COMPUTER AIDED DRUG DESIGN Prof K N RAJINI













- Slides: 20

COMPUTER AIDED DRUG DESIGN Prof. K. N. RAJINI KANTH M. Pharm. , (Ph. D) Head, Dept. of Pharmaceutical Analysis Chalapathi Institute of Pharmaceutical Sciences Chalapathi Nagar, Lam, Guntur

Drug Design • Drug design, is the inventive process of finding new medications based on the knowledge of a biological target. • Referred to as rational drug design or more simply rational design, • Multidisciplinary process that includes; Ø Identification of a drug target, Ø Bioassays, Ø Structural biology, Ø Synthetic medicinal chemistry, Ø Evaluation of the drug behaviour in the body.

• Each of these processes, in itself, is a complex task and there is a significant risk of failure at any step on the development path. • In the most basic sense, drug design involves the design of small molecules that are complementary in shape and charge to the biomolecular target with which they interact and therefore will bind to it.


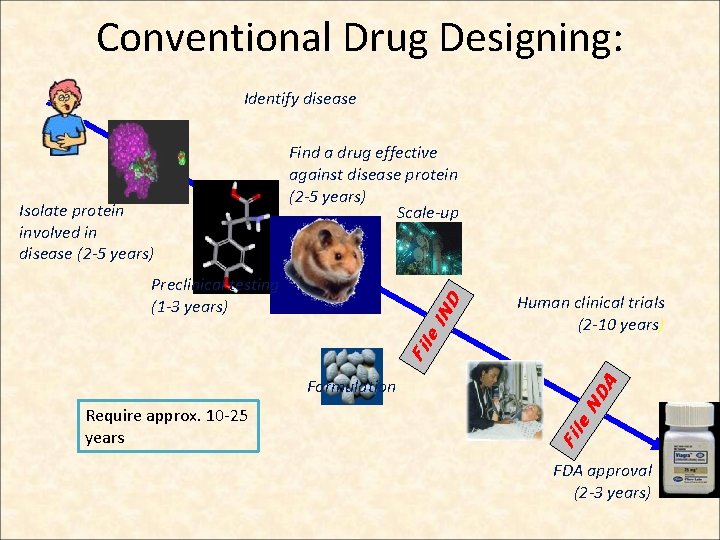
Conventional Drug Designing: Identify disease Isolate protein involved in disease (2 -5 years) Find a drug effective against disease protein (2 -5 years) Scale-up Human clinical trials (2 -10 years) ND le Require approx. 10 -25 years Fi Formulation A Fil e. I ND Preclinical testing (1 -3 years) FDA approval (2 -3 years)

Limitations of conventional drug designing: • Only traditional experimentation (in-vivo and in-vitro ) was done for therapeutic purpose • Animal and Human models were used • Laboratory tests were performed for assurance of desired pharmacological response. • Very costly • Time consuming : many years 10 -25 years needed for the approval of a single drug 6

Introduction of computer simulation in the field of drug designing: • By using the CADD software (computer simulations), time and expenses can be saved. 7

What does simulation means? ? ? • Imitative representation of the functioning of one system or process by means of the functioning of another. or • Simulation means mimicking of real life or potential situations, usually using computers.

Importance of using simulations: With simulation, • one can examine a problem that is often not subject to experimentation. • Gain better understanding of working of a system • Can Identify problems prior to implementation • Once built, the models can be run to give realistic results. • Provides a valuable support in making decisions on more logical & scientific basis. direct

The basic steps involved in simulation: 1. Define the process / problem 2. Collect Data on various events occurring in the process. 3. Build Computer models: • Which mimic independent events occurring in the process, the way they would occur in real processes. • Which give the user the flexibility to control events and parameters of the process the way he desires.

4. Run the simulation models through several recursions with a combination of real life 5. Observe the results and their variation and document them. 6. Make inferences and decisions based on the results of simulation.

CADD • Drug design which relies on computer modeling techniques is referred to as computer-aided drug design. • Computer-aided drug design uses computational chemistry to discover, enhance, or study drugs and related biologically active molecules. • The most fundamental goal is to predict whether a given molecule will bind to a target and if so how strongly. • Overcome the limitations of conventional methods

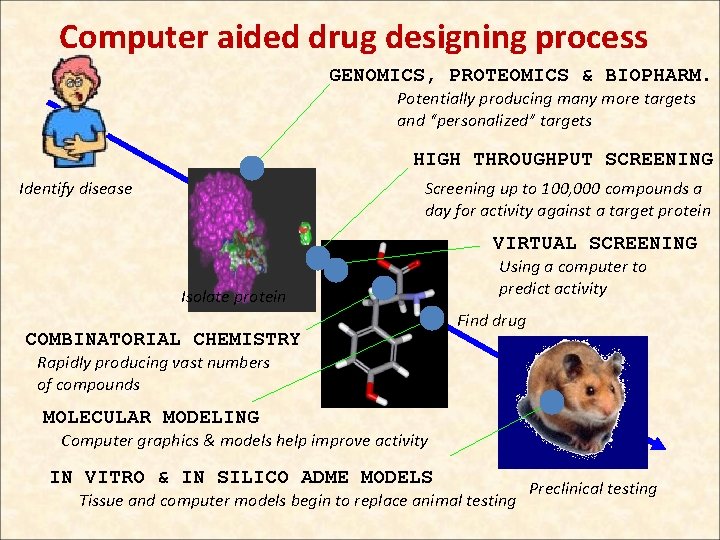
Computer aided drug designing process GENOMICS, PROTEOMICS & BIOPHARM. Potentially producing many more targets and “personalized” targets HIGH THROUGHPUT SCREENING Identify disease Screening up to 100, 000 compounds a day for activity against a target protein VIRTUAL SCREENING Isolate protein COMBINATORIAL CHEMISTRY Using a computer to predict activity Find drug Rapidly producing vast numbers of compounds MOLECULAR MODELING Computer graphics & models help improve activity IN VITRO & IN SILICO ADME MODELS Tissue and computer models begin to replace animal testing Preclinical testing

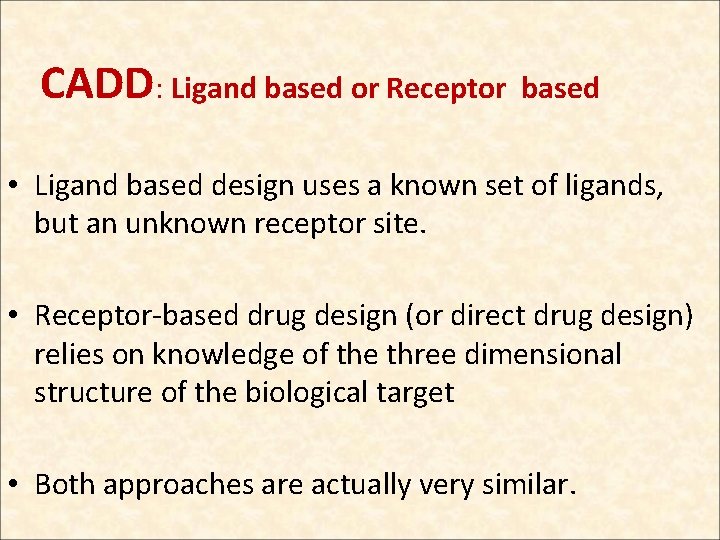
CADD: Ligand based or Receptor based • Ligand based design uses a known set of ligands, but an unknown receptor site. • Receptor-based drug design (or direct drug design) relies on knowledge of the three dimensional structure of the biological target • Both approaches are actually very similar.

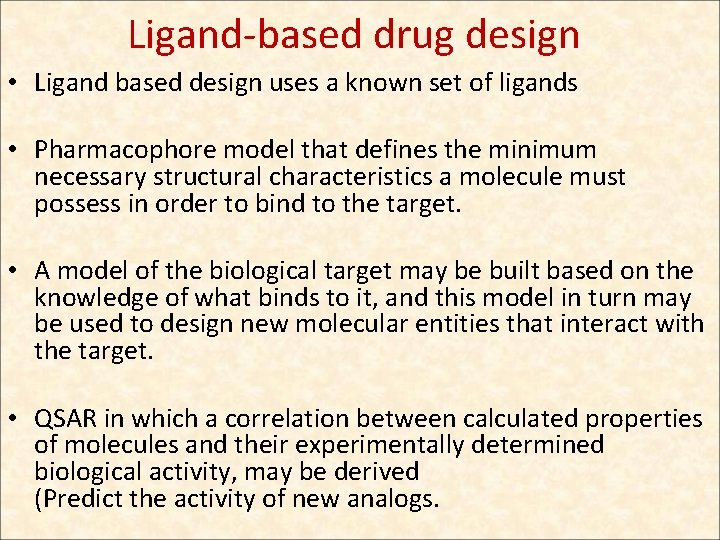
Ligand-based drug design • Ligand based design uses a known set of ligands • Pharmacophore model that defines the minimum necessary structural characteristics a molecule must possess in order to bind to the target. • A model of the biological target may be built based on the knowledge of what binds to it, and this model in turn may be used to design new molecular entities that interact with the target. • QSAR in which a correlation between calculated properties of molecules and their experimentally determined biological activity, may be derived (Predict the activity of new analogs.

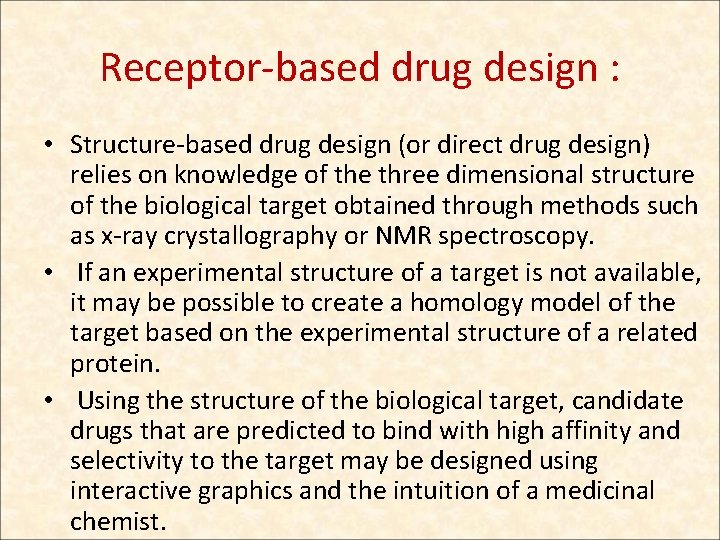
Receptor-based drug design : • Structure-based drug design (or direct drug design) relies on knowledge of the three dimensional structure of the biological target obtained through methods such as x-ray crystallography or NMR spectroscopy. • If an experimental structure of a target is not available, it may be possible to create a homology model of the target based on the experimental structure of a related protein. • Using the structure of the biological target, candidate drugs that are predicted to bind with high affinity and selectivity to the target may be designed using interactive graphics and the intuition of a medicinal chemist.

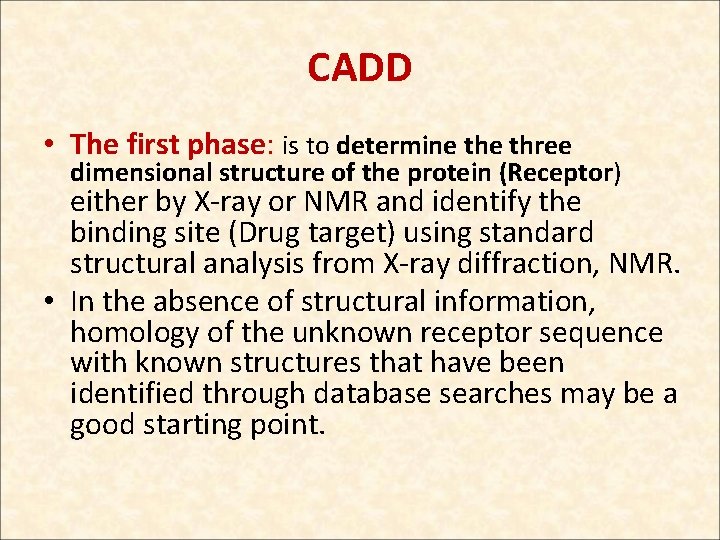
CADD • The first phase: is to determine three dimensional structure of the protein (Receptor) either by X-ray or NMR and identify the binding site (Drug target) using standard structural analysis from X-ray diffraction, NMR. • In the absence of structural information, homology of the unknown receptor sequence with known structures that have been identified through database searches may be a good starting point.

• The second phase: is to generate a query for database searching. • This model may be based on a pharmacophore (Functional group types (e. g. H bond donors, acceptors, hydrophobic regions) • The spatial arrangement of those groups on a molecule that interact with the receptor and are responsible for binding and biological activity). • The query is generated by building a simplified model of the receptor site

• The third phase: is to search databases for ligands that may bind to the chosen receptor. • The 3 D-pharmacophore is used in conformationally flexible searches for ligands that match the spatial distribution of the receptor. • Docking can be accomplished by either geometric matching of the ligand its receptor or by minimising the energy of interaction.

Benefits of CADD § Cost savings Based on CADD simulations, promosing experimental lines of enquiry followed and experimental dead-ends avoided § Time to market By focussing drug research on specific lead candidates companies can market the drugs quickly § Insight Non-quantifiable benefits of CADD