Clinical Events Committee Quality and Audit Readiness DukeStanford





































- Slides: 52

Clinical Events Committee Quality and Audit Readiness Duke/Stanford CEC Summit Chicago, IL Wednesday, September 26, 2018 Karen A. Hicks, M. D. Medical Officer Division of Cardiovascular and Renal Products (DCARP) Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER)

No disclosures. 2

This presentation reflects the views of the author and should not be construed to represent FDA’s views or policies. 3

Learning Objectives • Describe the role of the clinical events committee (CEC) in a development program • Describe the attributes of a quality CEC • Discuss how to prepare for a regulatory audit • Discuss pitfalls in CEC charters and adjudication • Discuss future challenges in CEC adjudication 4

Learning Objectives • Describe the role of the clinical events committee (CEC) in a development program • Describe the attributes of a quality CEC • Discuss how to prepare for a regulatory audit • Discuss pitfalls in CEC charters and adjudication • Discuss future challenges in CEC adjudication 5

Not all development programs (or clinical trials) use CECs. 6

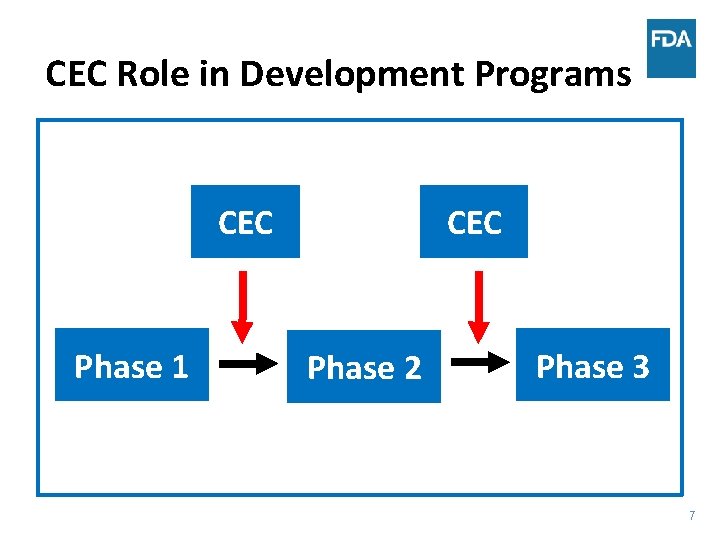
CEC Role in Development Programs CEC Phase 1 CEC Phase 2 Phase 3 7

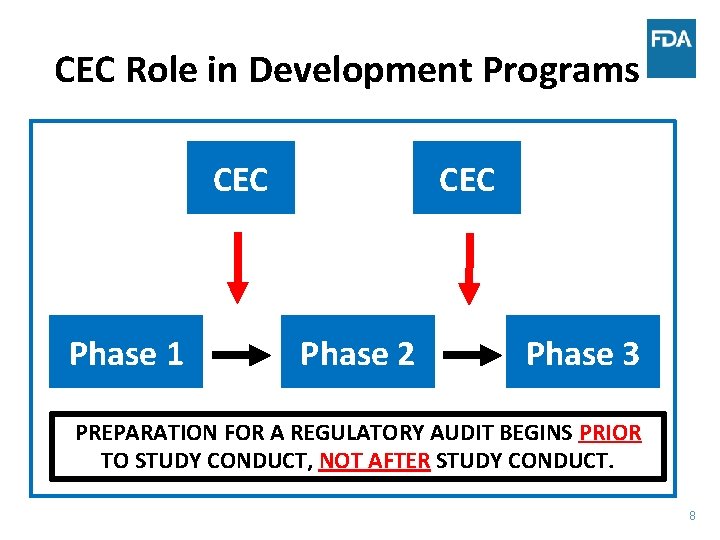
CEC Role in Development Programs CEC Phase 1 CEC Phase 2 Phase 3 PREPARATION FOR A REGULATORY AUDIT BEGINS PRIOR TO STUDY CONDUCT, NOT AFTER STUDY CONDUCT. 8

(J Am Coll Cardiol 2018; 71: 1021 -34)

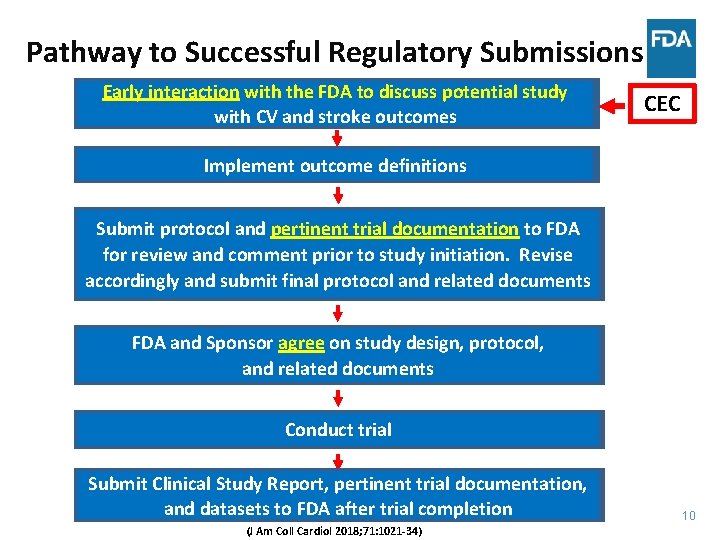
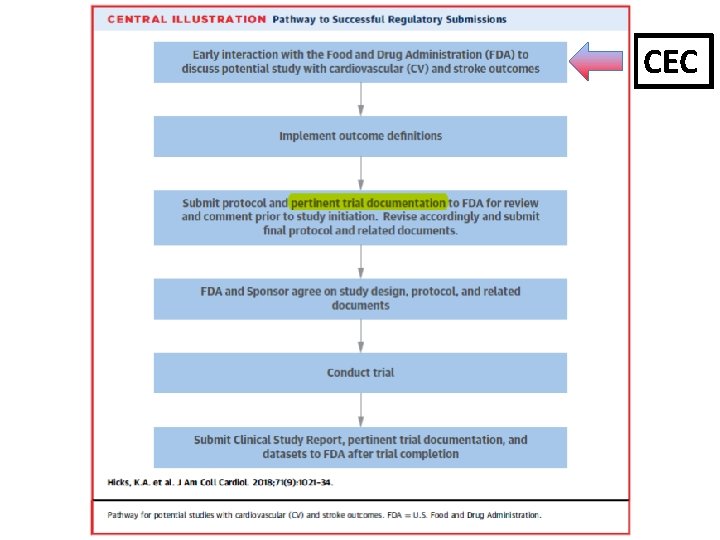
Pathway to Successful Regulatory Submissions Early interaction with the FDA to to discuss potential study with CV CV and stroke outcomes CEC Implement outcome definitions Submit protocol and pertinent trial documentation to to FDA for review and comment prior to to study initiation. Revise accordingly and submit final protocol and related documents FDA and Sponsor agree on on study design, protocol, and related documents Conduct trial Submit Clinical Study Report, pertinent trial documentation, and datasets to to FDA after trial completion (J Am Coll Cardiol 2018; 71: 1021 -34) 10

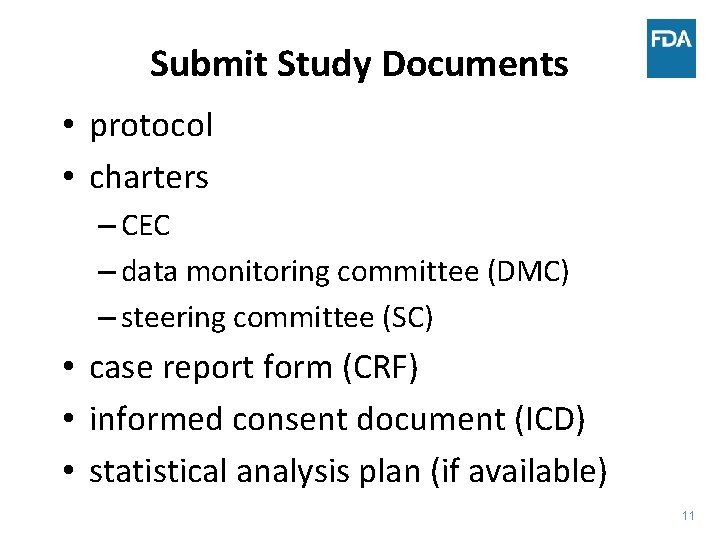
Submit Study Documents • protocol • charters – CEC – data monitoring committee (DMC) – steering committee (SC) • case report form (CRF) • informed consent document (ICD) • statistical analysis plan (if available) 11

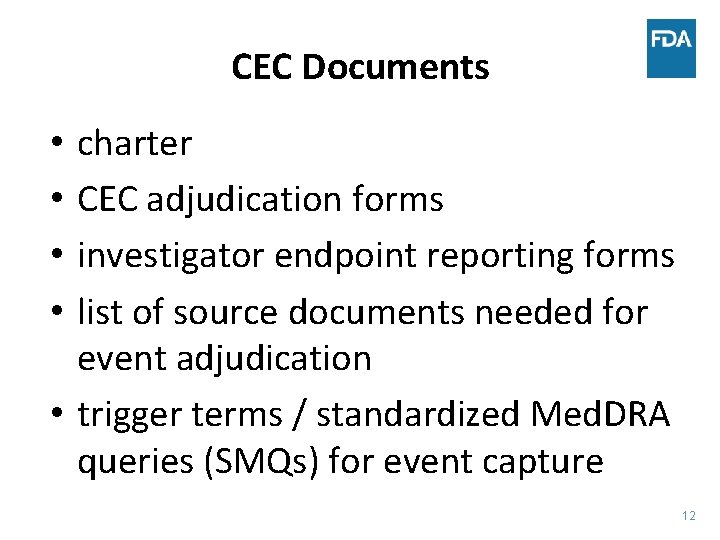
CEC Documents charter CEC adjudication forms investigator endpoint reporting forms list of source documents needed for event adjudication • trigger terms / standardized Med. DRA queries (SMQs) for event capture • • 12

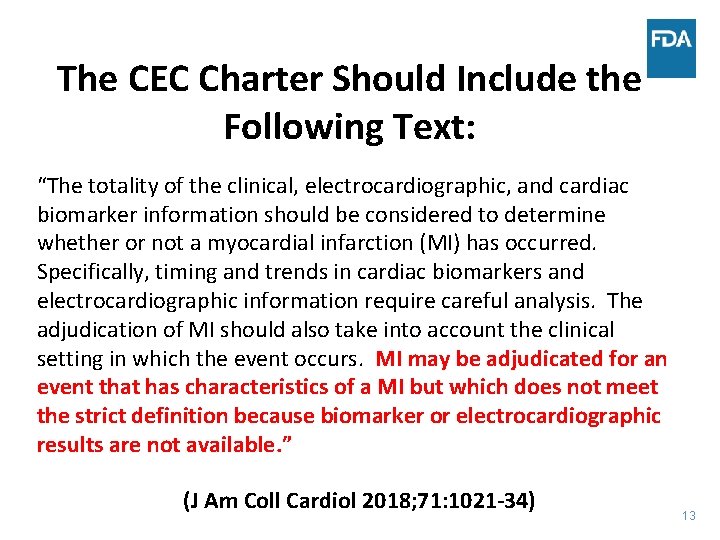
The CEC Charter Should Include the Following Text: “The totality of the clinical, electrocardiographic, and cardiac biomarker information should be considered to determine whether or not a myocardial infarction (MI) has occurred. Specifically, timing and trends in cardiac biomarkers and electrocardiographic information require careful analysis. The adjudication of MI should also take into account the clinical setting in which the event occurs. MI may be adjudicated for an event that has characteristics of a MI but which does not meet the strict definition because biomarker or electrocardiographic results are not available. ” (J Am Coll Cardiol 2018; 71: 1021 -34) 13

The CEC Charter Should Prespecify • whether silent MI will be adjudicated (and if so, the methodology) • whether undetermined deaths will be presumed to be cardiovascular deaths • source documents for event adjudication • trigger terms / SMQ queries for event capture • how to classify potential MI events where there are insufficient data to make a reliable determination (and these cases will need to be accounted for in a data analysis plan) 14

CEC Charter – Table of Contents - 1 • revision history • introduction • background on investigational product (IP)/ mechanism of action/dosing • protocol (title, IP, indication, primary objectives) • CEC organization (selection, qualifications, and roles/responsibilities of CEC chairman, members, and other team members) • clinical events requiring adjudication 15

CEC Charter – Table of Contents - 2 • event adjudication process – identification of clinical events – data collection/preparation of CEC packets – adjudication of events and process for handling disagreements – CEC meetings – quality control • clinical event definitions • source documents • CEC process flow diagram 16

CEC Adjudication Forms - 1 • If unable to adjudicate a case, indicate the reason: – – – event date and time not clear inadequate documentation of clinical studies inadequate information on symptoms/signs and/or duration inadequate description of physical examination findings insufficient cardiac biomarker results and reference limits (cardiac troponin [c. Tn]) inadequate ECG supportive evidence inadequate biomarker data (BNP, NT-pro. BNP) inadequate imaging reports inadequate information on therapies (drugs/devices) insufficient procedural documentation other (specify) 17

CEC Adjudication Forms - 2 • Rate adequacy of the documentation provided for the event definitions: – packet complete – packet incomplete but adequate for adjudication – packet inadequate for adjudication (specify what was missing and needed) 18

CEC Adjudication Forms - 3 • Select the option below which best describes the efforts required to adjudicate this event: – reviewers agreed without need for further discussion, committee, or consensus meeting – reviewers required further discussion to reach consensus but did not require a third party/committee to resolve – reviewers required a third party/committee to resolve the endpoint event 19

CEC Adjudication Forms - 4 • For all undetermined deaths, the CEC should indicate whether this classification is due to lack of information that should be available 20

Learning Objectives • Describe the role of the clinical events committee (CEC) in a development program • Describe the attributes of a quality CEC • Discuss how to prepare for a regulatory audit • Discuss pitfalls in CEC charters and adjudication • Discuss future challenges in CEC adjudication 21

Attributes of a Quality CEC - 1 • • • A CEC should be independent from the sponsor CEC members should be free of conflicts of interest A CEC should have the necessary expertise CEC members should be highly knowledgeable about the disease and treatments being studied A CEC chair should have prior CEC experience All CEC members should receive adequate training prior to adjudicating events in a clinical trial A CEC should have procedures to ensure confidentiality CEC meetings should have a uniform structure and occur at timely intervals A CEC should have a standard process for taking, circulating, and finalizing meeting minutes 22

Attributes of a Quality CEC - 2 • CEC members should adjudicate events prospectively and blindly in a uniform way and in a timely fashion. If additional information is needed, CEC members should make queries promptly, and queries should be addressed in a timely fashion. • A CEC should have a prespecified uniform process to manage disagreements • A CEC should make few changes to endpoint event definitions in the CEC charter during trial conduct. The CEC should document all changes and provide sufficient rationale for the changes. Revised CEC charters should be submitted to the IND (clean and track versions with a summary of changes/rationale) in real-time (not after study completion) 23

Attributes of a Quality CEC - 3 • A CEC should insist on quality and timely data collection • A CEC should respond promptly to FDA comments, questions, and recommendations 24

An Independent CEC is One in Which No Member Has • any basis for preferring the outcome to be in one or the other direction • any ability to influence the trial conduct in a role other than that of a CEC member 25

Who is Not “Independent”? • representatives of a pharmaceutical company sponsor • anyone involved with carrying out the study • anyone for whom the study outcome has financial implications 26

Learning Objectives • Describe the role of the clinical events committee (CEC) in a development program • Describe the attributes of a quality CEC • Discuss how to prepare for a regulatory audit • Discuss pitfalls in CEC charters and adjudication • Discuss future challenges in CEC adjudication 27

How to Prepare for a Regulatory Audit • Preparation begins PRIOR to study conduct, NOT AFTER study conduct. • Preparation continues during study conduct. THE CEC PROCESS MATTERS!! 28

Focus on the Process - 1 • Are all potential events being referred to the CEC for adjudication? Includes – Investigator-reported events; – Triggered events; – Other potential events, as identified by SMQs in the Adverse Event Database (“upgrades”); e. CRF listing, etc. • Is the CEC receiving all pertinent source data in a timely fashion to be able to adjudicate the event (i. e. , is the CEC seeing all the source documents available to the investigator? ) • Is the CEC blindly and prospectively adjudicating all events in a timely fashion and in a uniform way? Is there a uniform process to handle disagreements? If so, is this process being followed? • Are CEC queries being documented and addressed in a timely fashion so that additional source documents can be obtained to facilitate adjudication of the event? 29

Focus on the Process - 2 • Is the CEC Charter being followed regarding members, processes, and procedures? • Are CEC members qualified, not investigators in the study, and not otherwise directly associated with the sponsor or a member of another study committee (e. g. , Steering Committee)? • Have CEC members received adequate training prior to adjudication of the events for a clinical trial? • How are data from the site being collected to complete a Packet for the CEC? • Who is in charge of redaction? Has there been any accidental unblinding? • Are records being retained for the proper amount of time? (CEC should save all records which documented the initial independent review of all adjudicated events in addition to the final adjudication form) • Are there Good Clinical Practice (GCP) and site management/ monitoring issues? 30

Learning Objectives • Describe the role of the clinical events committee (CEC) in a development program • Describe the attributes of a quality CEC • Discuss how to prepare for a regulatory audit • Discuss pitfalls in CEC charters and adjudication • Discuss future challenges in CEC adjudication 31

CEC Pitfalls • Failure to address FDA comments, recommendations, and questions about the CEC Charter and other documents prior to study conduct • Endpoint Definitions – Changing definitions in CEC Charters during study conduct without sufficient rationale and not submitting revised Charters to the IND with clean/tracked changes and summary of changes with rationale – Subclassifying events into categories that are impossible to adjudicate – Including definitions for events in CEC Charters that overlap with other definitions in the Charter • Missing events – Failure to include all cardiac biomarker results (e. g. , c. Tn) with dates/times in one place – Failure to recognize myocardial ischemia in the setting of bundle branch blocks, pacemakers, biventricular pacemakers (cardiac resynchronization therapy) – Failure to recognize myocardial infarction in elderly women – Failure to use the totality of information to adjudicate a myocardial infarction in the event that cardiac biomarkers are incomplete/missing 32

Learning Objectives • Describe the role of the clinical events committee (CEC) in a development program • Describe the attributes of a quality CEC • Discuss how to prepare for a regulatory audit • Discuss pitfalls in CEC charters and adjudication • Discuss future challenges in CEC adjudication 33

Future Challenges in CEC Adjudication IT IS GETTING HARDER AND HARDER TO ADJUDICATE MYOCARDIAL INFARCTION! 34

Thygesen K, Alpert JS, Jaffe AS, Chaitman BR et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018; 138: e 1 – e 34.

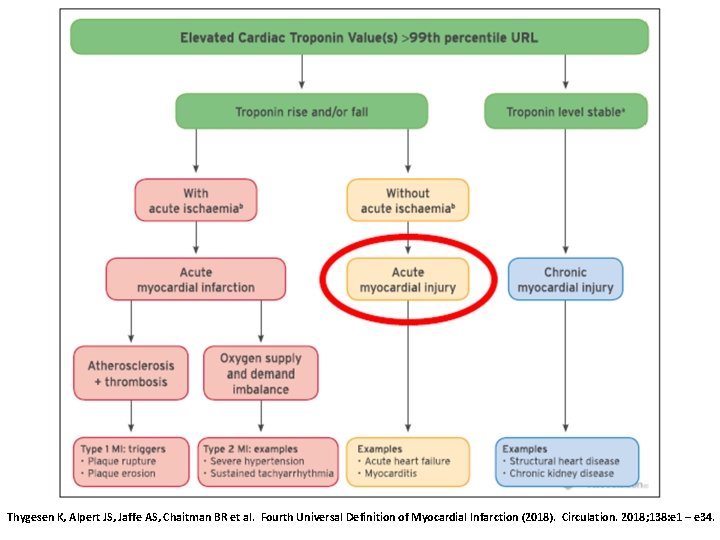
Adjudication of Myocardial Infarction • For the diagnosis of MI and reinfarction, the universal definition of MI (UDMI) definition recommends an immediate troponin measurement with a second sample at either 3 to 6 h or earlier with newer, more sensitive c. Tn assays. Later samples (> 6 h) are required in patients with – recurrent ischemia – high risk patients – when the timing of symptom onset is unclear (J Am Coll Cardiol 2018; 71: 1021 -34) 36

Adjudication of Myocardial Infarction • Diagnosis of MI should be based on the Fourth Universal Definition of Myocardial Infarction (UDMI; Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, et al. Fourth Universal Definition of Myocardial Infarction. Circulation. 2018; 138: e 1 -e 34). • A CEC should indicate whether the sampling times of cardiac biomarkers are adequate to adjudicate MI (i. e. , based on population, symptoms, timing of symptoms, and sampling times of cardiac biomarkers) 37

Summary - 1 • Not all development programs (or clinical trials) use CECs • When a development program (or clinical trial) uses a CEC, the CEC plays an important role • The CEC should be independent from the sponsor • Early interaction with FDA is critical to discuss a clinical trial • Preparation for a regulatory audit begins PRIOR to study conduct and continues DURING study conduct • The CEC should respond to all FDA comments, recommendations, and questions regarding the CEC documents PRIOR to study conduct • Ideally, the FDA, sponsor, and CEC should agree on the study design, protocol, CEC charter, and related documents PRIOR to study conduct 38

Summary - 2 • The best way to prepare for a regulatory audit is to focus on the process and whether the prespecified process is being followed • CEC training is important (e. g. , endpoint definitions, recognition of myocardial ischemia in various settings) • Avoid all potential pitfalls • A CEC should make few changes to endpoint definitions in the CEC charter during trial conduct. All changes should be carefully documented and the CEC should provide sufficient rationale for the changes. Revised CEC charters should be submitted in real-time (not after study completion) to the IND with clean/tracked changes and a summary of the changes with rationale. • Events should be adjudicated using the totality of information available 39

Thank you 40

karen. hicks@fda. hhs. gov

BACK-UP SLIDES 42

CEC

Protocol • Entry criteria • Study design • Endpoint definitions (Protocol, SAP, CEC Charter) • Silent MI (Protocol, SAP, CEC Charter) • Undetermined Death (Protocol, SAP, CEC Charter)(? Presumed CV death) • Withdrawal of Consent • Primary Endpoint/Vital Status 44

Withdrawal of Consent - 1 • For subjects who withdraw consent for followup, there should be documentation of the reason for withdrawal. Study staff should explicitly seek information about the possible contribution of adverse events to the subject’s desire to withdraw and document any adverse events that are identified in the Adverse Event section of the case record. 45

Withdrawal of Consent - 2 • Withdrawal of consent for treatment should be distinguished from withdrawal of consent for follow-up visits, telephone contacts, or medical records checks. Subjects requesting withdrawal should be informed that withdrawal of consent for follow-up will result in loss of important information about the benefits and risks of the drug product under study. 46

Withdrawal of Consent • Withdrawal of consent for follow-up should be accompanied by documentation of the reason for withdrawal. Withdrawal of consent for treatment should be distinguished from withdrawal of consent for follow-up visits and from withdrawal of consent for non-patient contact follow-up, e. g. , medical records checks. Subjects requesting withdrawal should be informed that withdrawal of consent for followup will jeopardize the public health value of the study. • Subjects who withdraw should be explicitly asked about the contribution of possible adverse events to their decision to withdraw consent, and any adverse event information elicited should be documented. • Preferably the subject should withdraw consent in writing and, if the subject or the subject’s representative refuses or is physically unavailable, the site should document and sign the reason for the subject’s failure to withdraw consent in writing. The informed consent for the study should note that although a subject is free to leave the study and stop taking study medication, the investigators hope the patient will remain for follow up status evaluations. 47

FDA Comments from Office of Scientific Investigation • Failure to ensure that an investigation was conducted in accordance with the general investigational plan and protocol as specified in the IND. • Failure to provide the FDA adequate descriptions and analyses of any other data or information relevant to the evaluation of the safety and effectiveness of the drug product obtained or otherwise received by the applicant from any source, foreign or domestic including information derived from clinical investigations (21 CFR 314. 50(d)(5)(iv)]. (GCP issues; site management/monitoring) • Records and reports were not retained for two years after discontinuance of the investigation and notification of FDA. (CEC should save all records which documented the initial independent review of all adjudicated events in addition to the final adjudication form) • Failure to ensure proper monitoring of the study. 48

Events • All investigator-reported events • All CEC-adjudicated events • All investigator-reported events that were also adjudicated by the CEC to be events • All investigator-reported events that were not thought to be events by the CEC (“downgrades”) • All CEC-adjudicated events that were not considered to be events by the investigator (“upgrades”) • Events identified by other methods 49

NDA Submission • Entire CEC packets with all CEC adjudication forms, investigator endpoint reporting forms, CRFs, source documents, queries, and results of queries for the adjudicated endpoints • SAS datasets – All investigator-reported events – All CEC-adjudicated events – All investigator-reported events that were also adjudicated as the same events by the CEC – “Downgrades” – “Upgrades” – Subject IDs, dates of onset, and the original and final adverse event (AE) terms for all adverse events. Include records for AEs that have been deleted – For each event submitted to the CEC for each subject, include subject ID, date of event, type of event, investigator description, and CEC adjudications (for each reviewer and final) 50

Red Flags for FDA • Investigator-reported results and CEC-adjudicated results go in opposite directions for the primary endpoint and major secondary endpoints • Investigator description of event is different from the CEC description of event OR there is disagreement about an event amongst CEC reviewers • Original verbatim term is mapped to a completely different final adverse event or endpoint 51

 Clinical events committee
Clinical events committee Audit readiness plan
Audit readiness plan Mutually exclusive vs non mutually exclusive
Mutually exclusive vs non mutually exclusive Audit committee institute
Audit committee institute Audit committee training
Audit committee training Audit committee self assessment
Audit committee self assessment Audit committee presentation
Audit committee presentation Mike gowell
Mike gowell Clinical audit example
Clinical audit example How to do clinical audit a brief guide
How to do clinical audit a brief guide Ndrkkm
Ndrkkm Advantages of auditing
Advantages of auditing Overall audit plan
Overall audit plan External quality assurance in clinical laboratory
External quality assurance in clinical laboratory Cql clinical quality language
Cql clinical quality language Clinical trials quality by design
Clinical trials quality by design Perbedaan audit konvensional dengan audit berbasis risiko
Perbedaan audit konvensional dengan audit berbasis risiko Audit klinik adalah
Audit klinik adalah Beda audit medis dan audit klinis
Beda audit medis dan audit klinis Penyelesaian audit dan tanggung jawab pasca audit
Penyelesaian audit dan tanggung jawab pasca audit Kerangka kerja audit manajemen
Kerangka kerja audit manajemen Perbedaan prosedur audit top-down dengan bottom-up
Perbedaan prosedur audit top-down dengan bottom-up Perbedaan audit konvensional dengan audit berbasis risiko
Perbedaan audit konvensional dengan audit berbasis risiko The word auditing is derived from the latin word
The word auditing is derived from the latin word Janette loveys
Janette loveys Quality control and quality assurance
Quality control and quality assurance Basic concepts of quality assurance
Basic concepts of quality assurance Quality audit definition
Quality audit definition Data quality audit tool
Data quality audit tool Pmp quality vs grade
Pmp quality vs grade Pmp gold plating
Pmp gold plating Quality assurance model in nursing management
Quality assurance model in nursing management Quality improvement vs quality assurance
Quality improvement vs quality assurance Quality management gurus
Quality management gurus Quality is free: the art of making quality certain
Quality is free: the art of making quality certain Old quality vs new quality
Old quality vs new quality Reading inventory scores
Reading inventory scores College and career readiness lexile chart
College and career readiness lexile chart Vocabulary power plus for college and career readiness
Vocabulary power plus for college and career readiness Bia airport
Bia airport Readiness through integrative science and engineering
Readiness through integrative science and engineering College and career readiness standards math
College and career readiness standards math The aspire test evaluates students in subject areas
The aspire test evaluates students in subject areas Young worker readiness
Young worker readiness Workplace readiness skills-positive work ethics
Workplace readiness skills-positive work ethics School readiness goals
School readiness goals The readiness is all
The readiness is all Employment readiness scale
Employment readiness scale Driver readiness
Driver readiness Rapid toilet training protocol
Rapid toilet training protocol Ndia systems engineering conference
Ndia systems engineering conference Test readiness review
Test readiness review School readiness goals
School readiness goals