Clinical Manifestations of Equine Motor Neuron Disease in



























- Slides: 51

Clinical Manifestations of Equine Motor Neuron Disease in Two Midwest Horses Rosemary Bayless Kansas State University College of Veterinary Medicine Class of 2014 Mentor: Elizabeth Davis, DVM, Ph. D, DACVIM Residents: Karie Vander Werf, DVM, MS and Cameon Ohmes, DVM Kansas State University Veterinary Medical Teaching Hospital Manhattan, Kansas

Case 1 Signalment 6 year old sorrel and white Paint mare Lives in northeast Kansas; used in riding lessons

Case 1 Background Environment: Previously kept in a dry lot; recently moved to a stall Diet: Alfalfa hay, equine pelleted concentrate (described by owner as similar to Purina Strategy®) Vaccination: EEE, WNV, rabies within previous 8 months Deworming: Ivermectin within past month Dentistry: No dental care in past two years

Case 1 History • Recurrent colic; 5 -6 episodes in previous 4 months • Prior episodes resolved with IM administration of flunixin meglumine • Marked weight loss in previous 4 months despite good appetite. • Referring veterinarian treated for suspected colic 4 days prior to presentation. • Profuse sweating and recumbency • Vital parameters and rectal palpation were within normal limits • Administration of flunixin meglumine and mineral oil did not result in significant improvement • Presented to KSU VMTH on November 16, 2010 for chronic colic and weight loss.

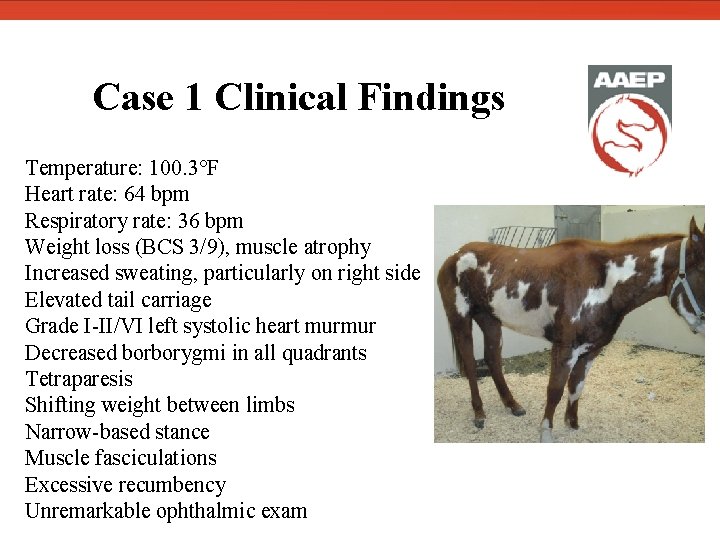
Case 1 Clinical Findings Temperature: 100. 3°F Heart rate: 64 bpm Respiratory rate: 36 bpm Weight loss (BCS 3/9), muscle atrophy Increased sweating, particularly on right side Elevated tail carriage Grade I-II/VI left systolic heart murmur Decreased borborygmi in all quadrants Tetraparesis Shifting weight between limbs Narrow-based stance Muscle fasciculations Excessive recumbency Unremarkable ophthalmic exam

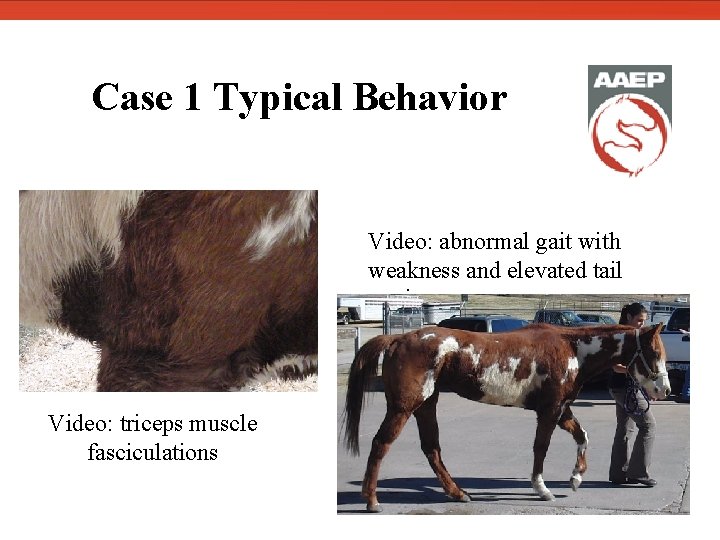
Case 1 Typical Behavior Video: triceps muscle fasciculations Video: abnormal gait with weakness and elevated tail carriage

Case 1 Preliminary Diagnostics Complete blood count Mild leukopenia 5. 5 K/µL (rr: 6 -14 K/µL) Mild lymphopenia 1. 3 K/µL (rr: 1. 5 -7. 7 K/µL) All other values within reference intervals Serum chemistry Mild elevation in BUN 25 mg/d. L (rr: 9 -22 mg/d. L) Increased aspartate transaminase (AST) 1693 U/L (rr: 255 -543 U/L) Increased creatine kinase (CK) 1488 U/L (rr: 192 -565 U/L) All other values within reference intervals Interpretation: Elevations in muscle enzymes in Case 1 are consistent with chronic (AST) and ongoing (CK) muscle damage.

Case 2 Signalment 11 year old dark brown Morgan gelding Lives in central Nebraska; used in competitive trail riding

Case 2 Background Environment: Turned out on pasture during the day, stalled at night No recent changes in environment Nine other apparently healthy horses on property Diet: Grass pasture, 80% grass/20% alfalfa hay, sweet feed/oats mixture, vitamin E supplement Vaccination: EEE, WEE, tetanus, WNV 8 months previously EEE, WEE, tetanus, influenza, EHV 2 months previously Deworming: Ivermectin approximately 2 months previously Dentistry: Teeth were floated in past month No current medications

Case 2 History • Presented to referring veterinarian approximately 2 months previously for episodes of weakness and excessive recumbency. • Interpreted by owner as signs of colic • Owner noted elevation of the tail to the side during presumed colic episodes • Appetite and fecal production remained normal • Recent health history included two incidents of esophageal obstruction.

Case 2 History: Referring Veterinarian Diagnostics • Complete blood count: all values within normal limits • Serum chemistry: all values within normal limits • EPM ELISA serum titer (SAG 1): <1: 16 (positive: ≥ 1: 32) • Interpreted as negative but treated for EPM with ponazuril (Marquis®) 5 mg/kg PO SID for one month

Case 2 History: Referring Veterinarian Therapeutics • Oxytetracycline: 6. 6 mg/kg IV SID for 5 days • Sulfadimethoxine: 30 mg/kg PO BID for one week • DMSO: 1 g/kg via nasogastric tube, once • Dexamethasone: 20 mg IV tapered to 10 mg IM for 3 doses • Ponazuril: 5 mg/kg PO SID for 28 days.

Case 2 History: Referring Veterinarian Therapeutics • Owner reported that clinical signs improved with ponazuril treatment but relapsed when ponazuril was discontinued. • Owner described increased ataxia and depression after tapering the dexamethasone dose, consistent with history of a similar episode treated by another veterinarian. • Presented to KSU VMTH on November 17, 2010 for suspected neurologic disease and chronic colic of 2 months duration.

Case 2 Clinical Findings Temperature: 99. 5°F Heart rate: 60 bpm Respiratory rate: 18 bpm Neurologic exam Slight loss of muscle mass No evidence of ataxia Increased sweating Normal mentation Shifting weight between limbs No cranial nerves deficits Muscle fasciculations Elevated tail carriage Narrow-based stance Tetraparesis Difficulty lying down Unremarkable ophthalmic exam

Case 2 Typical Behavior Video: prolonged attempt to lie down characterized by muscle tremors and stance with feet far underneath body

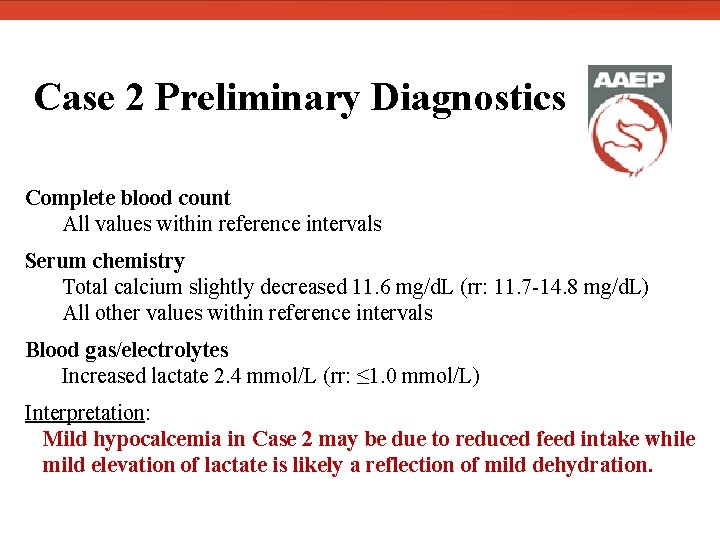
Case 2 Preliminary Diagnostics Complete blood count All values within reference intervals Serum chemistry Total calcium slightly decreased 11. 6 mg/d. L (rr: 11. 7 -14. 8 mg/d. L) All other values within reference intervals Blood gas/electrolytes Increased lactate 2. 4 mmol/L (rr: ≤ 1. 0 mmol/L) Interpretation: Mild hypocalcemia in Case 2 may be due to reduced feed intake while mild elevation of lactate is likely a reflection of mild dehydration.

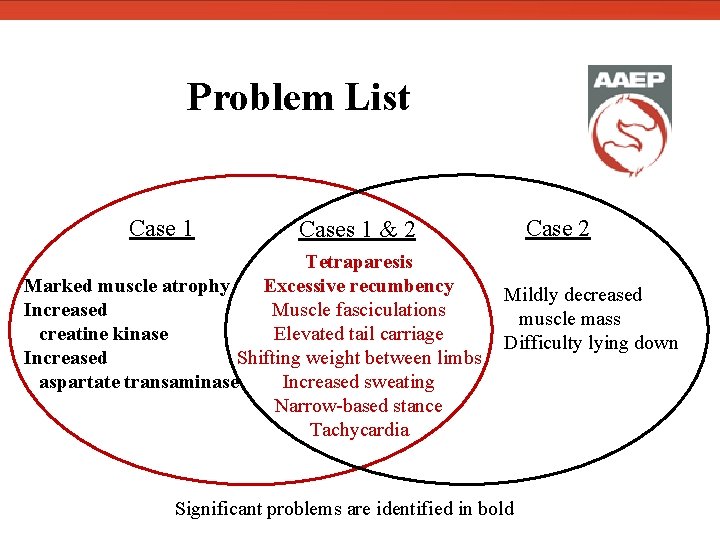
Problem List Case 1 Case 2 Cases 1 & 2 Tetraparesis Marked muscle atrophy Excessive recumbency Muscle fasciculations Increased creatine kinase Elevated tail carriage Increased Shifting weight between limbs aspartate transaminase Increased sweating Narrow-based stance Tachycardia Mildly decreased muscle mass Difficulty lying down Significant problems are identified in bold

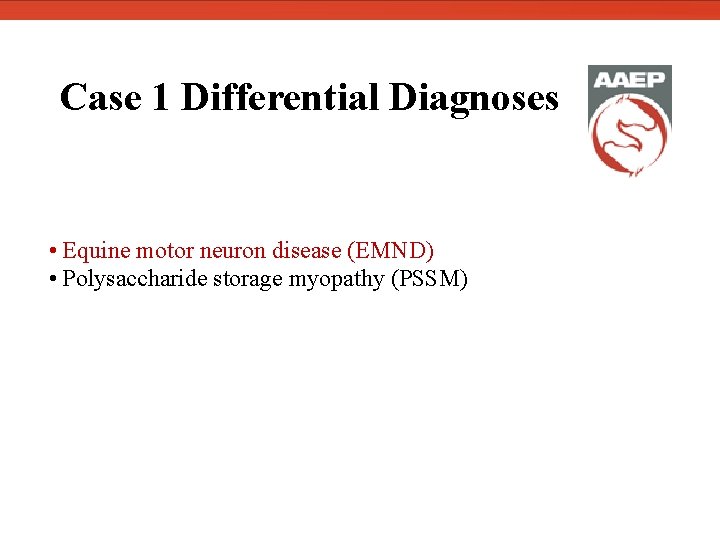
Case 1 Differential Diagnoses • Equine motor neuron disease (EMND) • Polysaccharide storage myopathy (PSSM)

Case 1 Diagnostic Plan • Qualitative and quantitative fecal exam • Rule out high parasite burden as cause for poor body condition • Measure vitamin E and selenium levels in serum • Check for evidence of nutritional myopathy Biopsy of sacrocaudalis dorsalis medialis muscle was declined by owner due to cost of testing

Case 1 Fecal Examination Qualitative exam No evidence of parasites seen Quantitative exam—Wisconsin technique No evidence of parasites seen Interpretation: A high parasite load does not appear to be the cause of poor body condition in Case 1.

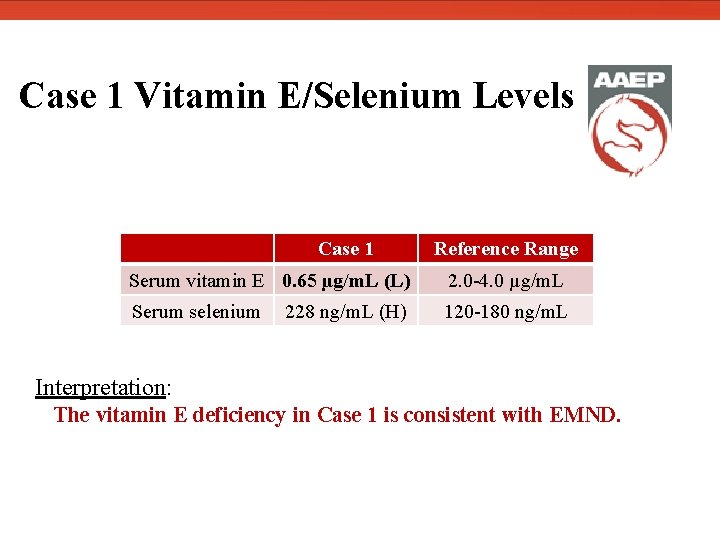
Case 1 Vitamin E/Selenium Levels Case 1 Reference Range Serum vitamin E 0. 65 µg/m. L (L) 2. 0 -4. 0 µg/m. L Serum selenium 120 -180 ng/m. L 228 ng/m. L (H) Interpretation: The vitamin E deficiency in Case 1 is consistent with EMND.

Case 2 Differential Diagnoses • Equine motor neuron disease (EMND) • Polysaccharide storage myopathy (PSSM) • Equine protozoal myeloencephalitis (EPM)

Case 2 Diagnostic Plan • Measure serum vitamin E and selenium • Check for evidence of nutritional myopathy • Muscle biopsy of sacrocaudalis dorsalis medialis muscle • Submit for histopathological assessment (Dr. S. Valberg, U. Minnesota) • Examine for antemortem diagnosis of EMND • Obtain cerebrospinal fluid • Cytology: KSU Clinical Pathology • Sarcocystis neurona Indirect Fluorescent Antibody Test (IFAT, UC Davis) • Rule out EPM (confirm diagnostics performed by referring veterinarian)

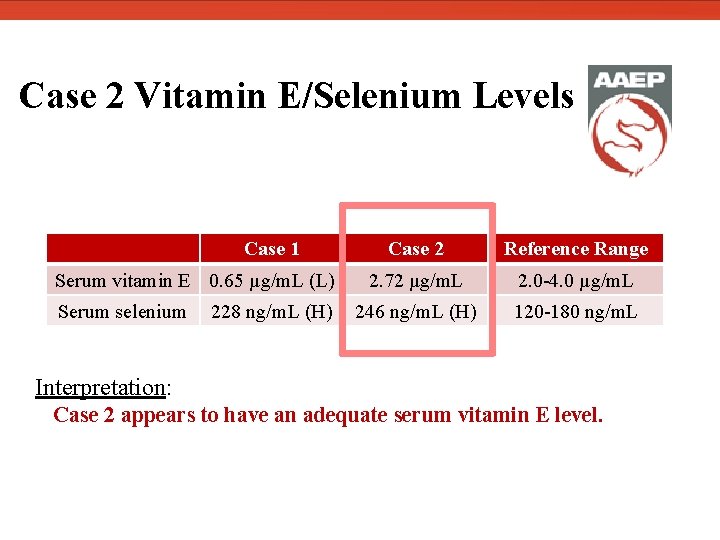
Case 2 Vitamin E/Selenium Levels Case 1 Serum vitamin E 0. 65 µg/m. L (L) Serum selenium 228 ng/m. L (H) Case 2 Reference Range 2. 72 µg/m. L 2. 0 -4. 0 µg/m. L 246 ng/m. L (H) 120 -180 ng/m. L Interpretation: Case 2 appears to have an adequate serum vitamin E level.

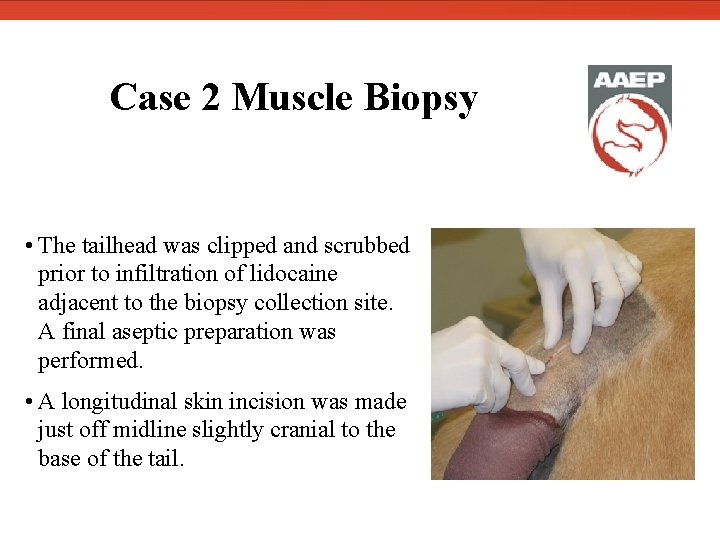
Case 2 Muscle Biopsy • The tailhead was clipped and scrubbed prior to infiltration of lidocaine adjacent to the biopsy collection site. A final aseptic preparation was performed. • A longitudinal skin incision was made just off midline slightly cranial to the base of the tail.

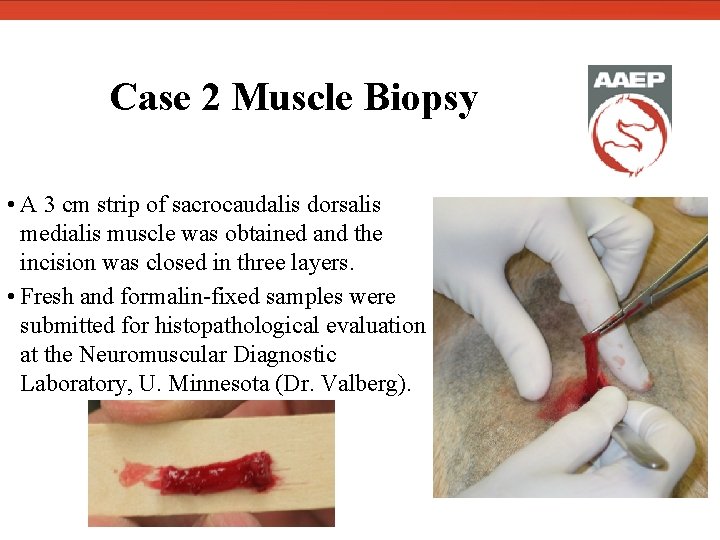
Case 2 Muscle Biopsy • A 3 cm strip of sacrocaudalis dorsalis medialis muscle was obtained and the incision was closed in three layers. • Fresh and formalin-fixed samples were submitted for histopathological evaluation at the Neuromuscular Diagnostic Laboratory, U. Minnesota (Dr. Valberg).

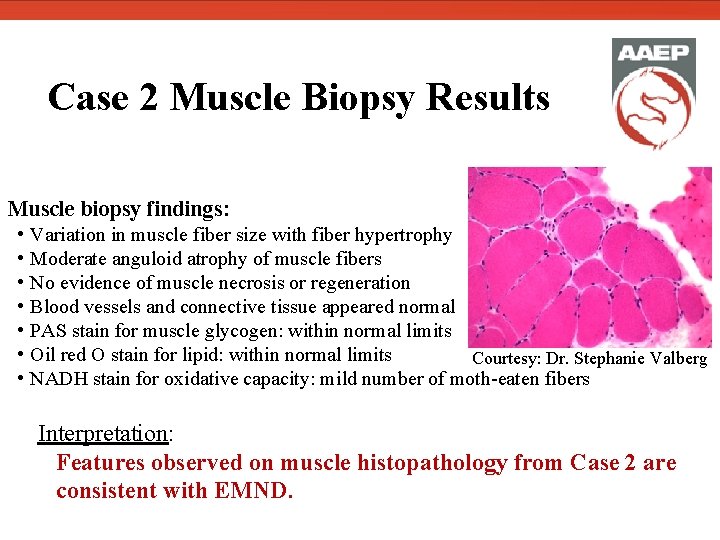
Case 2 Muscle Biopsy Results Muscle biopsy findings: • Variation in muscle fiber size with fiber hypertrophy • Moderate anguloid atrophy of muscle fibers • No evidence of muscle necrosis or regeneration • Blood vessels and connective tissue appeared normal • PAS stain for muscle glycogen: within normal limits • Oil red O stain for lipid: within normal limits Courtesy: Dr. Stephanie Valberg • NADH stain for oxidative capacity: mild number of moth-eaten fibers Interpretation: Features observed on muscle histopathology from Case 2 are consistent with EMND.

Case 2 Cerebrospinal Fluid Collection • The skin on the dorsal midline caudal to the poll was clipped and aseptically prepared. • With the horse in lateral recumbency under general anesthesia, a spinal needle was directed into the atlanto-occipital space, and a CSF sample was aspirated for analysis and Sarcocystis neurona indirect fluorescent antibody test (IFAT).

Case 2 Cerebrospinal Fluid Results CSF analysis: Gross appearance: colorless, clear Low cellularity (1 NCC/µL) 75% small mononuclear cells, 20% large mononuclear cells, 5% non-degenerate neutrophils Protein: 44 mg/d. L No organisms or malignant cells observed CSF S. neurona IFAT: negative Interpretation: EPM caused by Sarcocystis neurona infection is highly unlikely in Case 2.

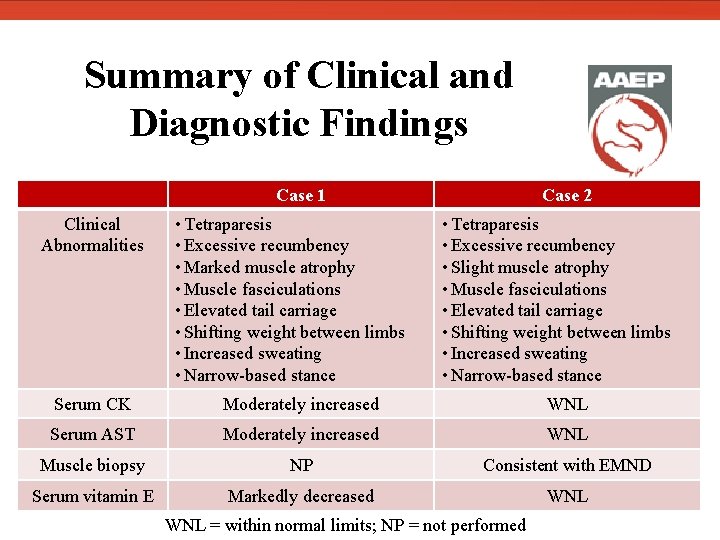
Summary of Clinical and Diagnostic Findings Clinical Abnormalities Case 1 • Tetraparesis • Excessive recumbency • Marked muscle atrophy • Muscle fasciculations • Elevated tail carriage • Shifting weight between limbs • Increased sweating • Narrow-based stance Case 2 • Tetraparesis • Excessive recumbency • Slight muscle atrophy • Muscle fasciculations • Elevated tail carriage • Shifting weight between limbs • Increased sweating • Narrow-based stance Serum CK Moderately increased WNL Serum AST Moderately increased WNL Muscle biopsy NP Consistent with EMND Serum vitamin E Markedly decreased WNL = within normal limits; NP = not performed

Diagnosis Equine Motor Neuron Disease • First reported in the United States in 1990. • Acquired lower motor neuron disorder that results in denervation skeletal muscle atrophy and generalized weakness. • Both type I and type II muscle fibers may be affected, but histologic changes are observed most commonly in type I fibers, suggesting an oxidative damage pathophysiology. • Etiology is unknown, but proposed factors include vitamin E deficiency, typically from lack of access to fresh grass or high-quality hay, and increased levels of copper and iron that may catalyze radical formation.

Equine Motor Neuron Disease Treatment • There is currently no specific treatment for EMND. • The possible relationship between low serum vitamin E levels and development of EMND suggests that vitamin E supplementation may be beneficial in stabilizing or improving clinical signs. • A therapeutic dose of 5000 -7000 IU/day has been suggested. Prognosis • Approximately 40% of horses with EMND respond to diet/management changes and vitamin E supplementation with improvement in clinical signs within 4 -6 weeks of treatment initiation. • Approximately 40% of horses with EMND show progression of clinical signs and are euthanized within 4 weeks of the onset of clinical disease. • The remaining 20% of affected horses display persistent muscle atrophy.

Equine Motor Neuron Disease Prevention • Horses with minimal or no access to grass or green hay should be supplemented with 1000 -2000 IU vitamin E (d-α-tocopherol) PO daily to reduce risk of EMND. • Though EMND typically occurs sporadically, testing other horses on the premises for vitamin E levels and supplementing with vitamin E as necessary is recommended due to the suggested role of diet and potential for subclinical disease. • Natural vitamin E (d-α-tocopherol) is preferred over synthetic vitamin E (dl-αtocopherol) for prophylactic and therapeutic purposes because the natural form reaches higher serum and CSF concentrations in horses after oral administration.

Typical EMND Clinical Signs • Weight loss due to muscle atrophy 1, 2 • Generalized weakness 1, 2 • Low head carriage • Feet positioned under body 1, 2 • Shifting of weight between limbs • Excessive recumbency 1, 2 • Excessive sweating 1, 2 • Muscle fasciculations 1, 2 • Elevated tail carriage 1, 2 1 present in Case 1 2 present in Case 2

Typical EMND Pathology Findings Clinical • Mild-moderate increase in serum creatine kinase 1 • Mild-moderate increase in serum aspartate transaminase 1 1 • Plasma vitamin E levels are frequently low (<1 g/m. L) • Plasma selenium levels are usually within normal limits 1, 2 • CSF protein levels are elevated in some cases 1 present in Case 1 2 present in Case 2

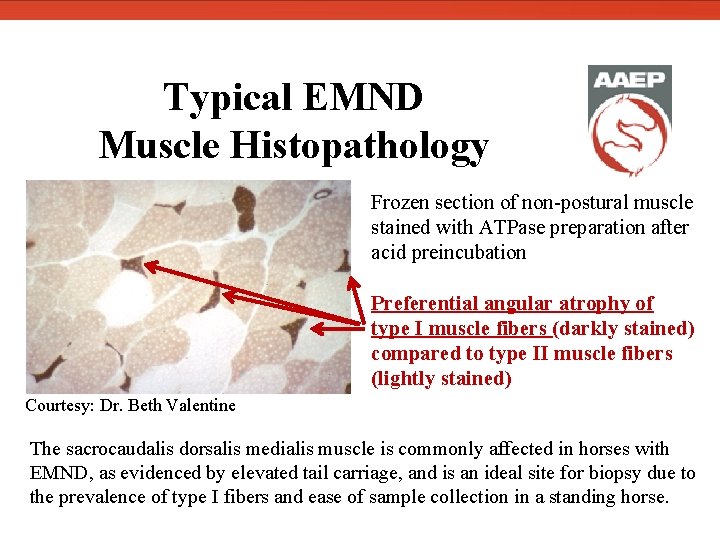
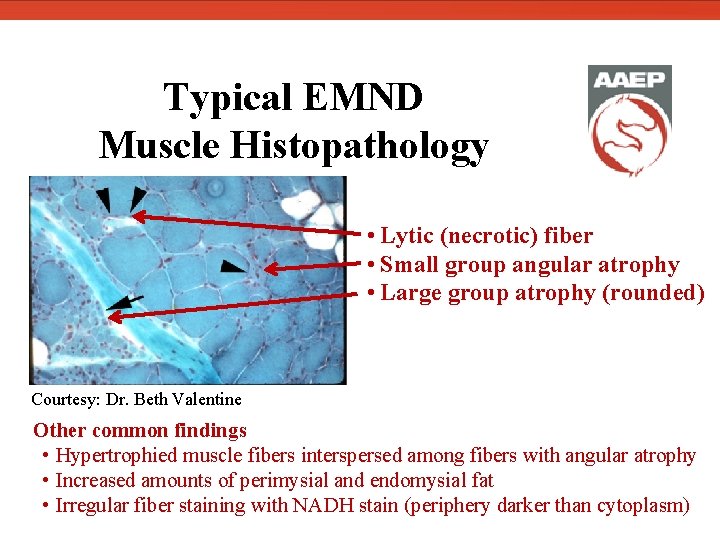
Typical EMND Muscle Histopathology Frozen section of non-postural muscle stained with ATPase preparation after acid preincubation Preferential angular atrophy of type I muscle fibers (darkly stained) compared to type II muscle fibers (lightly stained) Courtesy: Dr. Beth Valentine The sacrocaudalis dorsalis medialis muscle is commonly affected in horses with EMND, as evidenced by elevated tail carriage, and is an ideal site for biopsy due to the prevalence of type I fibers and ease of sample collection in a standing horse.

Typical EMND Muscle Histopathology • Lytic (necrotic) fiber • Small group angular atrophy • Large group atrophy (rounded) Courtesy: Dr. Beth Valentine Other common findings • Hypertrophied muscle fibers interspersed among fibers with angular atrophy • Increased amounts of perimysial and endomysial fat • Irregular fiber staining with NADH stain (periphery darker than cytoplasm)

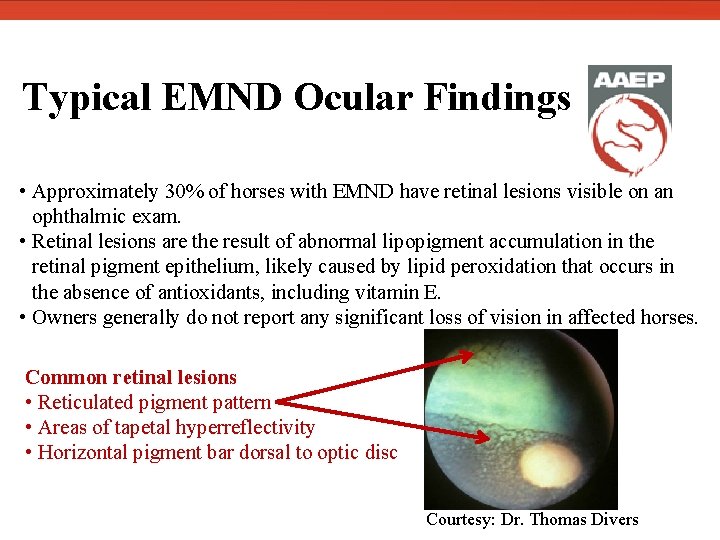
Typical EMND Ocular Findings • Approximately 30% of horses with EMND have retinal lesions visible on an ophthalmic exam. • Retinal lesions are the result of abnormal lipopigment accumulation in the retinal pigment epithelium, likely caused by lipid peroxidation that occurs in the absence of antioxidants, including vitamin E. • Owners generally do not report any significant loss of vision in affected horses. Common retinal lesions • Reticulated pigment pattern • Areas of tapetal hyperreflectivity • Horizontal pigment bar dorsal to optic disc Courtesy: Dr. Thomas Divers

Ancillary Diagnostics Antemortem Accessory nerve biopsy • Biopsy of the ventral branch of the spinal accessory nerve near its entrance into the sternocephalicus muscle under general anesthesia or with sedation and local anesthesia in a standing horse. • Findings of myelinated axonal degeneration and proliferation of Schwann cells (Büngner’s bands) are consistent with EMND. Electromyography • May be useful in assessing electrical activity of commonly affected muscle groups and the associated lower motor neurons. • Can be performed on the sacrocaudalis dorsalis medialis muscle in standing horse with sedation and caudal epidural anesthesia. • Spontaneous fibrillation potentials and series of positive sharp waves suggest a lower motor neuron disorder but are not specific for EMND.

Ancillary Diagnostics Postmortem CNS histopathology findings Brain • Neuronal degeneration in the facial nucleus and motor nucleus of the trigeminal nerve. • Swollen, pale eosinophilic neurons with central nuclei and cytoplasmic lipofuscin. Spinal cord, peripheral nerves, and cranial nerves • Degeneration of neurons in the ventral horn with morphology similar to affected brain neurons. • Increased depletion of ventral horn neurons and presence of glial scars with chronicity of clinical signs beyond 2 -3 months. • Axonal degeneration in ventral roots, spinal nerves, and select cranial nerves characterized by demyelination, Büngner’s bands, and the presence of macrophages and cellular debris.

Case 1 Therapeutic Plan In-hospital treatments • Vitamin E-selenium injection: 1 ml/100 lb IM once (1. 5 IU/kg vitamin E and 0. 055 mg/kg selenium) Administered on presentation to KSU VMTH Antioxidant to reduce oxidative damage • Vitamin E (d-α-tocopherol): 10, 000 IU PO SID Initiated treatment on presentation to KSU VMTH Antioxidant to reduce oxidative damage • Flunixin meglumine: 1. 1 mg/kg IV q 12 h for 2 doses Administered on presentation to KSU VMTH Analgesia to treat apparent pain • Phenylbutazone: 2. 2 mg/kg PO once Administered the morning of discharge Trial to assess whether analgesia improved comfort level

Case 1 Therapeutic Plan Long-term management recommendations Medications • Vitamin E (d-α-tocopherol): 10, 000 IU PO SID Continue for 60 days, at which time a reassessment is recommended • Phenylbutazone: 2. 2 mg/kg PO SID for 4 days Assess need for and efficacy of analgesics after 4 days If horse requires long-term NSAID treatment, consider use of omeprazole to reduce risk of gastric ulcers Diet • Free choice feeding of high quality grass hay and supplementation with alfalfa hay and pelleted concentrate feed (Purina Strategy®) to provide additional calories.

Case 1 Outcome 6 months post-treatment Owner reported improvement in clinical signs Successfully ridden at a walk and trot Photographs (6 months post-treatment) showing improved muscle mass and body condition Approximately 15 months post-treatment Horse changed ownership and was lost to follow-up

Case 2 Therapeutic Plan In-hospital treatments • Vitamin E (d-α-tocopherol): 10, 000 IU PO SID Initiated treatment on presentation to KSU VMTH Antioxidant to reduce oxidative damage • Phenylbutazone: 2. 2 mg/kg PO BID Initiated treatment on presentation to KSU VMTH Analgesic trial to assess role of pain in clinical signs • Trimethoprim sulfamethoxazole: 15 mg/kg PO BID Initiated after muscle biopsy was performed Prophylactic use to reduce risk of surgical site infection

Case 2 Therapeutic Plan Long-term management recommendations Medications • Vitamin E (d-α-tocopherol): 10, 000 IU PO SID Clinical signs appear to be vitamin E responsive despite apparent lack of vitamin E deficiency May be able to slowly taper dose down to 1000 IU PO SID for maintenance Diet • Current diet appears to be adequate; continue feeding normal diet.

Case 2 Outcome 19 months post-treatment Returned to use as a pleasure/recreational riding horse 29 months post-treatment Continues to be ridden recreationally No longer being used in trail riding competitions Maintained on 10, 000 IU vitamin E PO SID Owner is satisfied with recovery Photograph (10 months post-treatment) demonstrating return to normal posture and increased muscle mass Courtesy: Karen Nelson

Further Reading Bedford HE, Valberg SJ, Firshman AM, Lucio M, Boyce MK, Trumble TN. Histopathologic findings in the sacrocaudalis dorsalis medialis muscle of horses with vitamin E-responsive muscle atrophy and weakness. J Am Vet Med Assoc. 2013; 242: 1127 -1137. Divers TJ, Mohammed HO, Cummings JF. Equine motor neuron disease. Vet Clin N Am-Equine. 1997; 13: 97 -105 Divers TJ, Mohammed HO, Cummings JF, Valentine BA, de Lahunta A, Jackson CA, Summers BA. Equine motor neuron disease: findings in 28 horses and proposal of a pathophysiological mechanism for the disease. Equine Vet J. 1994; 26: 409 -415 Valentine, BA, de Lahunta A, George C, Summers BA, Cummings JF, Divers TJ, Mohammed HO. Acquired Equine Motor Neuron Disease. Vet Pathol. 1994; 31: 130 -138

Resources Cummings JF, de Lahunta A, George C, Fuhrer L, Valentine BA, Cooper BJ, Summers BA, Huxtable CR, Mohammed HO. Equine Motor Neuron Disease: A preliminary report. Cornell Vet. 1990; 80: 357 -379 Divers TJ, Mohammed HO, Hintz HF, de Lahunta A. Equine Motor Neuron Disease: A review of clinical and experimental studies. P Annu Conv Am Equin. 2003. Divers TJ, de Lahunta A, Hintz HF, Riis RC, Mohammed HO. Equine Motor Neuron Disease—Update. P Am Coll Vet Int Med. 2002. Jackson CA, de Lahunta A, Cummings JF, Divers TJ, Mohammed HO, Valentine BA, Hackett RP. Spinal accessory nerve biopsy as an ante mortem diagnostic test for equine motor neuron disease. Equine Vet J. 1996; 28: 215 -219. Kyles, KWJ, Mc. Gorum, BC, Fintl C, Hahn CN, Mauchline S, Mayhew IG. Electromyography under caudal epidural anesthesia as an aid to the diagnosis of equine motor neuron disease. Vet Rec. 2001; 148: 536 -538.

Resources Pusterla N, Puschner B, Steidl S, Collier J, Kane E, Stuart RL. alpha-Tocopherol concentrations in equine serum and cerebrospinal fluid after vitamin E supplementation. Vet Rec. 2010; 166: 366 -368. Riis RC, Jackson C, Rebhun W, Katz ML, Loew E, Summers B, Cummings J, de Lahunta A, Divers T, Mohammed H. Ocular manifestations of equine motor neuron disease. Equine Vet J. 1999; 31: 99 -110. Valentine BA, Divers TJ, Murphy DJ, Todhunter PG. Muscle biopsy diagnosis of equine motor neuron disease and equine polysaccharide storage myopathy. Equine Vet Educ. 1998; 10: 42 -50. Valentine, BA, de Lahunta A, George C, Summers BA, Cummings JF, Divers TJ, Mohammed HO. Acquired Equine Motor Neuron Disease. Vet Pathol. 1994; 31: 130 -138.

Resources Wijnberg ID. Equine motor neuron disease. Equine Vet Educ. 2006; 18: 126 -129. Other myopathies. http: //www. cvm. umn. edu/umec/lab/other_myop/. Univesrity of Minnesota Equine Center. Last modified on April 30, 2009. Accessed April 20, 2013.

Acknowledgements Special thanks to Dr. Elizabeth Davis for her time and guidance Dr. Karie Vander Werf and Dr. Cameon Ohmes for their assistance with case information Dr. Stephanie Valberg for diagnostic evaluation and photographs of slide from Case 2 muscle biopsy Dr. Beth Valentine for providing muscle histopathology photographs Dr. Thomas Divers for providing ocular pathology photographs Karen Nelson for providing photographs of Case 2 All other images and videos are courtesy of Dr. Elizabeth Davis.
 Equine motor neuron disease
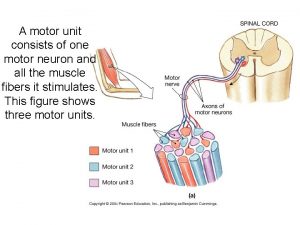
Equine motor neuron disease A motor unit consists of a motor neuron and
A motor unit consists of a motor neuron and Equine disease communication center
Equine disease communication center Lhermitte's sign
Lhermitte's sign Skeletal muscle autonomic nervous system
Skeletal muscle autonomic nervous system Umnl vs lmnl
Umnl vs lmnl Zapojenie alternatora
Zapojenie alternatora Somatic motor neuron
Somatic motor neuron Anal wink reflex
Anal wink reflex Lower
Lower Motor neuron adalah
Motor neuron adalah Neurotransmitters in somatic nervous system
Neurotransmitters in somatic nervous system Site of somatic motor neuron cell bodies
Site of somatic motor neuron cell bodies Upper motor neuron
Upper motor neuron Lower motor neuron
Lower motor neuron Somatic motor neuron
Somatic motor neuron Motor neuron
Motor neuron Somatic motor neuron
Somatic motor neuron Site of somatic motor neuron cell bodies
Site of somatic motor neuron cell bodies Motor neuron adalah
Motor neuron adalah Hypercalcemia nephrogenic di
Hypercalcemia nephrogenic di Boutonniere and swan neck deformity
Boutonniere and swan neck deformity Ocular sign
Ocular sign Chapitre 3 l'énergie et ses manifestations
Chapitre 3 l'énergie et ses manifestations Spirit of jealousy manifestations
Spirit of jealousy manifestations Euthyroid
Euthyroid Communicable disease and non communicable disease
Communicable disease and non communicable disease Parturition
Parturition Charlotte kin test
Charlotte kin test Uspc stall card
Uspc stall card Equine science
Equine science Equine rhinopneumonitis
Equine rhinopneumonitis Equine
Equine Equine encephalitis
Equine encephalitis Lingfield vets
Lingfield vets Electrical formula
Electrical formula Equine color calculator
Equine color calculator Equine
Equine Equine encephalitis
Equine encephalitis Equine definition
Equine definition Equine injury database
Equine injury database Equine large intestine
Equine large intestine Equine science
Equine science Pasture pals equine rescue
Pasture pals equine rescue Clinical observation of motor and postural skills pdf
Clinical observation of motor and postural skills pdf Motor neurone disease symptoms
Motor neurone disease symptoms Principle of operation of synchronous motor
Principle of operation of synchronous motor Ac motor vs dc motor
Ac motor vs dc motor Ee 216
Ee 216 Three phase synchronous generator
Three phase synchronous generator Neuron sterilizer
Neuron sterilizer Relay neuron
Relay neuron