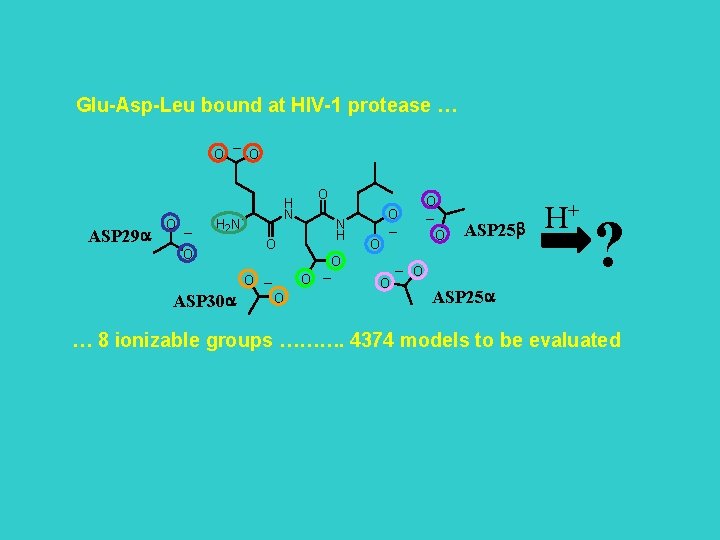
Glu-Asp-Leu bound at HIV-1 protease … O ASP 29 a O _ _ O H N H 2 N N H O O ASP 30 a O O O _ O _ O O O _ O ASP 25 b _ O H+ ? ASP 25 a … 8 ionizable groups ………. 4374 models to be evaluated

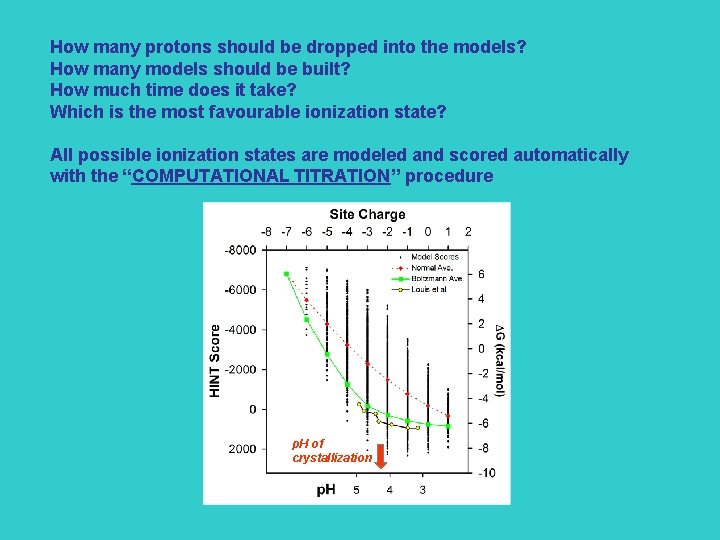
How many protons should be dropped into the models? How many models should be built? How much time does it take? Which is the most favourable ionization state? All possible ionization states are modeled and scored automatically with the “COMPUTATIONAL TITRATION” procedure p. H of crystallization

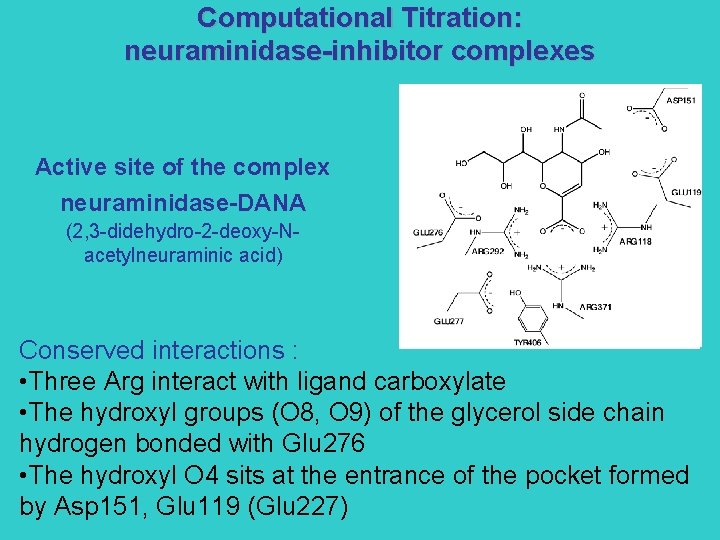
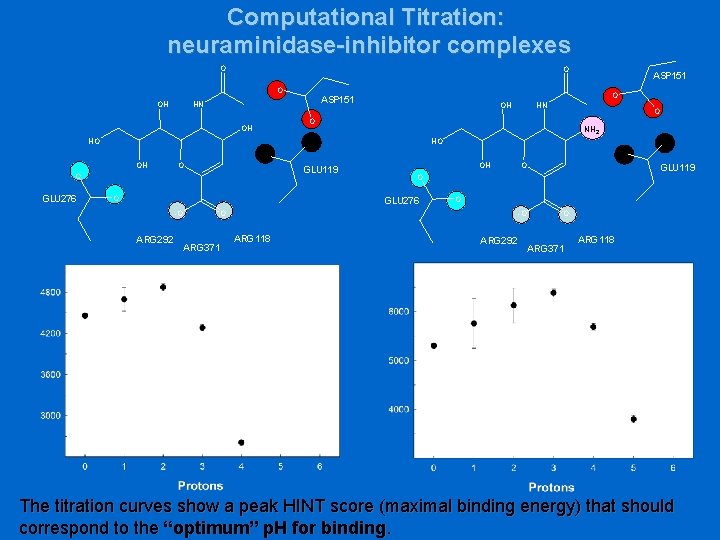
Computational Titration: neuraminidase-inhibitor complexes Active site of the complex neuraminidase-DANA (2, 3 -didehydro-2 -deoxy-Nacetylneuraminic acid) Conserved interactions : • Three Arg interact with ligand carboxylate • The hydroxyl groups (O 8, O 9) of the glycerol side chain hydrogen bonded with Glu 276 • The hydroxyl O 4 sits at the entrance of the pocket formed by Asp 151, Glu 119 (Glu 227)

Computational Titration: neuraminidase-inhibitor complexes O OH OH OH NH 2 O GLU 119 O OH -O ARG 118 GLU 119 O O ARG 371 O O GLU 276 ARG 292 O O -O O HO O OH HN O HO GLU 276 O ASP 151 HN ASP 151 ARG 292 O ARG 371 ARG 118 The titration curves show a peak HINT score (maximal binding energy) that should correspond to the “optimum” p. H for binding.

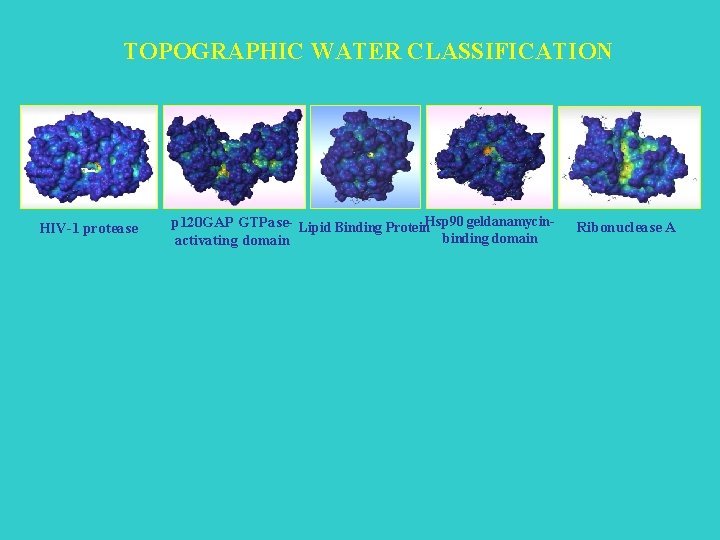
TOPOGRAPHIC WATER CLASSIFICATION HIV-1 protease p 120 GAP GTPase- Lipid Binding Protein. Hsp 90 geldanamycinbinding domain activating domain Ribonuclease A

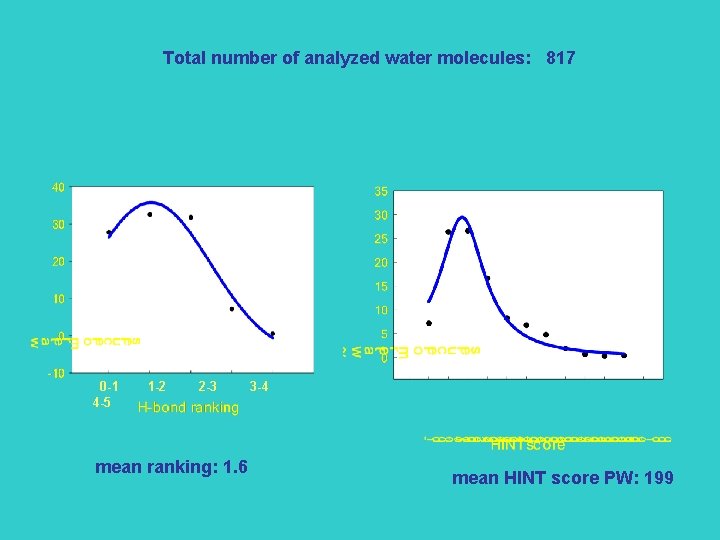
Total number of analyzed water molecules: 817 0 -1 4 -5 1 -2 2 -3 mean ranking: 1. 6 3 -4 mean HINT score PW: 199

Next: Analisi delle classi di molecole d’acqua (in cavità, in superficie, in siti attivi, …) Analisi dell’interazione tra proteina e DNA In silico screening Analisi dell’interazione proteina-proteina