CHME 420 Mass Transfer 2 a Differential Equations



- Slides: 17

CHME 420: Mass Transfer 2 a. Differential Equations of Mass Transfer Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

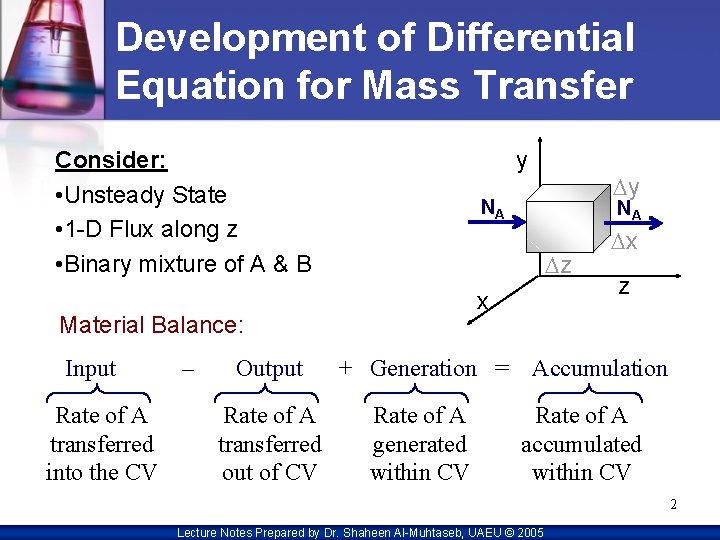
Development of Differential Equation for Mass Transfer Consider: • Unsteady State • 1 -D Flux along z • Binary mixture of A & B y NA Rate of A transferred into the CV – NA Dz x Material Balance: Input Dy Output + Generation = Rate of A transferred out of CV Rate of A generated within CV Dx z Accumulation Rate of A accumulated within CV 2 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

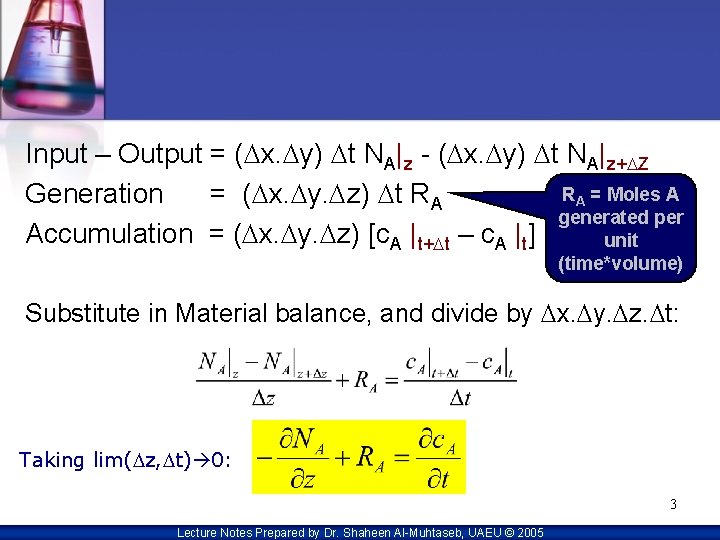
Input – Output = (Dx. Dy) Dt NA|z - (Dx. Dy) Dt NA|z+DZ RA = Moles A Generation = (Dx. Dy. Dz) Dt RA generated per Accumulation = (Dx. Dy. Dz) [c. A |t+Dt – c. A |t] unit (time*volume) Substitute in Material balance, and divide by Dx. Dy. Dz. Dt: Taking lim(Dz, Dt) 0: 3 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

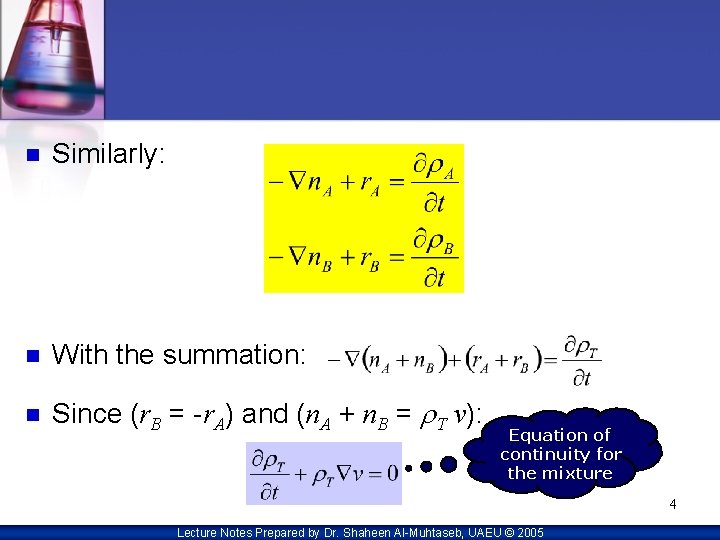
n Similarly: n With the summation: n Since (r. B = -r. A) and (n. A + n. B = r. T v): Equation of continuity for the mixture 4 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

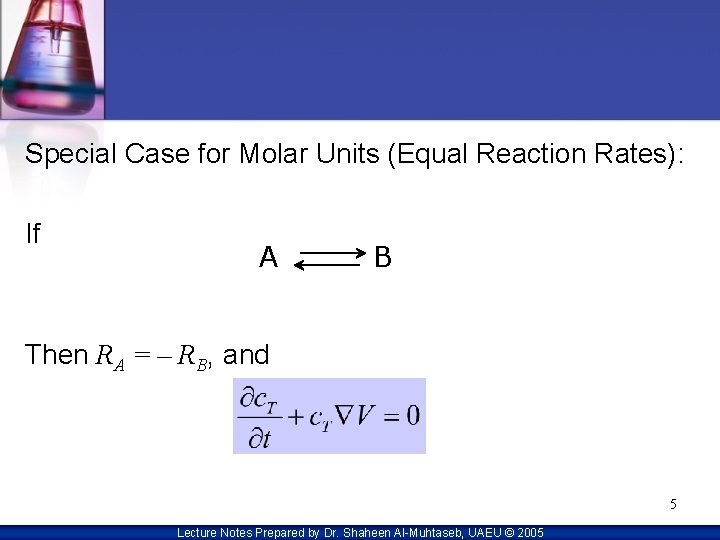
Special Case for Molar Units (Equal Reaction Rates): If A B Then RA = – RB, and 5 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

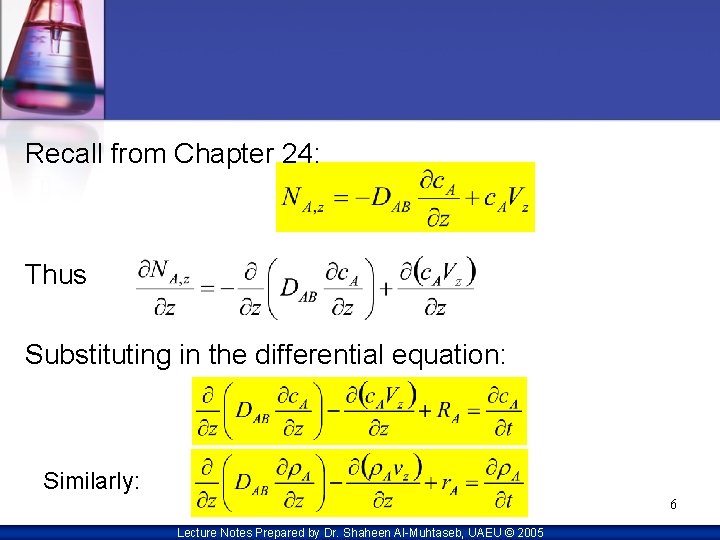
Recall from Chapter 24: Thus Substituting in the differential equation: Similarly: 6 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

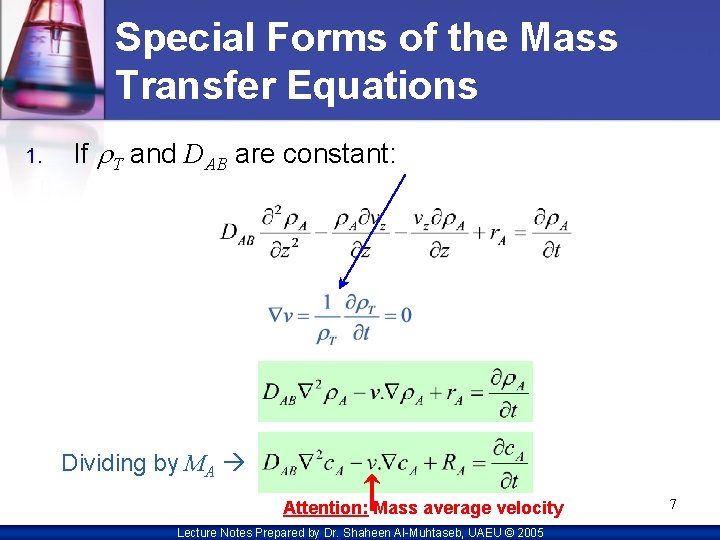
Special Forms of the Mass Transfer Equations 1. If r. T and DAB are constant: Dividing by MA Attention: Mass average velocity Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005 7

2. If r. T and DAB are constant with no bulk reaction (RA = r. A = 0): 0 Dividing by MA 8 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

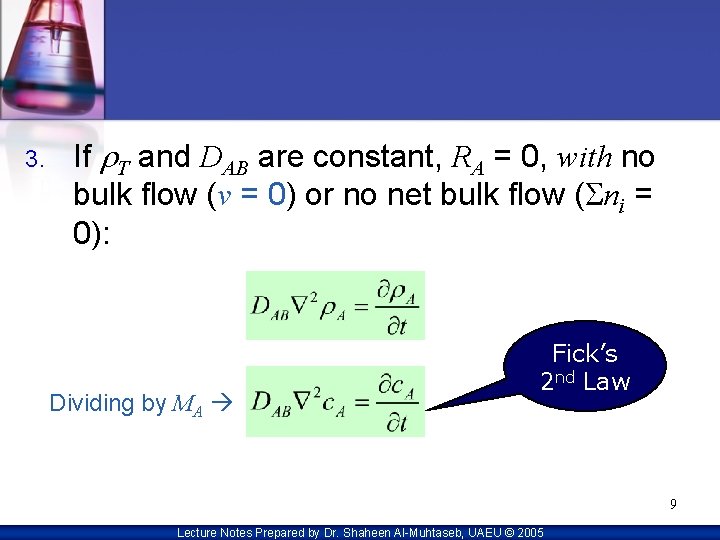
3. If r. T and DAB are constant, RA = 0, with no bulk flow (v = 0) 0 or no net bulk flow (Sni = 0): Dividing by MA Fick’s 2 nd Law 9 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

4. If we have steady state behavior (dr. A/dt = 0) with r. T and DAB are constant: Dividing by MA 5. 6. Case 4 with RA = r. A = 0 Case 4 with v = 0 10 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

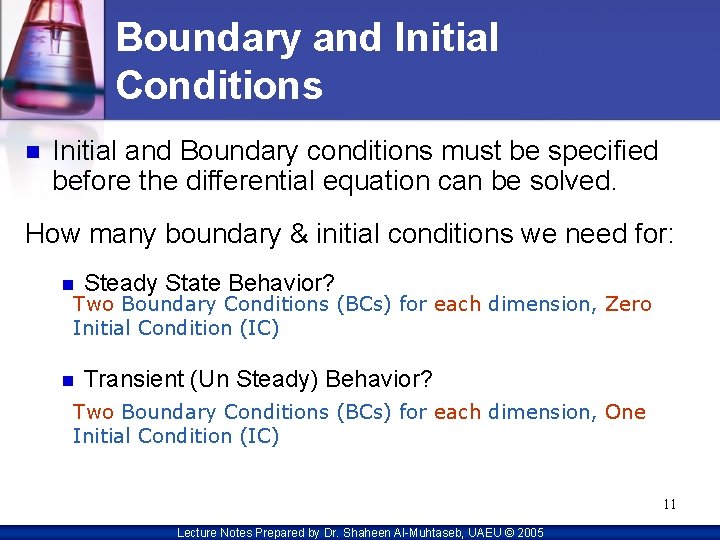
Boundary and Initial Conditions n Initial and Boundary conditions must be specified before the differential equation can be solved. How many boundary & initial conditions we need for: n Steady State Behavior? n Transient (Un Steady) Behavior? Two Boundary Conditions (BCs) for each dimension, Zero Initial Condition (IC) Two Boundary Conditions (BCs) for each dimension, One Initial Condition (IC) 11 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

Initial Condition (IC) IC IC: IC Concentration of the diffusing species at the start of the time interval n The IC can be either constant or a function of space variables n Examples (for one-dimensional (1 D) diffusion): (z, t = 0) = c. A 0 n c. A (z, t = 0) = f(z) n c. A or Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005 12

Examples of Boundary Conditions (BCs) BC 1. Concentration is known at z = 0 (surface) c. A (0, t) = c. As 2. Flux is known at z = 0 (surface) 3. Wall is impermeable to “A” NA (L, t) = 0 13 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

4. Rate of chemical reaction is specified at wall NA(L, t) = RA = kn c. A(L, t)n [nth order of reaction] 5. If instantaneous reaction at wall c. A(L, t) = 0 6. If fluid is flowing over the phase for which the differential equation is written species are lost by convective mass transfer 14 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

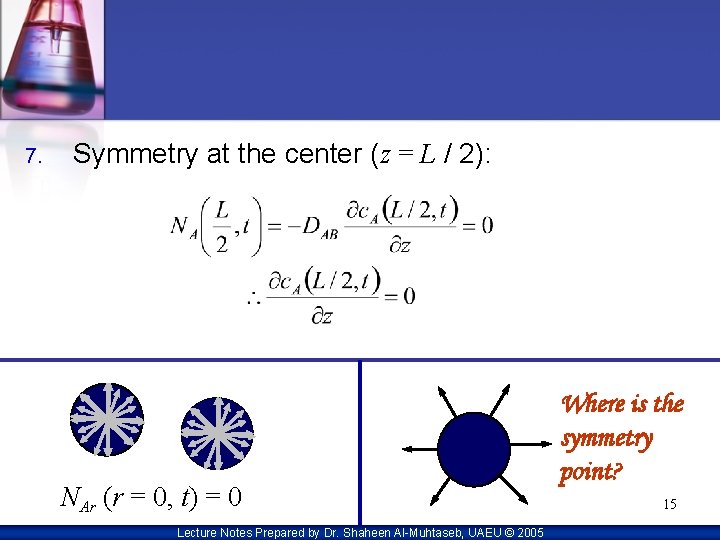
7. Symmetry at the center (z = L / 2): NAr (r = 0, t) = 0 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005 Where is the symmetry point? 15

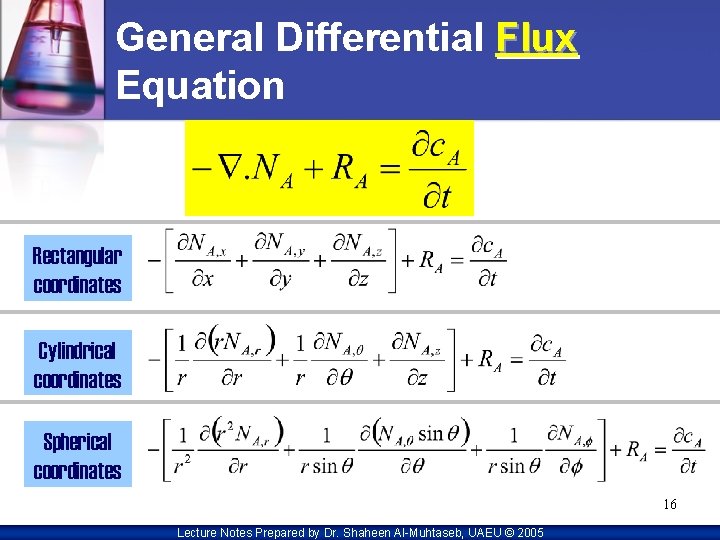
General Differential Flux Equation Rectangular coordinates Cylindrical coordinates Spherical coordinates 16 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005

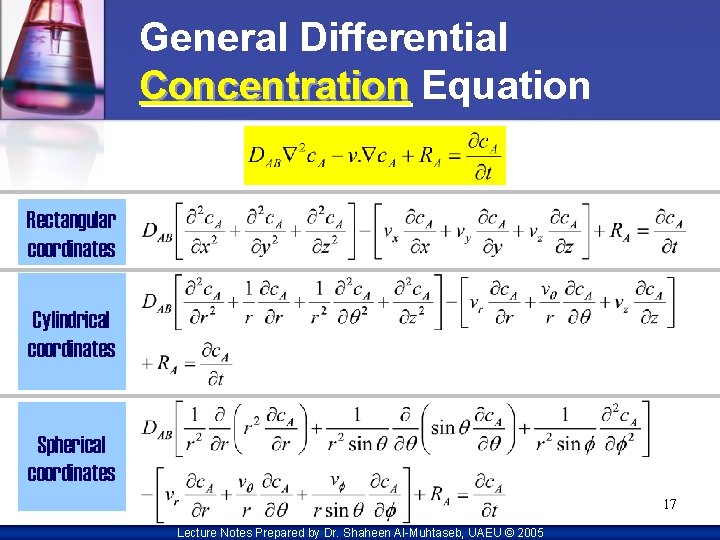
General Differential Concentration Equation Rectangular coordinates Cylindrical coordinates Spherical coordinates 17 Lecture Notes Prepared by Dr. Shaheen Al-Muhtaseb, UAEU © 2005