Chemical Reactions Chapter 7 Section 1 Chemical Reactions







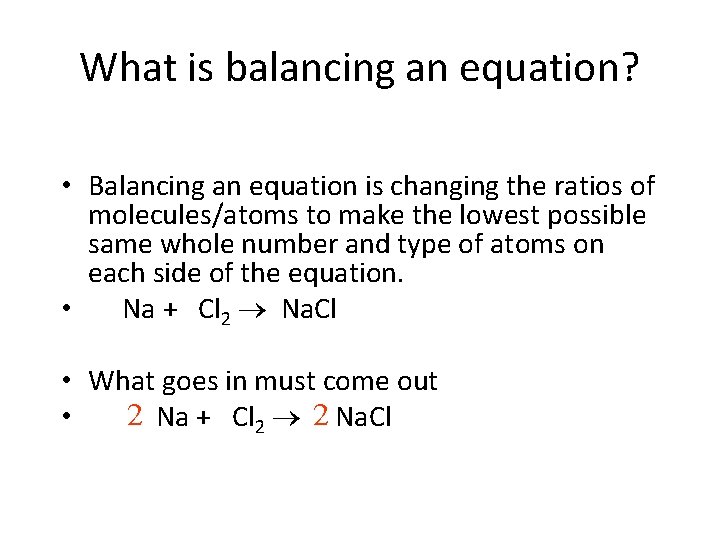


- Slides: 22

Chemical Reactions Chapter 7 Section 1

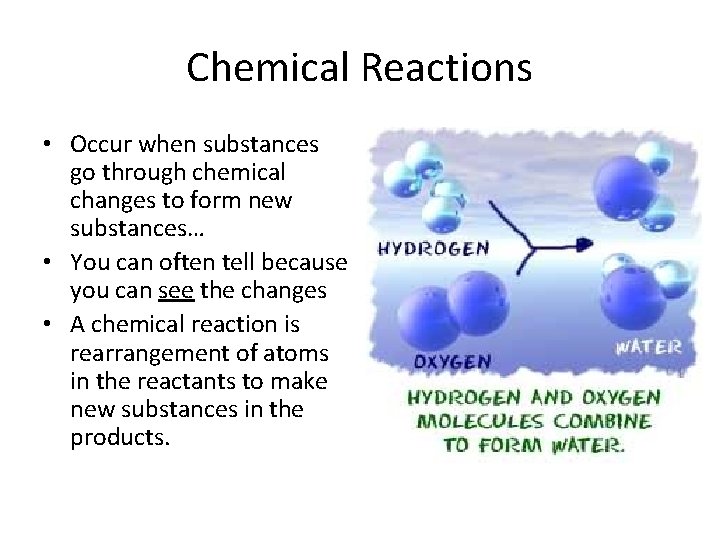
Chemical Reactions • Occur when substances go through chemical changes to form new substances… • You can often tell because you can see the changes • A chemical reaction is rearrangement of atoms in the reactants to make new substances in the products.

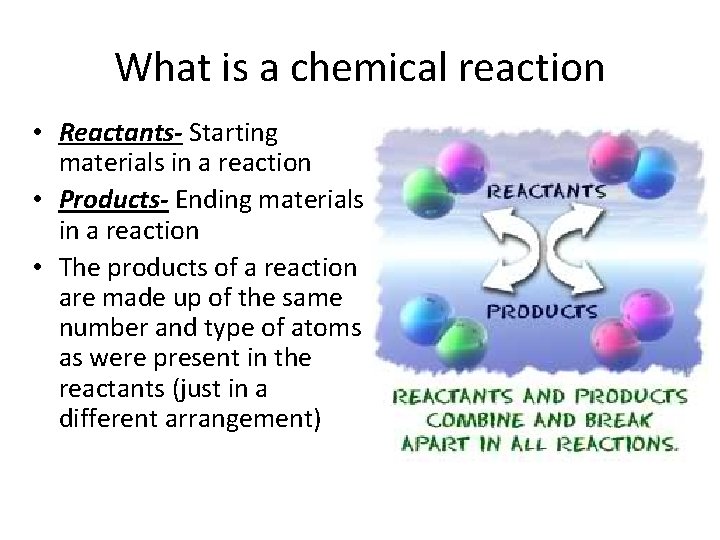
What is a chemical reaction • Reactants- Starting materials in a reaction • Products- Ending materials in a reaction • The products of a reaction are made up of the same number and type of atoms as were present in the reactants (just in a different arrangement)

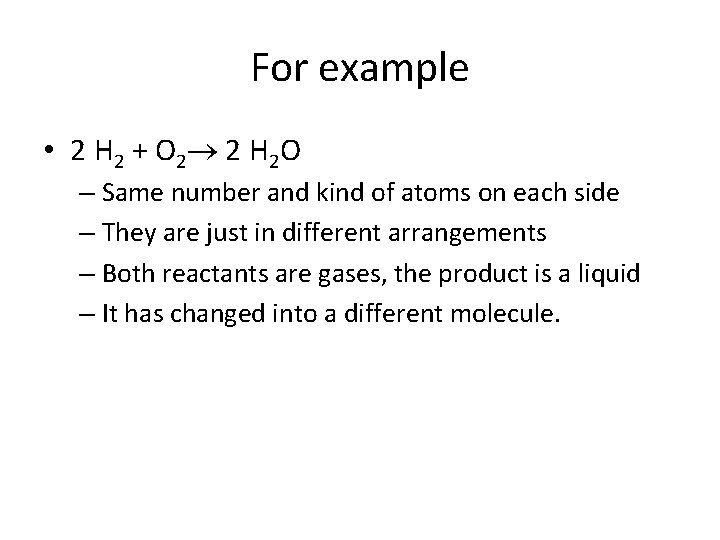
For example • 2 H 2 + O 2 2 H 2 O – Same number and kind of atoms on each side – They are just in different arrangements – Both reactants are gases, the product is a liquid – It has changed into a different molecule.

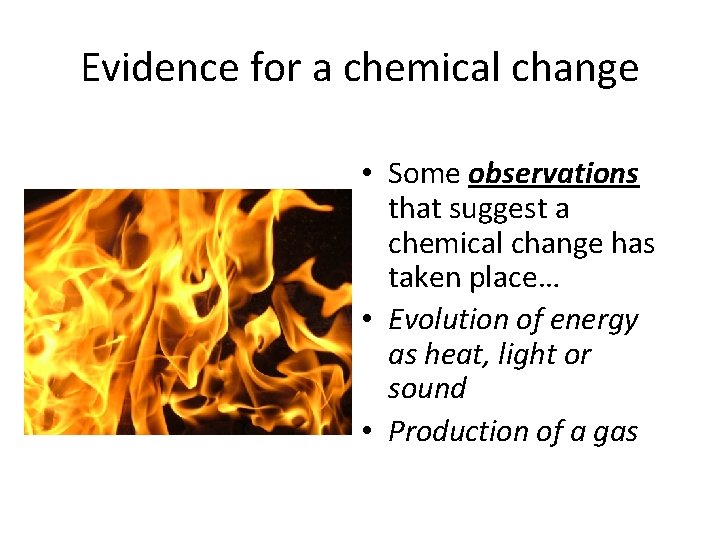
Evidence for a chemical change • Some observations that suggest a chemical change has taken place… • Evolution of energy as heat, light or sound • Production of a gas

More Evidence… • Formation of a precipitate (“stuff forming”) • Change in color • This is not conclusive proof though, it needs to be proven that some new compound is present.

Activation Energy • In order to start a chemical reaction you must break bonds (covalent, ionic or metallic) – To do this takes energy • The energy required to break enough bonds for a reaction to start is the activation energy of a reaction

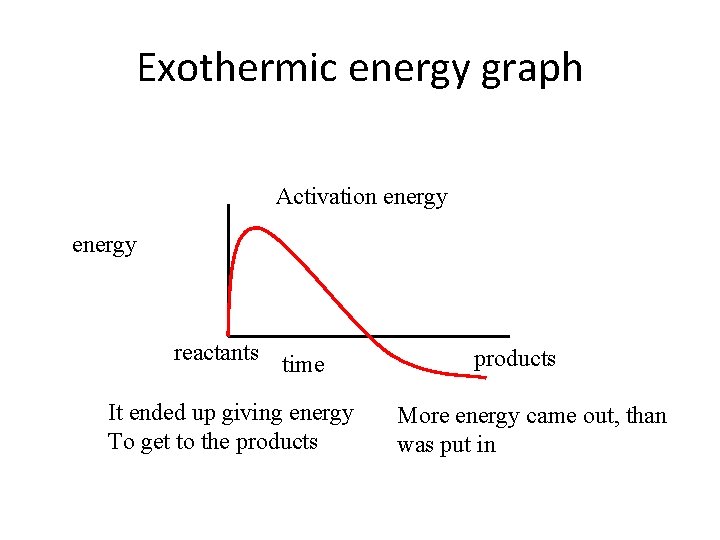
Exothermic • Exothermic reactions give off energy. . • When the bonds reform they will release energy • If more energy is released than is used to break more bonds (to continue) the reaction is exothermic

Exothermic energy graph Activation energy reactants time It ended up giving energy To get to the products More energy came out, than was put in

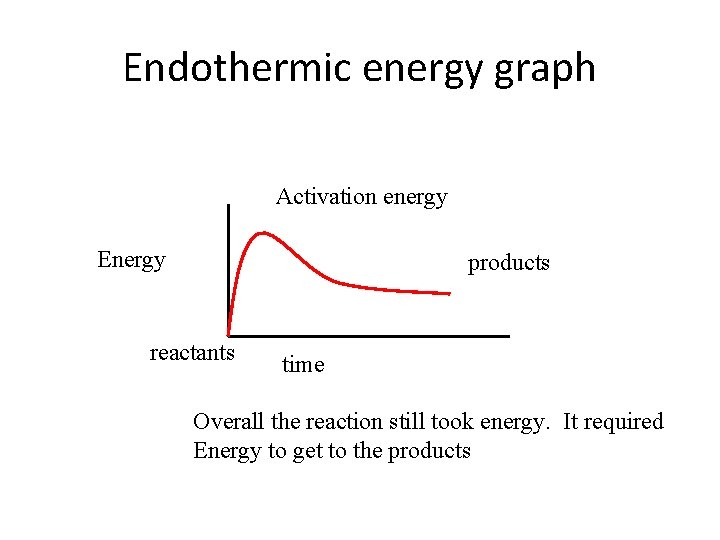
Endothermic • If more energy is required to make the reaction than is released the reaction is endothermic • Endothermic take in energy.

Endothermic energy graph Activation energy Energy products reactants time Overall the reaction still took energy. It required Energy to get to the products

Balancing Equations Chapter 7 section 2

Reminder: • All MATTER is conserved, but it can change forms. . – What goes in must come out, but it can be rearranged! – You have to account for all the “stuff” that goes into the reaction
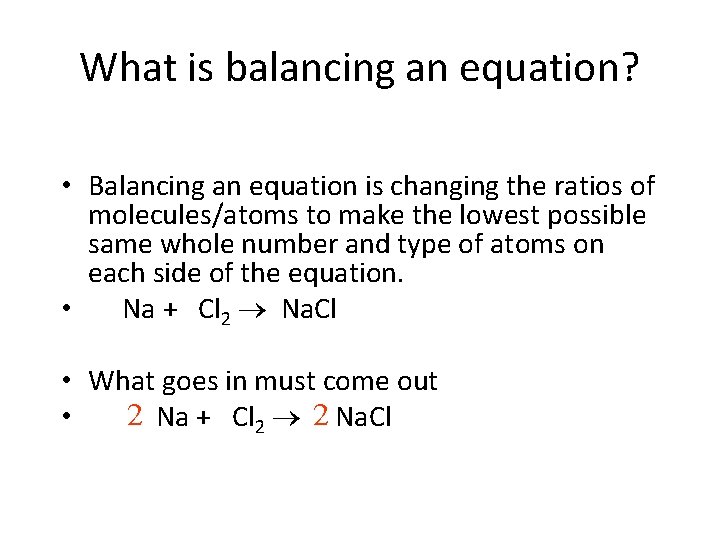
What is balancing an equation? • Balancing an equation is changing the ratios of molecules/atoms to make the lowest possible same whole number and type of atoms on each side of the equation. • Na + Cl 2 Na. Cl • What goes in must come out 2 Na + Cl 2 2 Na. Cl •

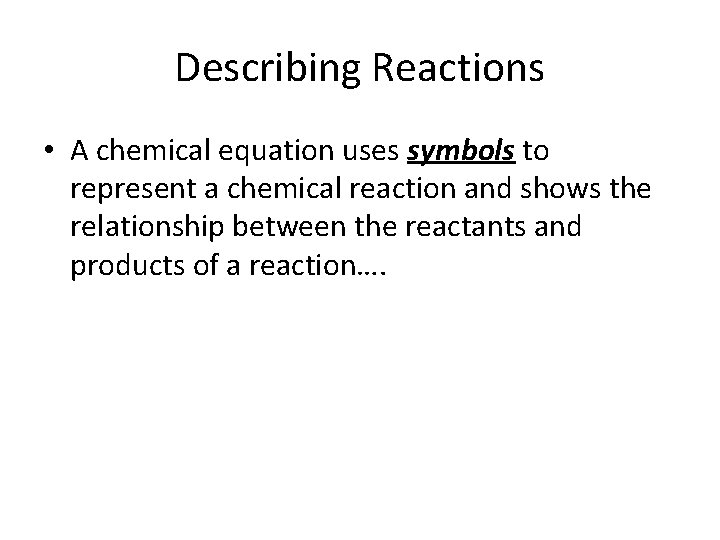
Describing Reactions • A chemical equation uses symbols to represent a chemical reaction and shows the relationship between the reactants and products of a reaction….

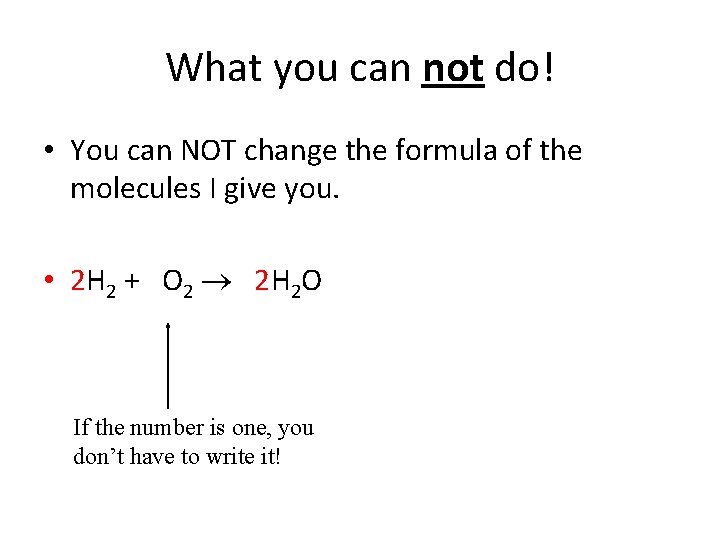
What you can not do! • You can NOT change the formula of the molecules I give you. • 2 H 2 + O 2 2 H 2 O If the number is one, you don’t have to write it!

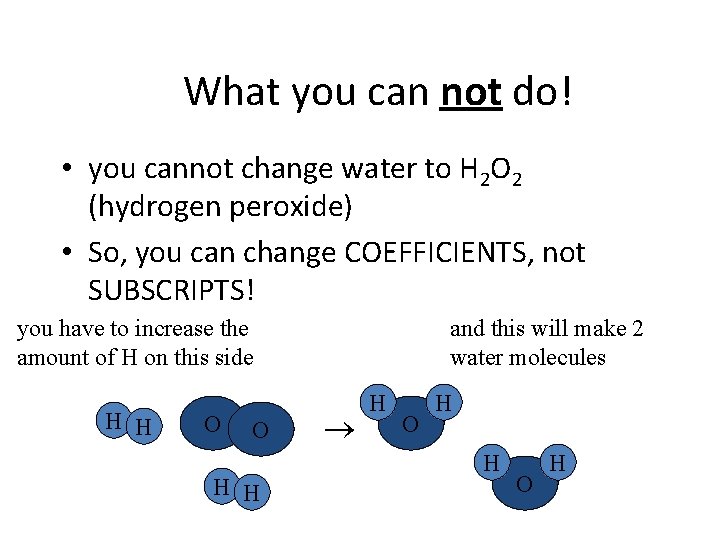
What you can not do! • you cannot change water to H 2 O 2 (hydrogen peroxide) • So, you can change COEFFICIENTS, not SUBSCRIPTS! you have to increase the amount of H on this side H H O O H H and this will make 2 water molecules H O H

What you are allowed to do • change the coefficient in front of any molecule/atom (changing the ratio of atoms) • ___ Ag + ___ Cl 2 ___ Ag. Cl • you can only write on those lines

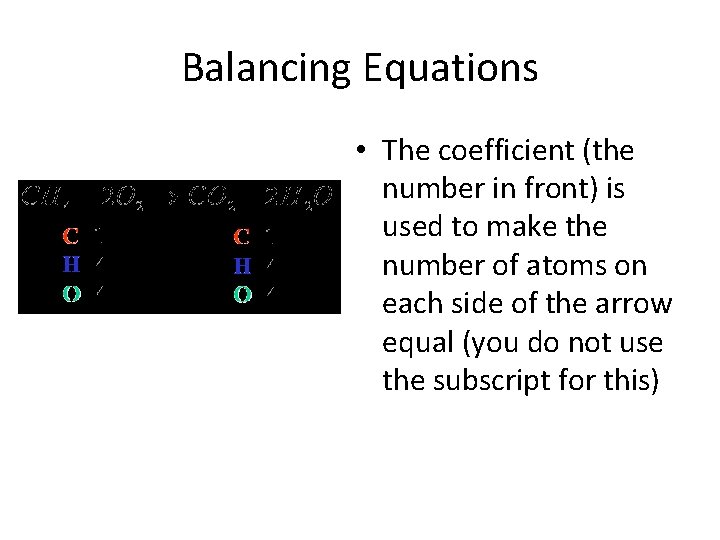
Balancing Equations • The coefficient (the number in front) is used to make the number of atoms on each side of the arrow equal (you do not use the subscript for this)

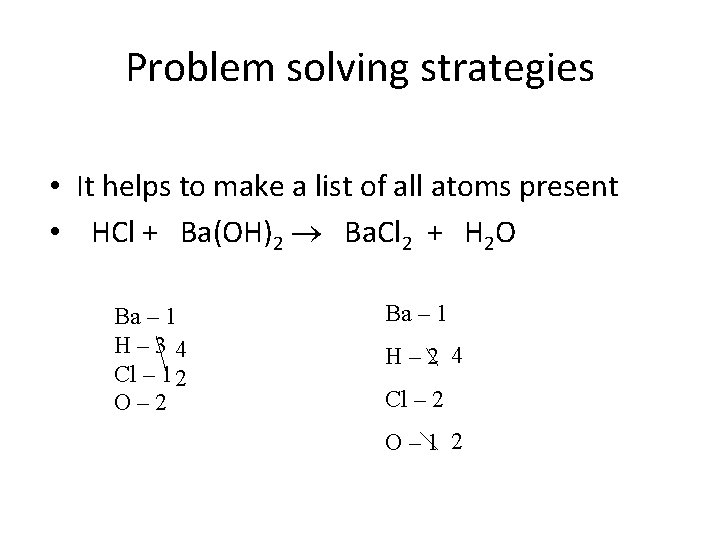
Problem solving strategies • It helps to make a list of all atoms present • HCl + Ba(OH)2 Ba. Cl 2 + H 2 O Ba – 1 H– 3 4 Cl – 1 2 O– 2 Ba – 1 H– 2 4 Cl – 2 O– 1 2

Problem solving strategies • It helps to make a list of all atoms present • 2 HCl + Ba(OH)2 Ba. Cl 2 + 2 H 2 O Ba – 1 H– 3 4 Cl – 1 2 O– 2 starting w/ Cl Start making the Ba – 1 numbers agree H– 2 4 one at a time (pick something, Cl – 2 that occurs in only O – 1 2 one molecule 1 st) ~not H in this case Now going to O Now everything is balanced!

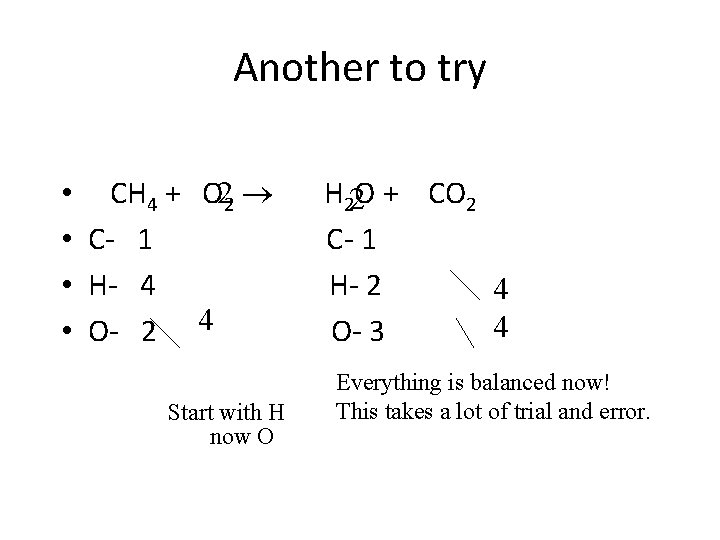
Another to try • CH 4 + O 22 • C- 1 • H- 4 • O- 2 4 Start with H now O H 22 O + CO 2 C- 1 H- 2 4 4 O- 3 Everything is balanced now! This takes a lot of trial and error.
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Combination reaction equation
Combination reaction equation Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 6 section 1 atoms elements and compounds answer key
Chapter 6 section 1 atoms elements and compounds answer key Section 2 chemical reactions answer key
Section 2 chemical reactions answer key Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Balancing equations chapter 8
Balancing equations chapter 8 Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Chapter 11 chemical reactions practice problems
Chapter 11 chemical reactions practice problems Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Types of reactions
Types of reactions