Motor Neuron Diseases n Motor Neuron Diseases n































- Slides: 50

Motor Neuron Diseases

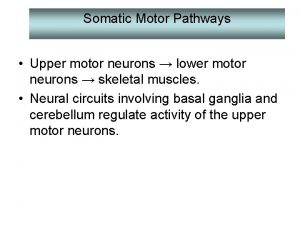
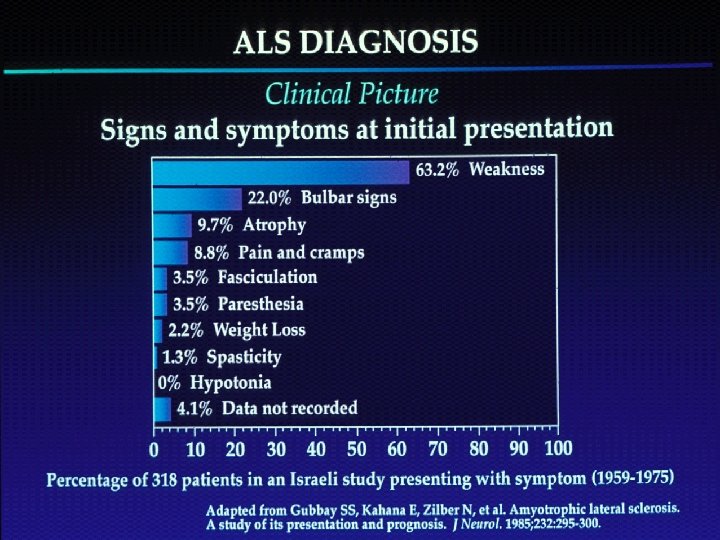
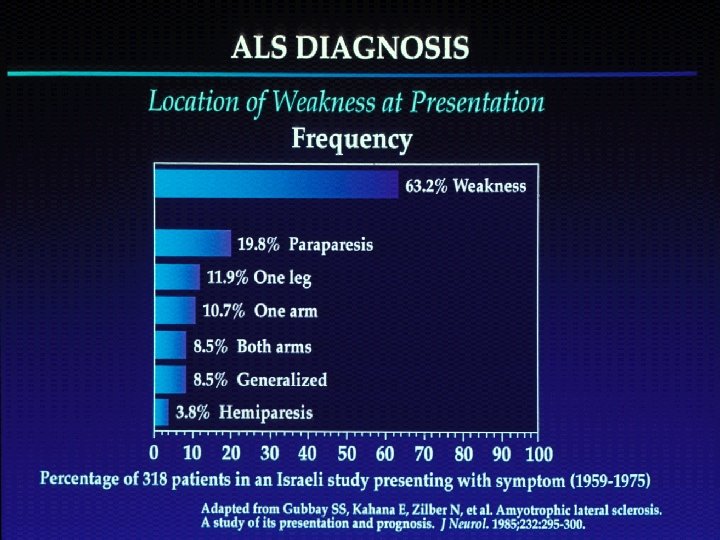
n Motor Neuron Diseases n n n group of diseases which include progressive degeneration and loss of motor neurons with or without similar lesion of the motor nuclei of the brain replacement of lost cells with gliosis “Motor Neuron Disease” = ALS (Charcot’s Disease, Lou Gehrig’s Disease) n n LMN - limbs (PMA), bulbar (progressive bulbar palsy) UMN – limbs (PLS), bulbar (progressive pseudobulbar palsy)

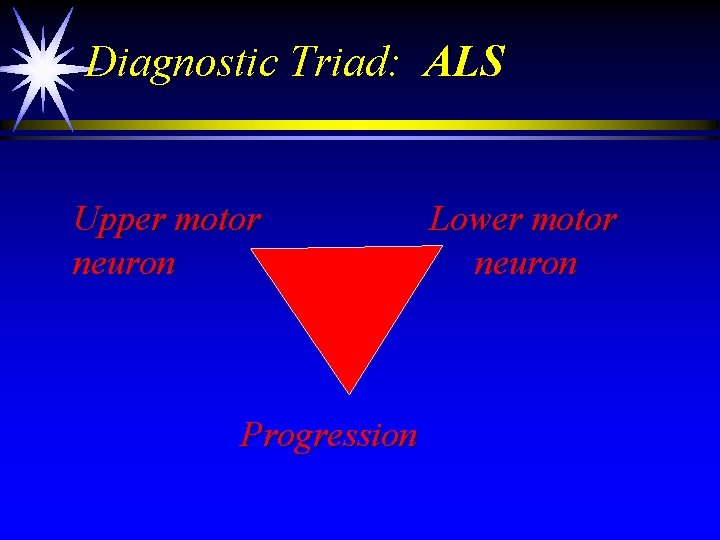
Diagnostic Triad: ALS Upper motor neuron Progression Lower motor neuron

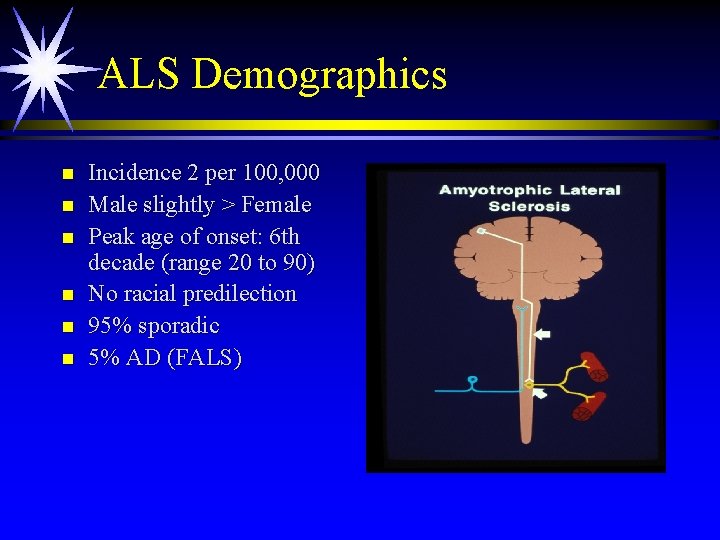
ALS Demographics n n n Incidence 2 per 100, 000 Male slightly > Female Peak age of onset: 6 th decade (range 20 to 90) No racial predilection 95% sporadic 5% AD (FALS)

ALS Diagnosis: Upper Motor Neuron Symptoms n n n Loss of dexterity Slowed movements Loss of muscle strength Stiffness Emotional lability

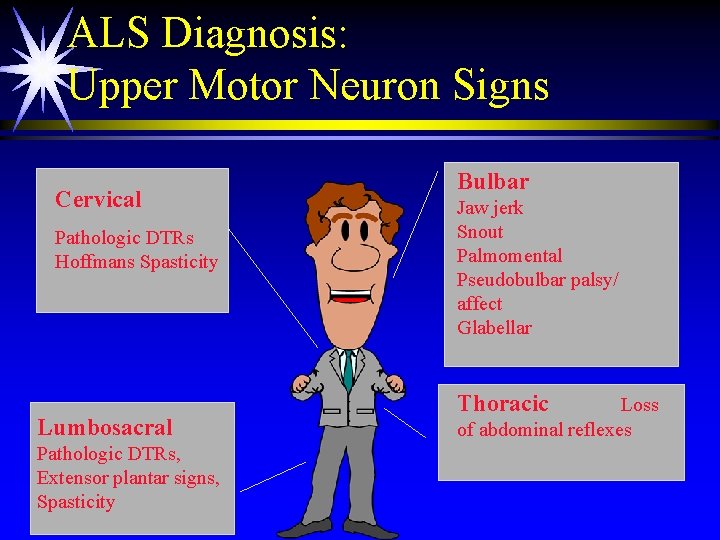
ALS Diagnosis: Upper Motor Neuron Signs Cervical Pathologic DTRs Hoffmans Spasticity Lumbosacral Pathologic DTRs, Extensor plantar signs, Spasticity Bulbar Jaw jerk Snout Palmomental Pseudobulbar palsy/ affect Glabellar Thoracic Loss of abdominal reflexes

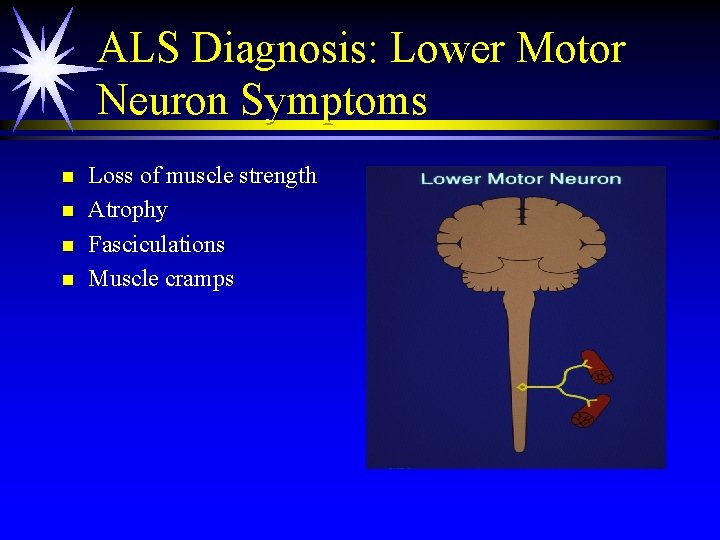
ALS Diagnosis: Lower Motor Neuron Symptoms n n Loss of muscle strength Atrophy Fasciculations Muscle cramps




ALS: Inconsistent Clinical Features n n n n Sensory dysfunction Bladder and bowel sphincter dysfunction Autonomic nervous system dysfunction Visual pathway abnormalities Movement disorders Cognitive abnormalities Bedsores

Pathology n n n Precentral gyrus atrophy Sparing of nucleus of Onuf Neuronal loss of cranial nuclei Degeneration of corticospinal tract Chromatin dissolution (chromatolysis), atrophy, shrinkage, cell loss, gliosis

Pathology n n n Bunina’s bodies – intracytoplasmic, easinophilic dense granular Hirano’s bodies – rod shaped, contain parallel filaments Lewy bodies Neuritic plaques Neurofibrillary tangles

Familial ALS n n n n n AD inheritance, variable penetrance Male = Female Higher incidence of cognitive changes Chorea Younger onset Reported spongiform changes, plaques, tangles 15 year survival One type maps to chromosome 2 20 % are SOD

ALS: Differential Diagnosis n n n Toxins (lead, mercury, ? aluminum) Metabolic (hyperthyroidism, hyperparathyroidism, hypoglycemia) Enzyme deficiency (Hexosaminidase A) Paraneoplastic (lymphoma, small cell lung) Cervical spondylosis

ALS: Differential Diagnosis n n n Immunologic (paraproteinemia) Multi-system degeneration (Creutzfeldt-Jacob, ALS-PDDementia, Spinocerebellar Degeneration) Viral (Post-polio) Bacterial (Lyme disease) Vitamin B 12 deficiency

ALS: Laboratory Studies n n n CK levels are typically normal but may be increased 2 -3 x normal in almost half of patients. * CSF may show mild protein elevation (less than 100 mg/dl). * All other laboratory studies should be normal.

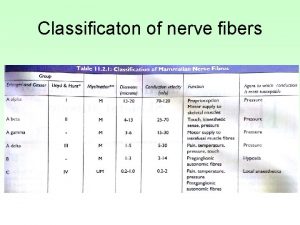
ALS: Electrodiagnostic Testing n n Normal SNAPs CMAPs may be normal or show decreased amplitude* n n NCV rarely < 80% LLN DL rarely > 1. 5 x normal F response rarely > 1. 3 x normal Fibrillations/fasciculations in 2 muscles in 3 extremities (head and paraspinals count as an extremity)*

ALS: Prognosis n n Prognosis n 50% dead in 3 years n 20% live 5 years n 10% live 10 years Worse prognosis if: n Bulbar onset n Simultaneous arm/leg onset n Older age at diagnosis (onset < 40: 8. 2 yr duration, onset 61 -70: 2. 6 yr duration)

Anatomical Variants

Primary Lateral Sclerosis n n n Upper motor neuron syndrome Rare disorder (2% of MND cases) with survival ranging between years - decades Weakness is typically distal, asymmetrical Patients present with slowly progressive spastic paralysis/bulbar palsy EMG should not reveal evidence of active or chronic denervation

Primary Lateral Sclerosis n n Patients may develop clinical LMN abnormalities over the course of their disease. Frequently, patients may have subtle evidence of active or chronic denervation on EMG (rare fibs/decreased recruitment), and/or muscle biopsy at diagnosis

Progressive Muscular Atrophy n n n Lower motor neuron syndrome Literature suggests 8 -10% of patients with MND Much better prognosis than ALS (mean duration 3 -14 years) Bulbar involvement is rare Weakness is typically distal, asymmetrical

Lower Motor Neuron Syndromes n n n Multi-focal motor neuropathy Mononeuropathy multiplex CIDP Polyneuropathy/ radiculopathy Plexopathy Kennedy’s n n n n Hexosaminidase A deficiency Spinal muscular atrophy Post-polio syndrome Polymyositis Inclusion body myositis LMN onset ALS PMA

Progressive Muscular Atrophy n n n The majority of patients presenting with PMA eventually develop clinical UMN signs. Post-mortem examinations of PMA patients frequently show pathologic evidence of UMN degeneration. In some FALS families, the same gene mutation causes the phenotypes of PMA and ALS in different individuals.

Spinobulbar Muscular Atrophy n n Originally reported by Kennedy in 1966 – 11 males in 2 families Age of onset n n Usually begins in 3 rd or 4 th decade Genetics n n Most common form of adult onset SMA X-linked recessive >40 CAG repeats in the androgen receptor gene Number of repeats correlates with age of onset

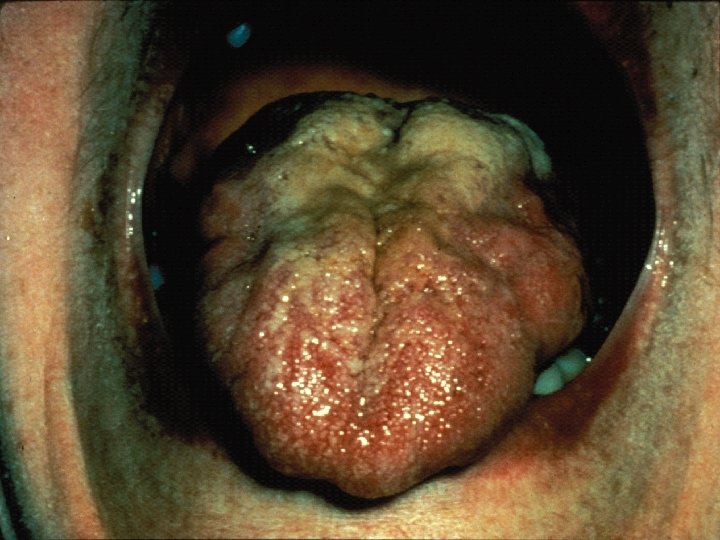
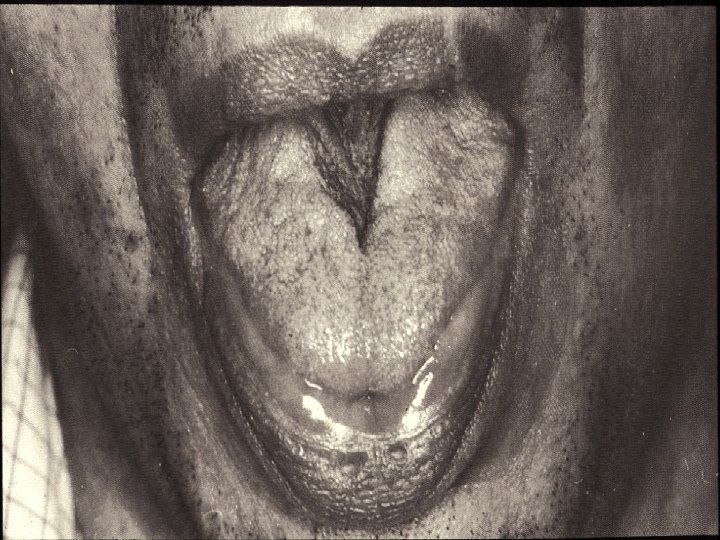
Spinobulbar Muscular Atrophy n n n Lower motor neuron syndrome with limb-girdle distribution of weakness/bulbar palsy* Facial or perioral fasciculations (90%) Tongue atrophy with longitudinal midline furrowing Prominent muscle cramps Generalized fasciculations and atrophy Rarely causes respiratory muscle weakness


Spinobulbar Muscular Atrophy n n Reflexes are decreased or absent Cognitive impairment may occur Hand tremor Sensory exam may be normal or minimally abnormal

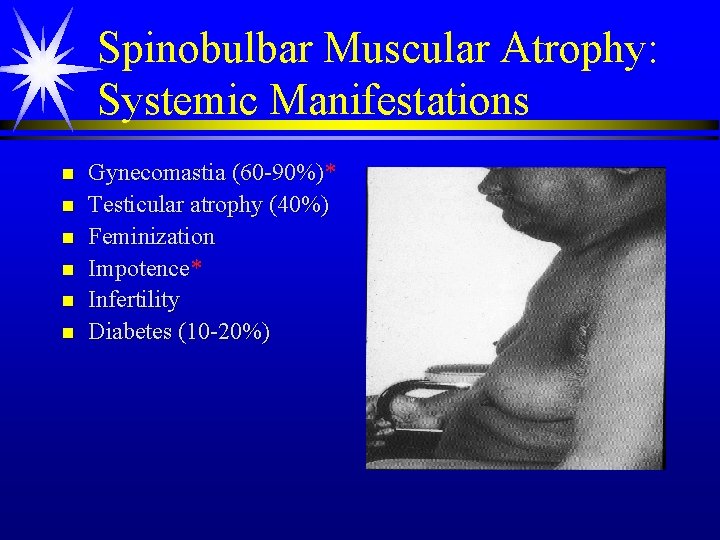
Spinobulbar Muscular Atrophy: Systemic Manifestations n n n Gynecomastia (60 -90%)* Testicular atrophy (40%) Feminization Impotence* Infertility Diabetes (10 -20%)

Spinobulbar Muscular Atrophy: Laboratory Studies n n n Markedly abnormal sensory NCS Sural nerve bx: significant loss of myelinated fibers* Elevated CK (may be 10 x normal) Abnormal sex hormone levels (androgen nl or decreased; estrogen may be elevated, FSH/LH may be mildly elevated)* Increased expansion of CAG repeats in the androgen receptor gene*

Conclusions n n n Although some patients with MND variants evolve into “classic” ALS over time, others continue to show restricted clinical features even late in the course of their disease. In daily clinical practice, precise definitions may not be crucial but recognition of the “variants” is important since each has a different course and prognosis. The “treatment cocktail” should be the same until we learn more about pathogenesis.

Treatment Issues to Consider n n n Symptom management Nutritional management Respiratory management Palliative care Therapies to slow disease progression

Symptoms Associated with Motor Neuron Disease n n n n Dysarthria Dysphagia Sialorrhea Emotional lability Depression Weight Loss Bladder urgency Sleep dysfunction n n n n Constipation Edema Pain Spasticity Cramps Weight loss Fatigue Weakness

Sialorrhea n n Symptoms result from inability to clear oropharyngeal secretions Common pharmacologic treatments: n n n Glycopyrrolate (Robinul) 1 -2 mg q 4 h Amitriptyline (Elavil) 25 -100 mg qhs Hyoscyamine sulfate (Levsin) 1 -2 tsp q 4 h Transdermal scopolamine Suction machines

Management of Emotional Lability n Common pharmacologic treatments: n n n Amitriptyline (Elavil) 25 -150 mg qhs* SSRIs Common nonpharmacologic treatments: n Counseling/support groups

Spasticity n Common pharmacologic treatments*: n n n Baclofen (Lioresal) 10 -40 mg TID-QID Dantrolene sodium (Dantrium) 25 mg qd - QID Tizanidine HCL (Zanaflex) 12 -36 mg TID Diazepam (Valium) 2 -5 mg TID Botox ? Common nonpharmacologic treatments: n n Physical therapy Occupational therapy

Management of Weakness: Assistive Devices n n n n Cane Roll-aided walker AFOs Wheelchair Hoyer lift Cervical collar Hospital bed Ramps n n n Built-up utensils Velcro fasteners Raised toilet seat Shower chair Resting hand splints Grab bars

Management of Dysphagia: Consideration for PEG n Consider n n n n n Significant weight loss Inadequate fluid or caloric intake Difficulty swallowing medications Frequent choking during meals Prolonged meal times FVC < 50% Aspiration pneumonia* Does not prolong survival Malnutrition independent risk factor for worse prognosis

Respiratory Insufficiency: Early Symptoms n n n n Dyspnea on exertion Supine dyspnea Marked fatigue Excessive daytime somnolence Frequent nocturnal arousals Vivid dreams Morning headaches

Management of Respiratory Muscle Weakness n Consider initiation of support when: n n n Symptoms of nocturnal hypoventilation FVC <50% of predicted MIP < -60 cm H 2 O Evidence of significant O 2 desaturations May prolong time to death/trach in longitudinal studies


Pathogenesis n Nucleic acid metabolism – decreased nucleolus staining, reduced m. RNA/r. RNA content n Glutamate – activation NMDA type receptor, Ca influx, free radical production (NO/ROS/protein misfolding by endoplasmic reticulum) n n Increased in CSF and plasma Decreased in brain and spinal cord Decreased active transport of glutamate into synaptosomes Loss of glial glutamate transporters

Pathogenesis n n Loss of muscarinic cholinergic repectors of anterior horns Decreased choline acetyltransferase in spinal cord Decreased glycine and BZD receptors Immunology n n CSF Ig. G ? Elevated in spinal cord C 3, C 4 deposits in spinal cord Reported abnormal glycolipid antibodies in serum Elevated antibodies to voltage gated calcium channels – disturbance of calcium homeostasis (binding proteins parvalbumin/calbindin. D 28)

Pathogenesis n n Viral? – amantadine not effective SOD 1 – loss of function mutation? n n n 20% of FALS Free radical toxicity Chromosome 21 Cytosolic enzyme Transgenic mouse model

Pathogenesis n n n Heat shock proteins – chaperones, influence shape, shuttle proteins Apoptosis – programmed cell death CNS glial cells – retain some reproductive capacity n n Microglial – specialized macrophages Macroglia – astrocytes, oligodendrocytes, ependymal cells, radial glial (neurogenesis/migration)

Treatment n n n Riluzole IGF-1 - growth factor Ceftriaxone – glutamate transporter Co-Q 10 Statins Memantine with riluzole

Treatment n n n Tamoxifen with riluzole Celebrex Thalidomide - TNF alpha Buspirone – neurotrophic effect Stem cell*

Western Pacific ALS n n ALS-PD-Dementia Guam, West New Guinea, Honshu Island Earlier onset UMN precedes LMN features Bulbar weakness more common

Hexosaminidase A Deficiency n n n AR Onset childhood SMA-like picture Mild dementia, neuropathy, ataxia, psychosis Atrophy on imaging (cerebellum)
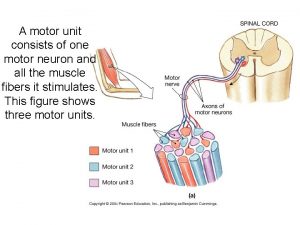
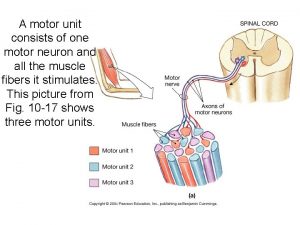
 A motor unit consits of
A motor unit consits of Chart
Chart Lower motor neuron lesion
Lower motor neuron lesion Motor neuron adalah
Motor neuron adalah Somatic nervous system neurotransmitters
Somatic nervous system neurotransmitters Spinal cord diagram
Spinal cord diagram Lower motor neuron
Lower motor neuron Somatic motor neuron
Somatic motor neuron Motor neuron
Motor neuron Somatic motor pathway
Somatic motor pathway Site of somatic motor neuron cell bodies
Site of somatic motor neuron cell bodies Upper motor neuron
Upper motor neuron Lhermitte’s sign
Lhermitte’s sign Equine motor neuron disease
Equine motor neuron disease Autonomic nervous system skeletal muscle
Autonomic nervous system skeletal muscle Umnl vs lmnl
Umnl vs lmnl Princip alternatora
Princip alternatora Motor neuron adalah
Motor neuron adalah Somatic motor neuron
Somatic motor neuron Principle of synchronous motor
Principle of synchronous motor Ac motor vs dc motor
Ac motor vs dc motor Pony motor starting synchronous motor
Pony motor starting synchronous motor Pony motor starting method
Pony motor starting method Adrenergic neuron blockers
Adrenergic neuron blockers Hbs 2 2
Hbs 2 2 Neuron sheath
Neuron sheath Structure of neuron ppt
Structure of neuron ppt What are neuron processes
What are neuron processes All or none law
All or none law Qrs complex duration
Qrs complex duration Neuron fibers
Neuron fibers Neuron diagram
Neuron diagram Fauziah othman
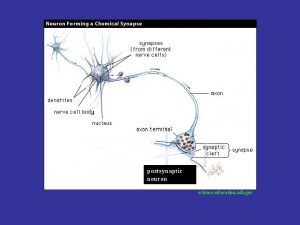
Fauziah othman Sinapsis merupakan sambungan antara dua neuron
Sinapsis merupakan sambungan antara dua neuron Bipolar neuron function
Bipolar neuron function What causes depolarization of a neuron
What causes depolarization of a neuron All or none principle of action potential
All or none principle of action potential Buatlah model neuron mcp untuk menyatakan fungsi logika or
Buatlah model neuron mcp untuk menyatakan fungsi logika or Multipolar neuron
Multipolar neuron Neuron sterilizer
Neuron sterilizer Neuron n
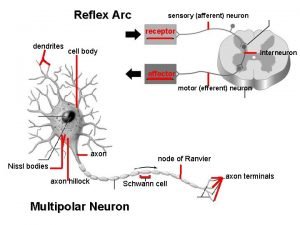
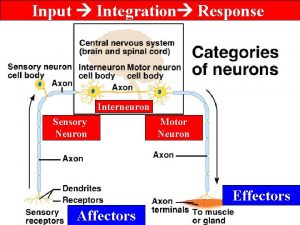
Neuron n What is the correct sequence in a typical reflex arc
What is the correct sequence in a typical reflex arc Relay neuron
Relay neuron Na+ equilibrium potential
Na+ equilibrium potential Neuron def
Neuron def Tonic receptors
Tonic receptors A neuron without terminal buttons would be unable to
A neuron without terminal buttons would be unable to Spinal cord structure
Spinal cord structure Bipolar neuron is found in
Bipolar neuron is found in Postsynaptic neuron
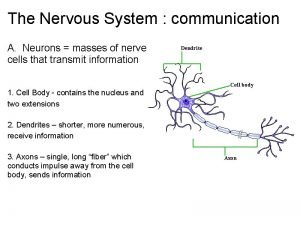
Postsynaptic neuron Primary functions of the nervous system
Primary functions of the nervous system