CME Disclosure Statement The North Shore LIJ Health



















- Slides: 33


CME Disclosure Statement • The North Shore LIJ Health System adheres to the ACCME's new Standards for Commercial Support. Any individuals in a position to control the content of a CME activity, including faculty, planners and managers, are required to disclose all financial relationships with commercial interests. All identified potential conflicts of interest are thoroughly vetted by the North Shore-LIJ for fair balance and scientific objectivity and to ensure appropriateness of patient care recommendations. • Course Director, Kevin Tracey, has disclosed a commercial interest in Setpoint, Inc. as the cofounder, for stock and consulting support. He has resolved his conflicts by identifying a faculty member to conduct content review of this program who has no conflicts. • The speaker, Martin L. Lesser, Ph. D, has no conflicts. 2


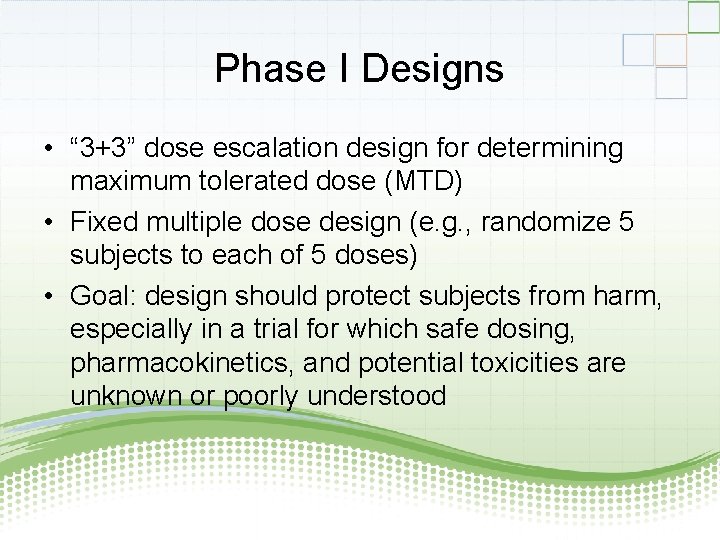
Phase I Designs • “ 3+3” dose escalation design for determining maximum tolerated dose (MTD) • Fixed multiple dose design (e. g. , randomize 5 subjects to each of 5 doses) • Goal: design should protect subjects from harm, especially in a trial for which safe dosing, pharmacokinetics, and potential toxicities are unknown or poorly understood

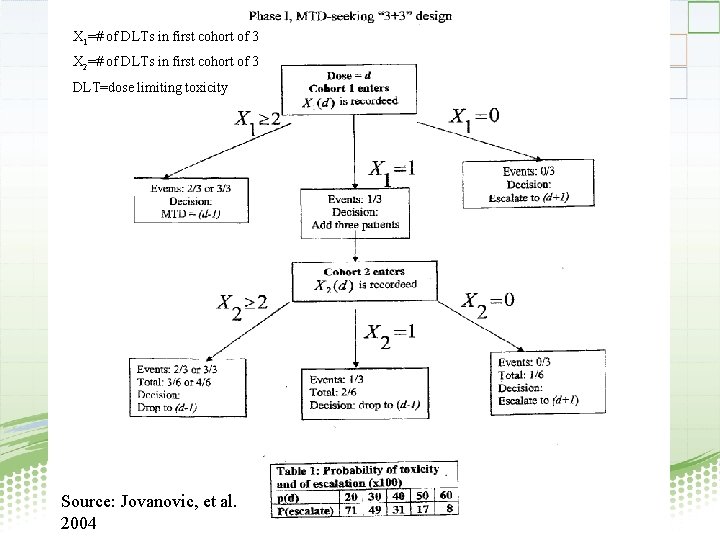
X 1=# of DLTs in first cohort of 3 X 2=# of DLTs in first cohort of 3 DLT=dose limiting toxicity Source: Jovanovic, et al. 2004

Phase II Designs • Applied to a specific disease entity • Fixed dose is used • Simple primary outcome: response, measurement of some parameter • Single arm, open label (traditional) • Single arm, blinded evaluator (uncommon) • Simon 2 -stage design • Randomized Phase II trial (for selection of best therapy)

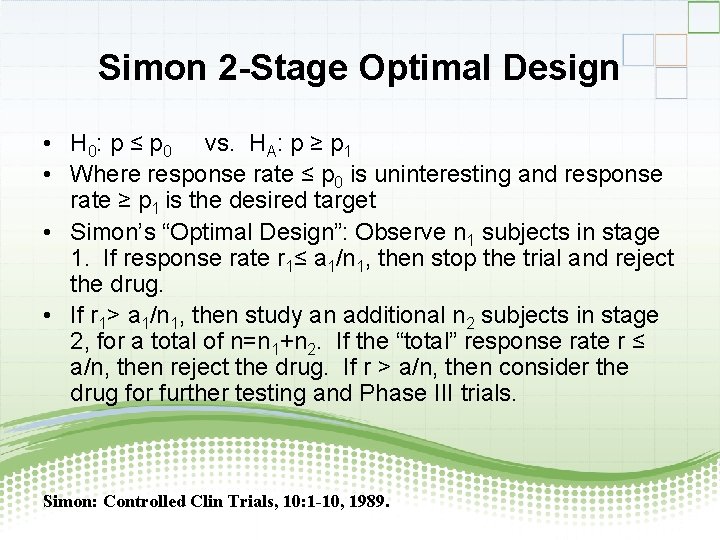
Simon 2 -Stage Optimal Design • H 0: p ≤ p 0 vs. HA: p ≥ p 1 • Where response rate ≤ p 0 is uninteresting and response rate ≥ p 1 is the desired target • Simon’s “Optimal Design”: Observe n 1 subjects in stage 1. If response rate r 1≤ a 1/n 1, then stop the trial and reject the drug. • If r 1> a 1/n 1, then study an additional n 2 subjects in stage 2, for a total of n=n 1+n 2. If the “total” response rate r ≤ a/n, then reject the drug. If r > a/n, then consider the drug for further testing and Phase III trials. Simon: Controlled Clin Trials, 10: 1 -10, 1989.

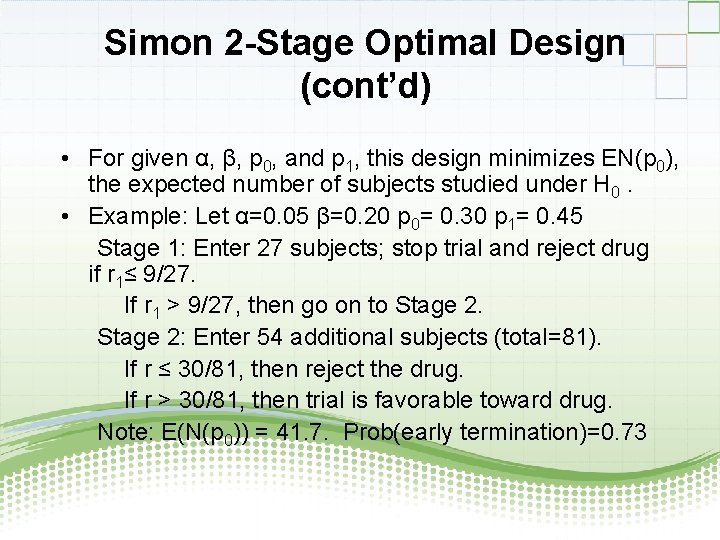
Simon 2 -Stage Optimal Design (cont’d) • For given α, β, p 0, and p 1, this design minimizes EN(p 0), the expected number of subjects studied under H 0. • Example: Let α=0. 05 β=0. 20 p 0= 0. 30 p 1= 0. 45 Stage 1: Enter 27 subjects; stop trial and reject drug if r 1≤ 9/27. If r 1 > 9/27, then go on to Stage 2: Enter 54 additional subjects (total=81). If r ≤ 30/81, then reject the drug. If r > 30/81, then trial is favorable toward drug. Note: E(N(p 0)) = 41. 7. Prob(early termination)=0. 73

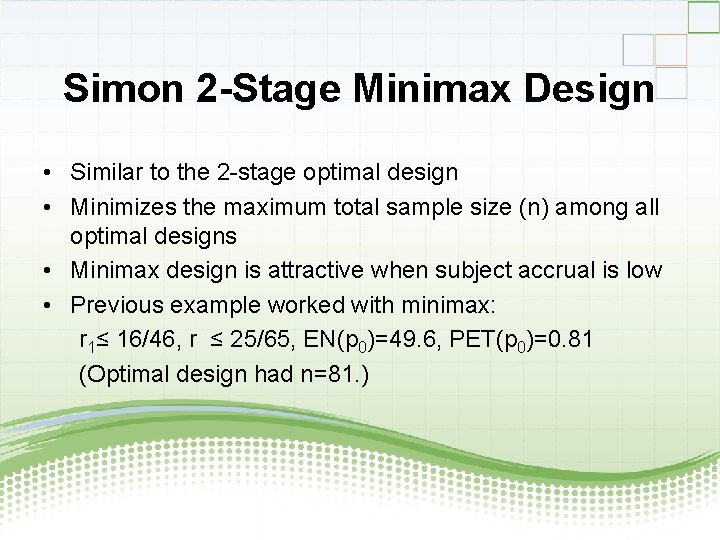
Simon 2 -Stage Minimax Design • Similar to the 2 -stage optimal design • Minimizes the maximum total sample size (n) among all optimal designs • Minimax design is attractive when subject accrual is low • Previous example worked with minimax: r 1≤ 16/46, r ≤ 25/65, EN(p 0)=49. 6, PET(p 0)=0. 81 (Optimal design had n=81. )

Phase III Trials

Design Considerations (continued) • Treatment Plan • Blinding • Use of Placebo Control • Criteria for evaluation of treatment effect (comparability of patient follow-up)






Design Considerations (continued) • Blinding • Placebo control • Stratification • The process of randomization • Handling dropouts and non-compliance • Statistical methods for data analysis • Sample size and power • Interim analysis and early stopping

Blinding • Any attempt to make study participants unaware of which treatment is offered • Is indicated when the occurrence and reporting of outcomes can be easily influenced by knowledge of treatment (subjective responses, behavior change) • May be either single blind or double blind • Blinding is not always feasible • Blinding may be unsuccessful (ability to break the blind)

Placebo Control • Appropriate when no effective standard treatment exists for the control group • Makes subject’s attitudes to the trial as similar as possible in the treatment and control groups • Major uses: − Controls for psychological factors − Maintains double blind design − Controls for spontaneous disease variability • Ethical issues: - May be unethical to withhold treatment in order to administer placebo






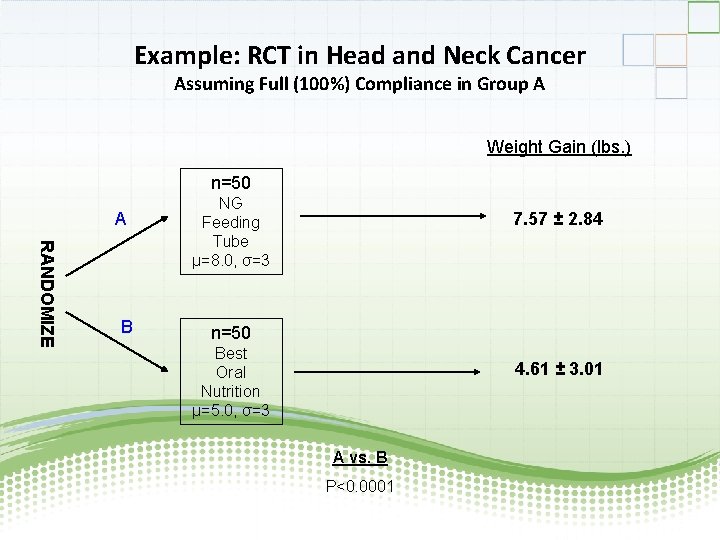
Example: RCT in Head and Neck Cancer Assuming Full (100%) Compliance in Group A Weight Gain (lbs. ) n=50 A RANDOMIZE B NG Feeding Tube µ=8. 0, σ=3 7. 57 ± 2. 84 n=50 Best Oral Nutrition µ=5. 0, σ=3 4. 61 ± 3. 01 A vs. B P<0. 0001

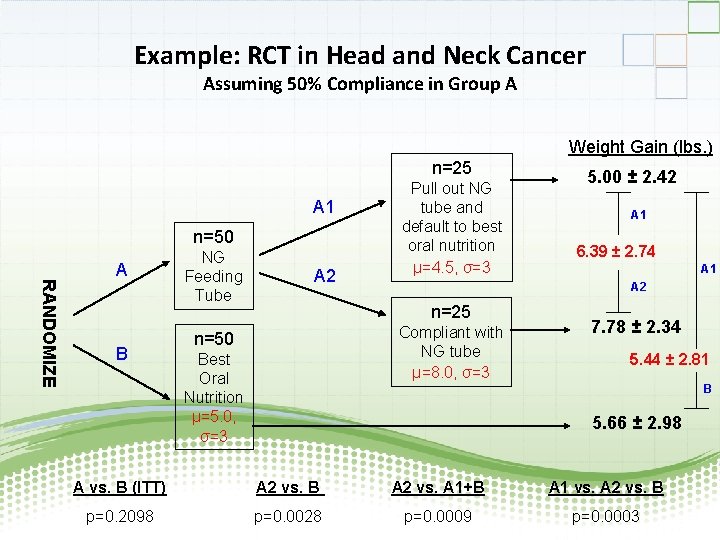
Example: RCT in Head and Neck Cancer Assuming 50% Compliance in Group A Weight Gain (lbs. ) n=25 A 1 n=50 RANDOMIZE A B NG Feeding Tube A 2 Pull out NG tube and default to best oral nutrition µ=4. 5, σ=3 Compliant with NG tube µ=8. 0, σ=3 Best Oral Nutrition µ=5. 0, σ=3 A 1 6. 39 ± 2. 74 A 1 A 2 n=25 n=50 5. 00 ± 2. 42 7. 78 ± 2. 34 5. 44 ± 2. 81 B 5. 66 ± 2. 98 A vs. B (ITT) A 2 vs. B A 2 vs. A 1+B A 1 vs. A 2 vs. B p=0. 2098 p=0. 0028 p=0. 0009 p=0. 0003






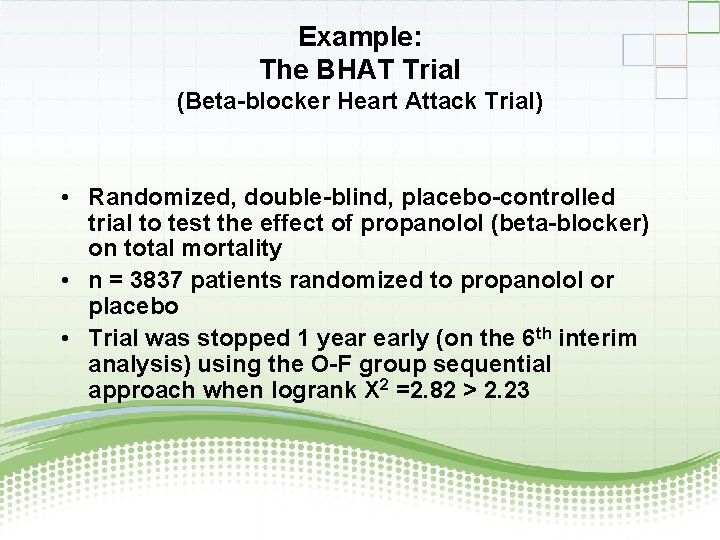
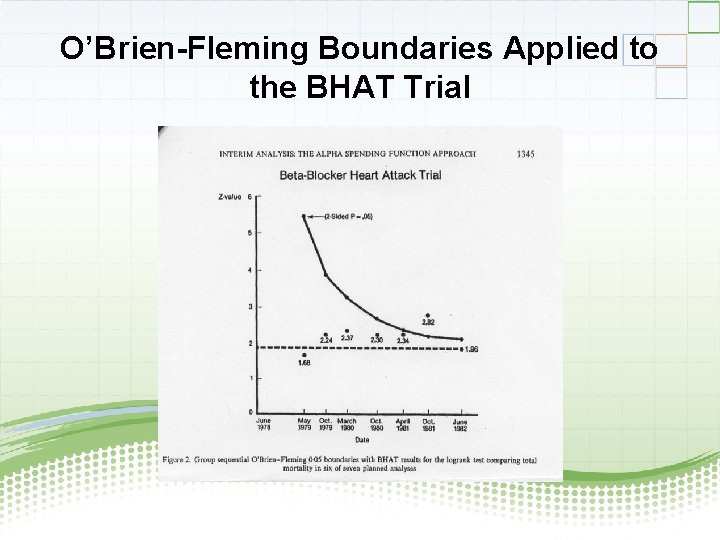
Example: The BHAT Trial (Beta-blocker Heart Attack Trial) • Randomized, double-blind, placebo-controlled trial to test the effect of propanolol (beta-blocker) on total mortality • n = 3837 patients randomized to propanolol or placebo • Trial was stopped 1 year early (on the 6 th interim analysis) using the O-F group sequential approach when logrank X 2 =2. 82 > 2. 23

O’Brien-Fleming Boundaries Applied to the BHAT Trial
 Steamship america a north shore legend
Steamship america a north shore legend Piranha triathlon
Piranha triathlon North shore travel
North shore travel Mean girls cafeteria map
Mean girls cafeteria map No disclosures
No disclosures School ethics commission personal disclosure statement
School ethics commission personal disclosure statement Disclosure statement example
Disclosure statement example Albuminuria
Albuminuria Pedialink cme
Pedialink cme Cme 2012
Cme 2012 Faculty invitation letter
Faculty invitation letter Emory cme
Emory cme Lente intensificadora de imagem para cme
Lente intensificadora de imagem para cme Market profile day types
Market profile day types Cme task
Cme task Cme fec
Cme fec Nccpa self assessment cme
Nccpa self assessment cme Www nyp org nursing news cme
Www nyp org nursing news cme Felix carabello
Felix carabello Cme semi centralizada
Cme semi centralizada Cme tracking tool
Cme tracking tool Dr jeffrey sklar
Dr jeffrey sklar Penicillin allergy cme
Penicillin allergy cme Cme tbond futures
Cme tbond futures Uic cme
Uic cme True north vs magnetic north
True north vs magnetic north North east and north cumbria integrated care system
North east and north cumbria integrated care system Chapter 14 lesson 4 people of the south
Chapter 14 lesson 4 people of the south The north pole ____ a latitude of 90 degrees north
The north pole ____ a latitude of 90 degrees north Open disclosure in aged care
Open disclosure in aged care No disclosure slide
No disclosure slide Nothing to disclose slide
Nothing to disclose slide Disclosure slide example
Disclosure slide example Class disclosure
Class disclosure