Clinical trials facts and myths Why do clinical


















































- Slides: 69

Clinical trials - facts and myths! Why do clinical trials 2. How did it all start 3. Do we really need to do trials in India 4. SWOT Analysis reenanair@email. com 1.

Why do Clinical trials? Ø Academic Investigators / Caregivers ~ Increased ability to publish results ↑ professional stature, earlier promotion, ↑ salary ~ Desire to offer more therapeutic options to patients Ø Government Sponsors ~ Claims of success in advancing health care ~ Leverage for ↑ in government funding Ø Industry Sponsors ~ Company profits, ↑ value of stock options, promotion …. Wide Spread & Significant Conflicts of Interest

Clinical Trial Gains! l Gains for mankind l National gains l Institutional gains Departmental gains l Personal gains

How did it all start 1639 The surgeons Mate, by John Woodall The cures of scurvy 1753 Two sailors (2 X 6) allocated to each of: a quart of cider daily 25 gutts of elixir vitriol thrice daily 2 spoonfuls of vinegar thrice daily half a pint of sea water daily two oranges and a lemon daily the bigness of a nutmeg thrice daily Diet was constant 1795 Approved in all ships

The Flexner Report - the Standardization of American Medical Education 1900 s l If the sick are to reap the full benefit of recent progress in medicine, a more uniformly arduous and expensive medical education is demanded. l The AMA sought to eliminate schools that failed to adopt this rigorous brand of systematized, experiential medical education. l Editors of JAMA declared, “It is to be hoped that with higher standards universally applied their number will soon be adequately reduced, and that only the fittest will survive, ”

American Medical Education – 100 Years after the Flexner Report l Flexner envisioned a clinical phase of education in academically oriented hospitals, where thoughtful clinicians would pursue research stimulated by the questions that arose in the course of patient care and teach their students to do the same. In academic hospitals, research quickly outstripped teaching in importance. l A “publish or perish” culture emerged in American universities and medical schools. l

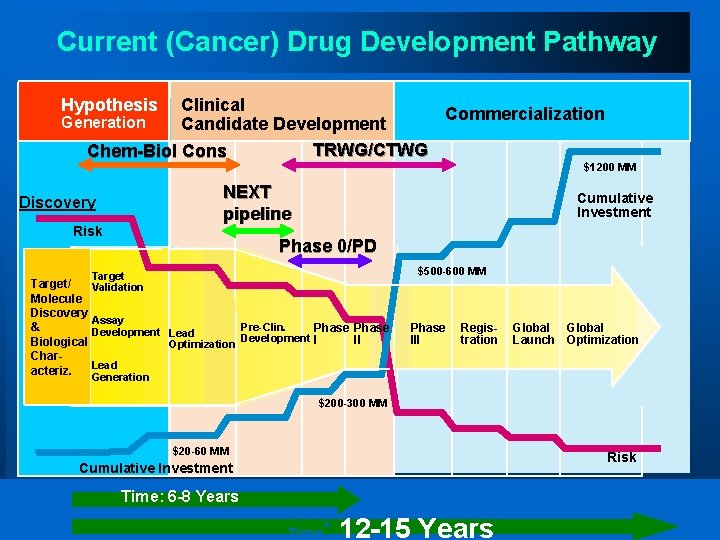
Current (Cancer) Drug Development Pathway Hypothesis Clinical Commercialization Generation Candidate Development TRWG/CTWG Chem-Biol Cons $1200 MM NEXT pipeline Discovery Risk Cumulative Investment Phase 0/PD $500 -600 MM Target Validation Target/ Molecule Discovery Assay Pre-Clin. & Phase Development Lead Development I II Biological Optimization Char. Lead acteriz. Phase III Registration Global Launch Optimization Generation $200 -300 MM $20 -60 MM Risk Cumulative Investment Time: 6 -8 Years Time : 12 -15 Years

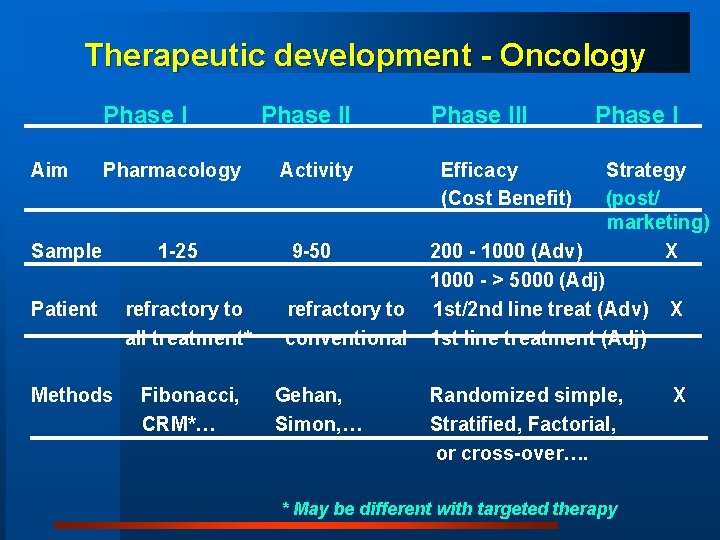
Therapeutic development - Oncology Phase I Aim Phase II Pharmacology Activity 1 -25 9 -50 Sample Patient Methods refractory to all treatment* Fibonacci, CRM*… refractory to conventional Gehan, Simon, … Phase III Efficacy (Cost Benefit) Phase I Strategy (post/ marketing) X 200 - 1000 (Adv) 1000 - > 5000 (Adj) 1 st/2 nd line treat (Adv) 1 st line treatment (Adj) Randomized simple, Stratified, Factorial, or cross-over…. * May be different with targeted therapy X X


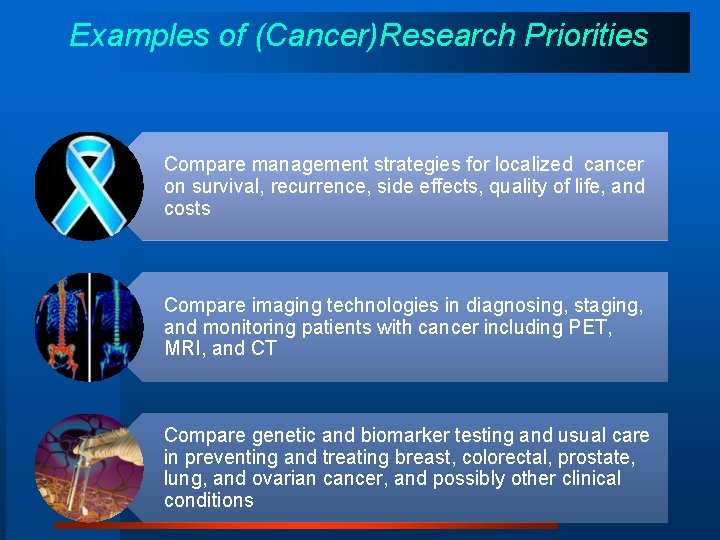
Examples of (Cancer)Research Priorities Compare management strategies for localized cancer on survival, recurrence, side effects, quality of life, and costs Compare imaging technologies in diagnosing, staging, and monitoring patients with cancer including PET, MRI, and CT Compare genetic and biomarker testing and usual care in preventing and treating breast, colorectal, prostate, lung, and ovarian cancer, and possibly other clinical conditions

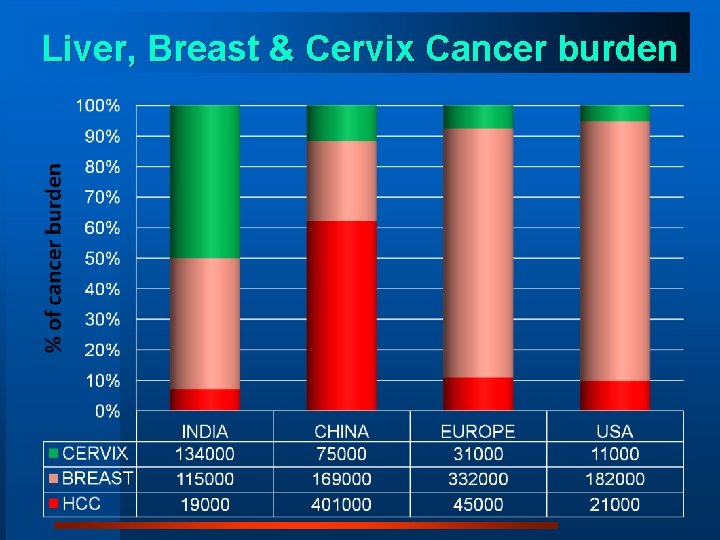
1. Disease (Cancer) burden

Liver, Breast & Cervix Cancer burden

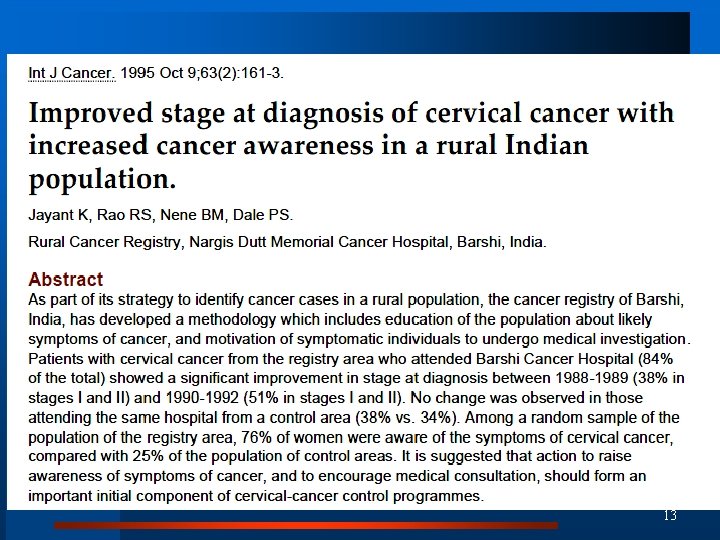
13

Health education improves survival 3 -year survival improved from 26. 6% to 44. 0% 14

2. Natural history varies


3. Needs of our populations vary

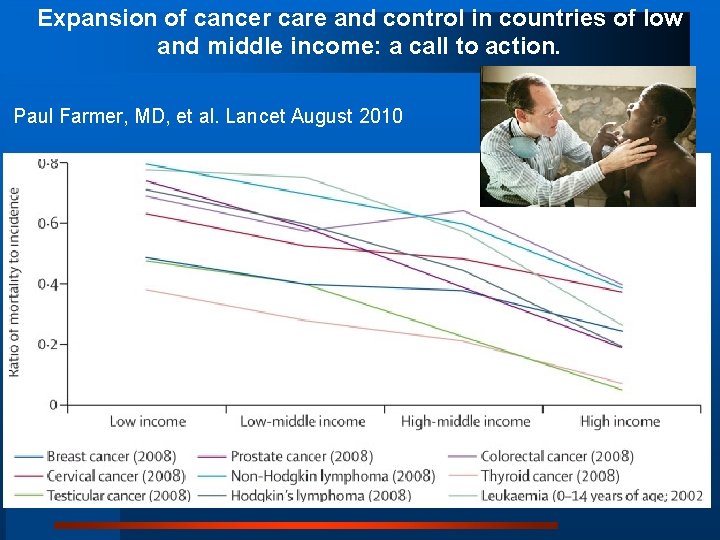
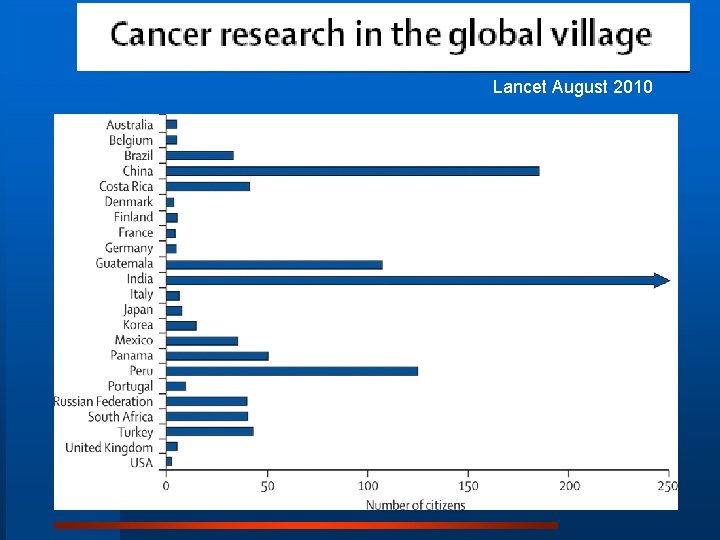
Expansion of cancer care and control in countries of low and middle income: a call to action. Paul Farmer, MD, et al. Lancet August 2010

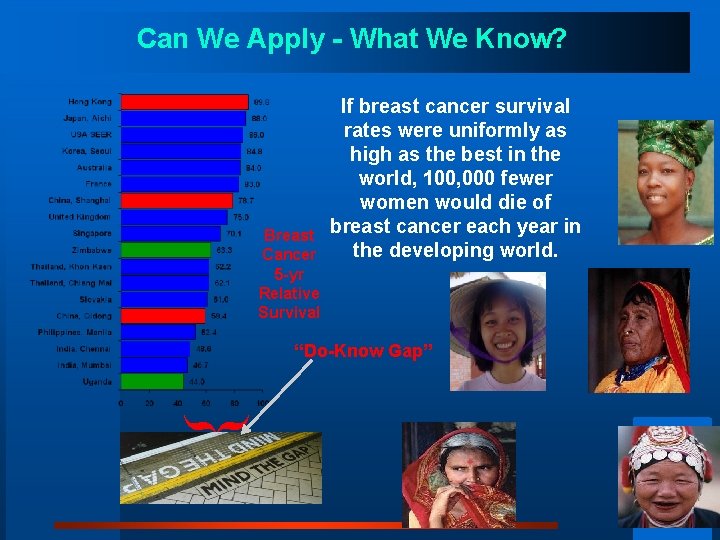
Can We Apply - What We Know? Breast Cancer 5 -yr Relative Survival If breast cancer survival rates were uniformly as high as the best in the world, 100, 000 fewer women would die of breast cancer each year in the developing world. “Do-Know Gap” }


4. Co-morbidity varies

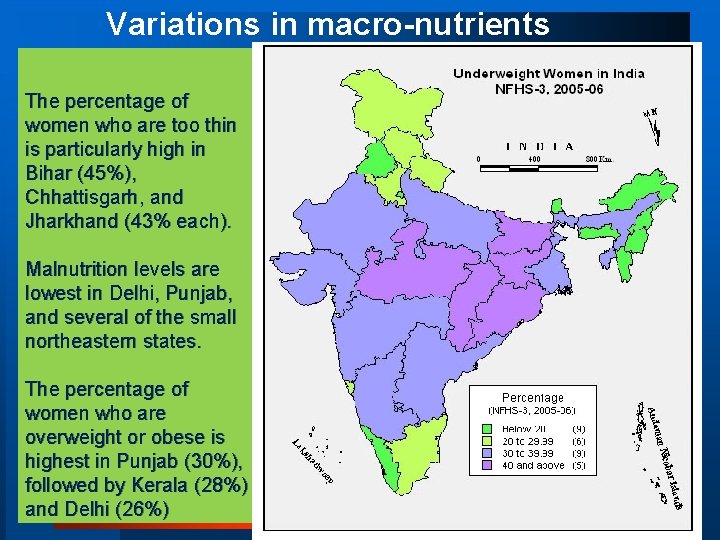
Variations in macro-nutrients The percentage of women who are too thin is particularly high in Bihar (45%), Chhattisgarh, and Jharkhand (43% each). Malnutrition levels are lowest in Delhi, Punjab, and several of the small northeastern states. The percentage of women who are overweight or obese is highest in Punjab (30%), followed by Kerala (28%) and Delhi (26%)

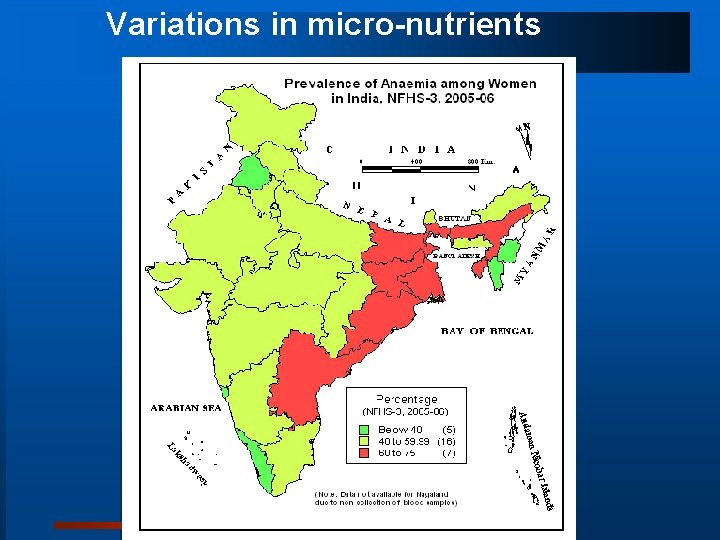
Variations in micro-nutrients

5. Infections are very common and the bugs are different


6. PK/PD can also vary. Toxicity and effectiveness varies


7. Tumor response varies

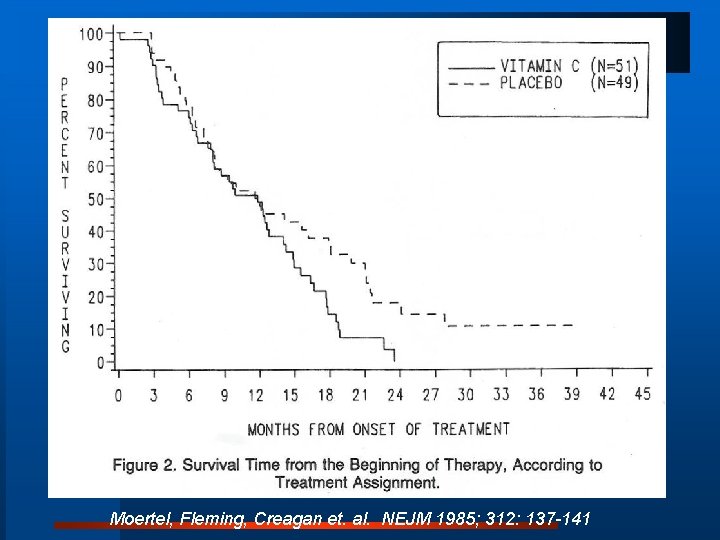
Hypothesis generation observational data vs confirmation by clinical research Mega doses of Vitamin C: What is the effect on duration of survival in preterminal cancer patients? Ø Nobel Laureate Linus Pauling: Loch Lomanside, Scotland Cameron, Pauling. Proc Natl Acad Sci 1976; 1978 Median Survival: 50 vs. 210 days; 38 vs. 293 days Ø Mayo Clinic sponsored randomized trial

Moertel, Fleming, Creagan et. al. NEJM 1985; 312: 137 -141

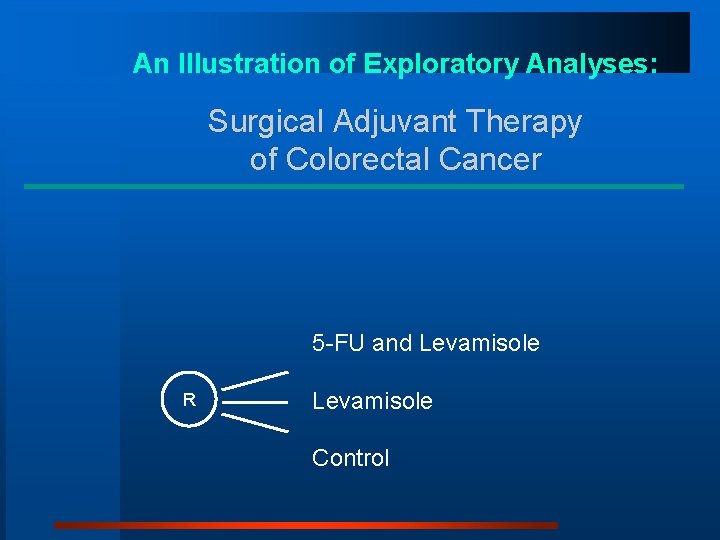
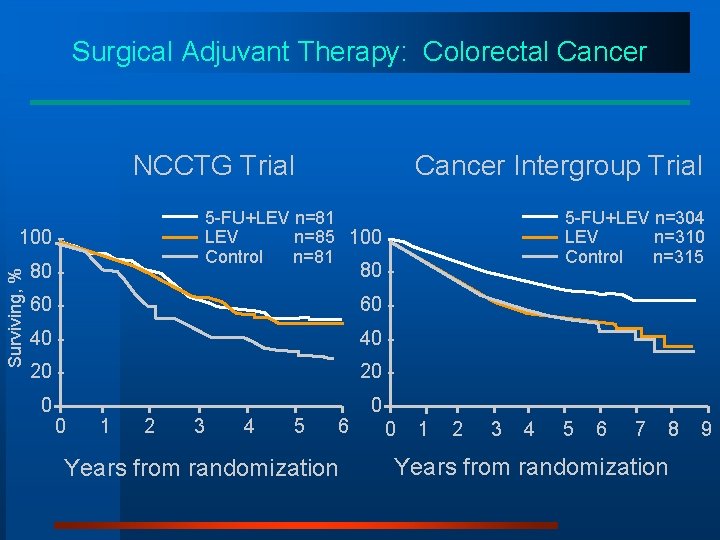
An Illustration of Exploratory Analyses: Surgical Adjuvant Therapy of Colorectal Cancer 5 -FU and Levamisole R Levamisole Control

Surgical Adjuvant Therapy: Colorectal Cancer NCCTG Trial 5 -FU+LEV n=81 LEV n=85 100 Control n=81 100 Surviving, % Cancer Intergroup Trial 80 - 80 60 - 40 - 20 - 0 0 0 5 -FU+LEV n=304 LEV n=310 Control n=315 1 2 3 4 5 6 Years from randomization 0 1 2 3 4 5 6 7 8 Years from randomization 9

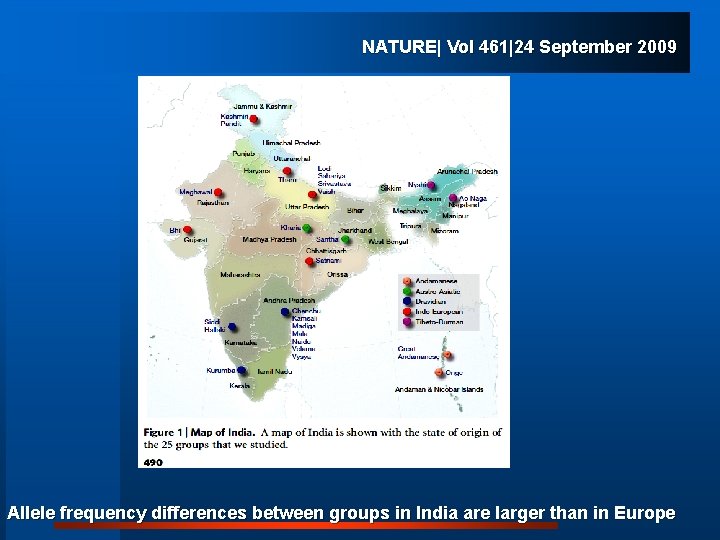
8. Genetic make up also varies. This is going to be important in the era of personalized medicine

NATURE| Vol 461|24 September 2009 Allele frequency differences between groups in India are larger than in Europe

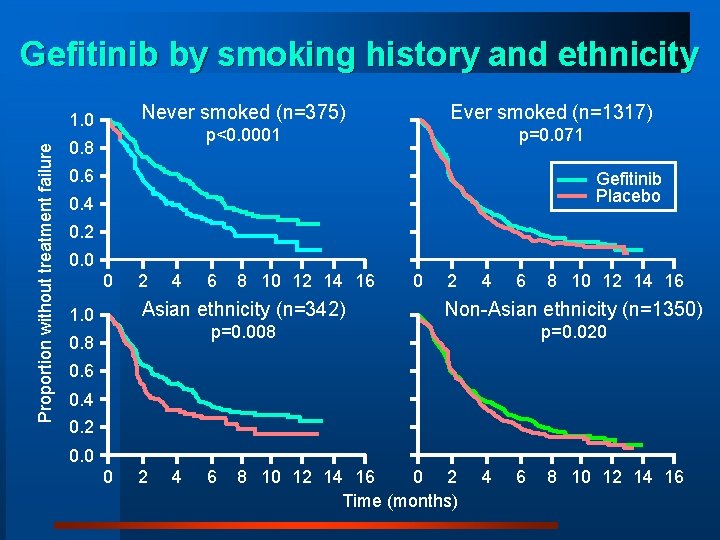
Gefitinib by smoking history and ethnicity Proportion without treatment failure 1. 0 Never smoked (n=375) Ever smoked (n=1317) p<0. 0001 p=0. 071 0. 8 0. 6 Gefitinib Placebo 0. 4 0. 2 0. 0 0 1. 0 2 4 6 8 10 12 14 16 Asian ethnicity (n=342) Non-Asian ethnicity (n=1350) p=0. 008 p=0. 020 0. 8 0. 6 0. 4 0. 2 0. 0 0 2 4 6 0 2 8 10 12 14 16 Time (months) 4 6 8 10 12 14 16

9. Creating affordable treatments

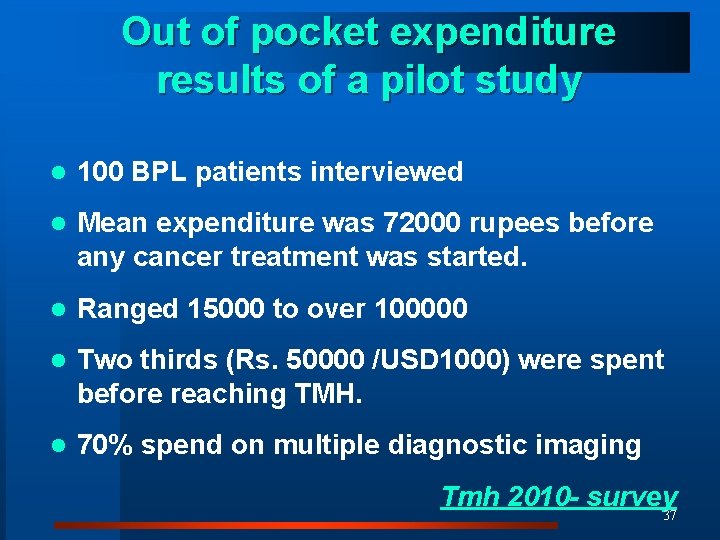
Out of pocket expenditure results of a pilot study l 100 BPL patients interviewed l Mean expenditure was 72000 rupees before any cancer treatment was started. l Ranged 15000 to over 100000 l Two thirds (Rs. 50000 /USD 1000) were spent before reaching TMH. l 70% spend on multiple diagnostic imaging Tmh 2010 - survey 37

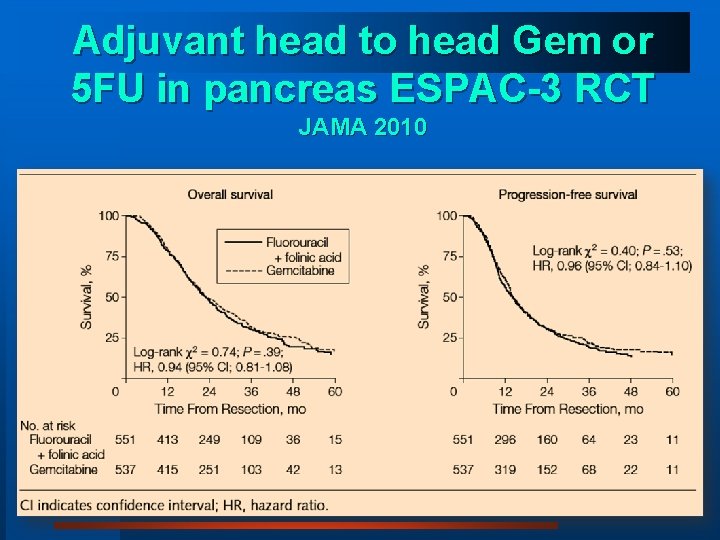
Outcome following adjuvant chemotherapy for pancreas cancer- recent trials 5 FU costs 5% of Gemcitabine CONKO-001: Disease-Free Survival ESPAC-1: Survival 75% 100% 50% Survival (%) Cumulative Disease Free Survival 100% gemcitabine 25% 75% 50% LV+ 5 FU 25% observation 0% No chemotherapy 0% 0 12 24 36 48 Months 60 72 84 Oettle H, et al. J Am Med Assoc. 2007; 297: 267 -77. Assoc. 0 12 24 36 Months 48 60 Neoptolemos JP, et al. NEJM. 2004; 350: 1200 -10. 72

Adjuvant head to head Gem or 5 FU in pancreas ESPAC-3 RCT JAMA 2010


Clinical Research in Cancer A SWOT ANALYSIS Speaking for myself!!

CLINICAL TRIALS

STRENGTHS l Very large patient pool l Untreated patients l High volume services l World class facilities l Good record keeping l Operating costs are low l English speaking l Research culture is improving




Lancet August 2010


WEAKNESS l Lack of formal training in clinical research l We give up easily (like our cricket team) – We also need foreign coaches l Very large (migrant) patient pool – Lost to follow up l High volume (overburdened) services l Cheap (untrained and incompetent) labor l Regulatory affairs personnel lack experience l Illiterate or vernacular speaking l Drop out and lost to follow up rates are high

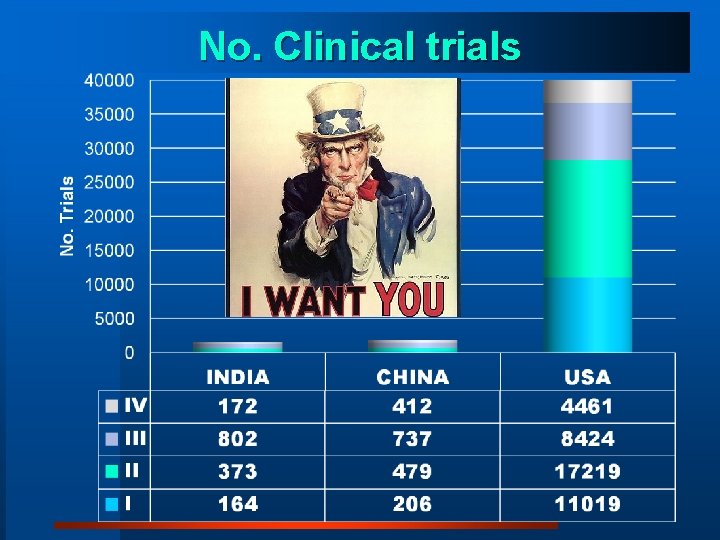
No. Clinical trials

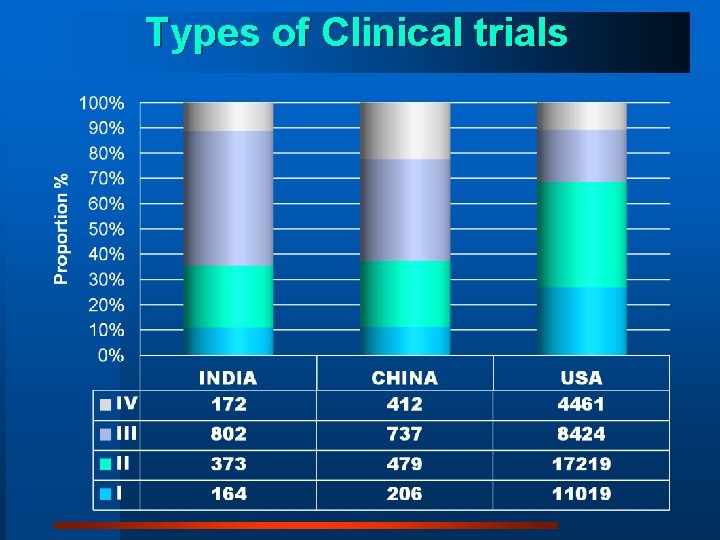
Types of Clinical trials


WEAKNESS l Few trials published in high impact journals l Still struggling with regulatory aspects of trials l Professional jealousy has crept in l Inter & Intra departmental bottlenecks l We do not collaborate [within TMC & out side] – Divide and rule hangover still exits l Less than 1% patients on clinical trials. l Routine care is starting to suffer l Education & training is loosing out







Opportunities l Training in trial methodology l Recognition and opinion leadership l Numerous trials help patients l Funding has increased l International exposure & network l HRD in clinical trials – Youngsters are getting opportunity

THREATS l Competitive enrollment – Many small groups enrolling l Cheaper than us options l Collaboration is higher l Competing trials l Professional rivalry l Failure to comply with regulators l Ethics/ Blacklisting l Move away from core competence

When a great profession and the forces of capitalism interact, drama is likely to result.



Clinical trials losing the plot in India l Mc. Kinsey had earlier projected that by 2011, over 3, 000 patients would be enrolled for clinical trials in India and 1, 500 to 2, 000 studies conducted here each year. l As against this, the Indian clinical trial industry did only 240 -260 trials from MNCs and another 180 -200 trials of domestic companies last year. l Recession, regulatory issues, lack of laws, concerns on data protection, skill sets, infrastructure and delay in approvals are among the many reasons given by sector experts for the decline. l If a trial is approved in the US within a month, it takes six to eight weeks for the apex drug regulator, Drug Controller General of India, to respond. Normally 12 -16 weeks are needed to get approval for a trial. l Not only the trial sites: quality and infrastructure of CROs are another area of concern. Of the 120 -plus CROs, only about 20 comply with the global benchmark ICH- GCP. l DCGI had, a few days earlier, come out with a comprehensive clinical trial inspection programme, with specific guidelines and checklists to make trial regulations more stringent and uniform. At present, trials are based on guidelines brought out by the Indian Council of Medical Research and the office of DCGI. India had amended Schedule Y of the Drugs and Cosmetics Act in 2005 to create a conducive environment for doing trials in India, but specific laws are yet to be in place to effectively regulate trials in the country.

SUMMARY l Do only those trials that are necessary l Have a portfolio of short and long term projects l Allocate time for each trial l Plan your act- Act your plan l Reinforce enthusiasm in your team l Reinforce competition among investigators by sending newsletters or holding investigator meets.

Winning in resource limited settings? AR Rahman’s Mantra s a e id / e r co s l a n i g i Or Passion/ Commitment Struggle/ Hard work Sel f im pro vem ent win t ’ n o d s r e uitt Q / e c n e i t Pa

So that every prospective idea does not become a retrospective study

Thank You!
 Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Difference between inspection and audit
Difference between inspection and audit Hurricane myths and legends
Hurricane myths and legends Nida clinical trials network
Nida clinical trials network Site initiation visit
Site initiation visit Clinicaltrails.gov api
Clinicaltrails.gov api Role of statistician in clinical trials
Role of statistician in clinical trials Mrc clinical trials unit
Mrc clinical trials unit Randomization
Randomization Mpn clinical trials
Mpn clinical trials Clinicaltrials gov prs
Clinicaltrials gov prs Clinical trials
Clinical trials Clinical trials quality by design
Clinical trials quality by design Professor claire harrison
Professor claire harrison Dhl atyrau
Dhl atyrau Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Ohsu clinical trials office
Ohsu clinical trials office Prs clinical trial
Prs clinical trial Clinical trials.gov login
Clinical trials.gov login Clinical trial iwr
Clinical trial iwr York clinical trials unit
York clinical trials unit Andreas carlsson bye bye bye
Andreas carlsson bye bye bye Salem witch trials facts
Salem witch trials facts Why do people tell myths
Why do people tell myths Myths and legends quiz questions and answers
Myths and legends quiz questions and answers Dont ask why why why
Dont ask why why why Tulpar
Tulpar The flower myths narcissus hyacinth adonis
The flower myths narcissus hyacinth adonis Polish legends and myths
Polish legends and myths Legends and myths difference
Legends and myths difference Myth legend fable
Myth legend fable Myth legend fable
Myth legend fable Myths and legends of king arthur
Myths and legends of king arthur Greece
Greece Elements of science fiction and myths legends folktales
Elements of science fiction and myths legends folktales Examples of fables
Examples of fables Irish myths and legends lesson plans
Irish myths and legends lesson plans Equation for drag
Equation for drag Myths and legends jeopardy
Myths and legends jeopardy Sipapuni
Sipapuni What are legends?
What are legends? Myth and fallacies about non-communicable diseases
Myth and fallacies about non-communicable diseases Myths and legends robin hood
Myths and legends robin hood Heroes myths and legends
Heroes myths and legends Multiplication facts and division facts
Multiplication facts and division facts Do we our life done
Do we our life done Design and analysis of cross over trials
Design and analysis of cross over trials Creation myth definition
Creation myth definition What is management myths in software engineering
What is management myths in software engineering Myths about pirates
Myths about pirates Myths about comets
Myths about comets 5 myths about attic ventilation
5 myths about attic ventilation Types of myths
Types of myths Chapter 10:3 psychosocial changes of aging
Chapter 10:3 psychosocial changes of aging Dogon creation myth
Dogon creation myth Myths about business ethics
Myths about business ethics Mythological allusions examples
Mythological allusions examples Generic viagra myths
Generic viagra myths Ethics and business is relative
Ethics and business is relative Bulgarian myths
Bulgarian myths Where does this come from
Where does this come from In the beginning there was chaos odyssey
In the beginning there was chaos odyssey If a software production gets behind schedule
If a software production gets behind schedule Practitioner myths in software engineering
Practitioner myths in software engineering Genre is a french word meaning
Genre is a french word meaning Practitioner myths in software engineering
Practitioner myths in software engineering Greek mythology essay
Greek mythology essay Famous myths
Famous myths Myths about termites
Myths about termites What is myth
What is myth