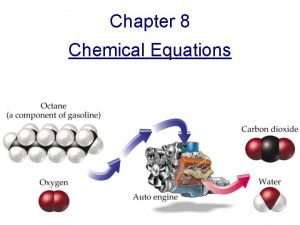
Chemical Equations and Reactions Chemical Equations Law of


- Slides: 22

Chemical Equations and Reactions

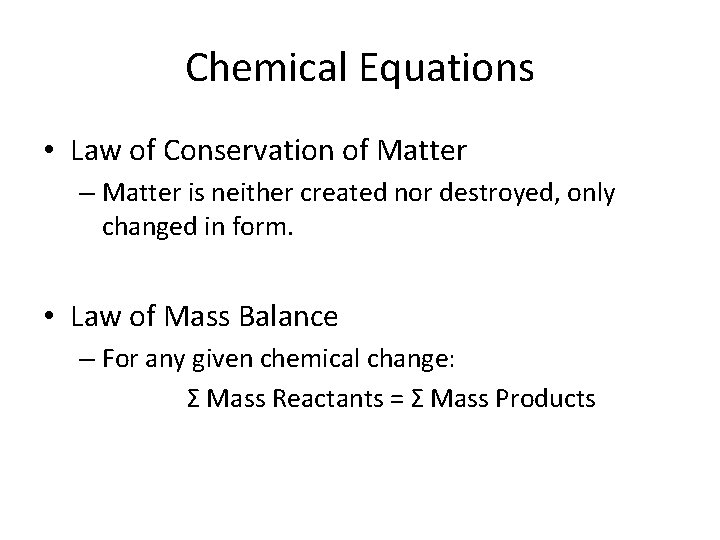
Chemical Equations • Law of Conservation of Matter – Matter is neither created nor destroyed, only changed in form. • Law of Mass Balance – For any given chemical change: Σ Mass Reactants = Σ Mass Products

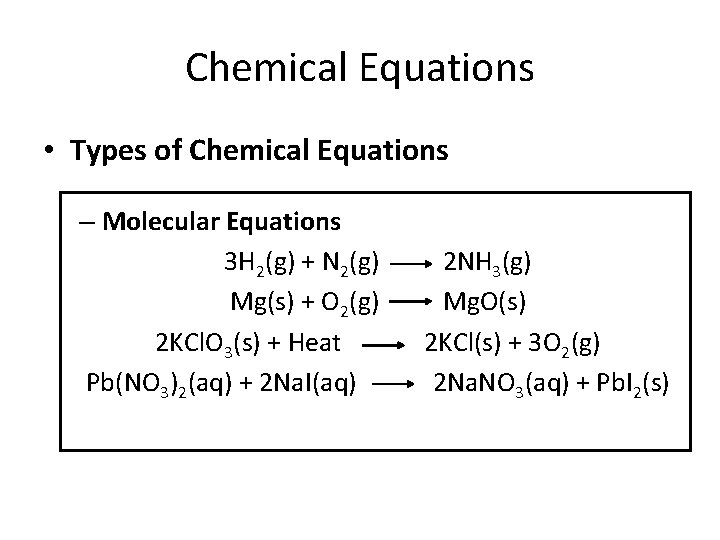
Chemical Equations • Types of Chemical Equations – Molecular Equations 3 H 2(g) + N 2(g) Mg(s) + O 2(g) 2 KCl. O 3(s) + Heat Pb(NO 3)2(aq) + 2 Na. I(aq) 2 NH 3(g) Mg. O(s) 2 KCl(s) + 3 O 2(g) 2 Na. NO 3(aq) + Pb. I 2(s)

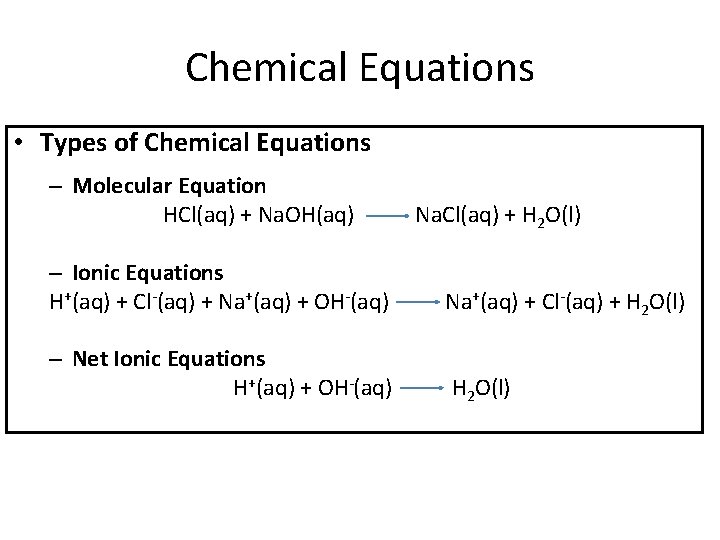
Chemical Equations • Types of Chemical Equations – Molecular Equation HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) – Ionic Equations H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) Na+(aq) + Cl-(aq) + H 2 O(l) – Net Ionic Equations H+(aq) + OH-(aq) H 2 O(l)

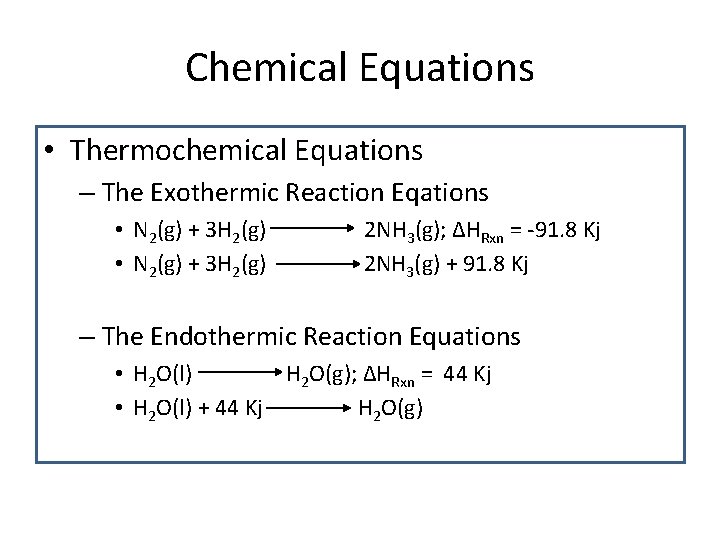
Chemical Equations • Thermochemical Equations – The Exothermic Reaction Eqations • N 2(g) + 3 H 2(g) 2 NH 3(g); ΔHRxn = -91. 8 Kj 2 NH 3(g) + 91. 8 Kj – The Endothermic Reaction Equations • H 2 O(l) H 2 O(g); ΔHRxn = 44 Kj • H 2 O(l) + 44 Kj H 2 O(g)

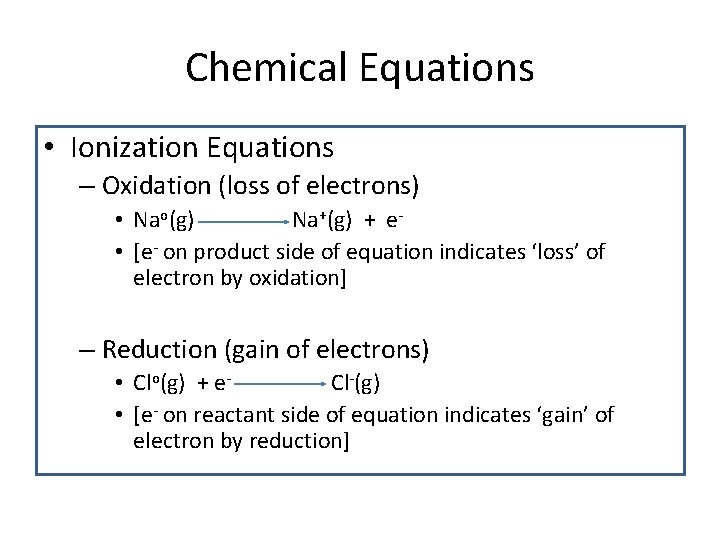
Chemical Equations • Ionization Equations – Oxidation (loss of electrons) • Nao(g) Na+(g) + e • [e- on product side of equation indicates ‘loss’ of electron by oxidation] – Reduction (gain of electrons) • Clo(g) + e. Cl-(g) • [e- on reactant side of equation indicates ‘gain’ of electron by reduction]

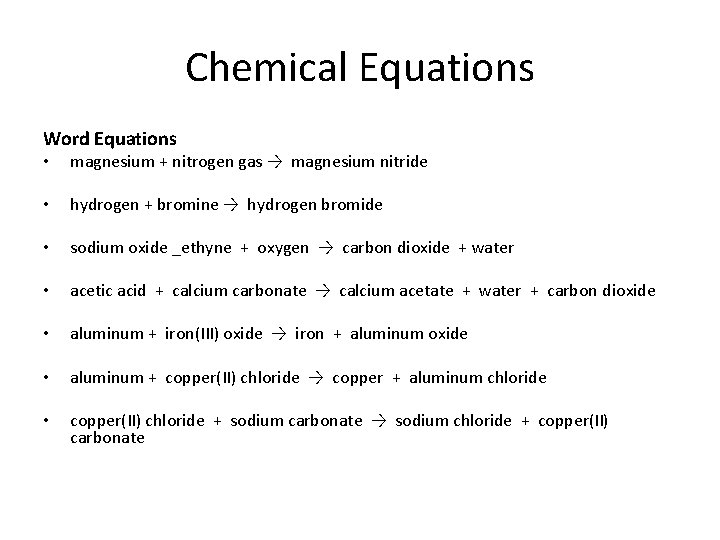
Chemical Equations Word Equations • magnesium + nitrogen gas → magnesium nitride • hydrogen + bromine → hydrogen bromide • sodium oxide _ethyne + oxygen → carbon dioxide + water • acetic acid + calcium carbonate → calcium acetate + water + carbon dioxide • aluminum + iron(III) oxide → iron + aluminum oxide • aluminum + copper(II) chloride → copper + aluminum chloride • copper(II) chloride + sodium carbonate → sodium chloride + copper(II) carbonate

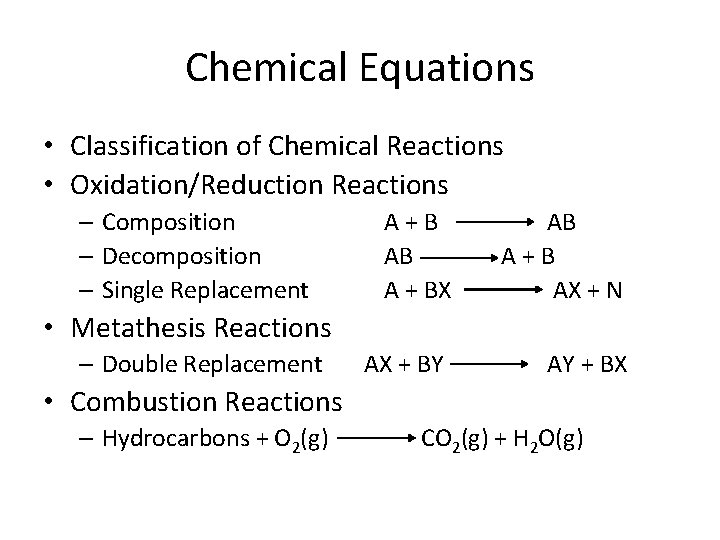
Chemical Equations • Classification of Chemical Reactions • Oxidation/Reduction Reactions – Composition – Decomposition – Single Replacement A+B AB A + BX AB A+B AX + N • Metathesis Reactions – Double Replacement AX + BY AY + BX • Combustion Reactions – Hydrocarbons + O 2(g) CO 2(g) + H 2 O(g)

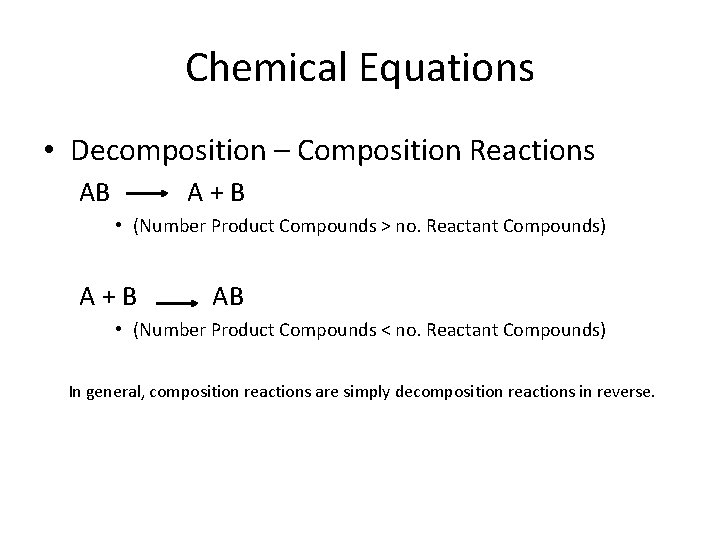
Chemical Equations • Decomposition – Composition Reactions AB A+B • (Number Product Compounds > no. Reactant Compounds) A+B AB • (Number Product Compounds < no. Reactant Compounds) In general, composition reactions are simply decomposition reactions in reverse.

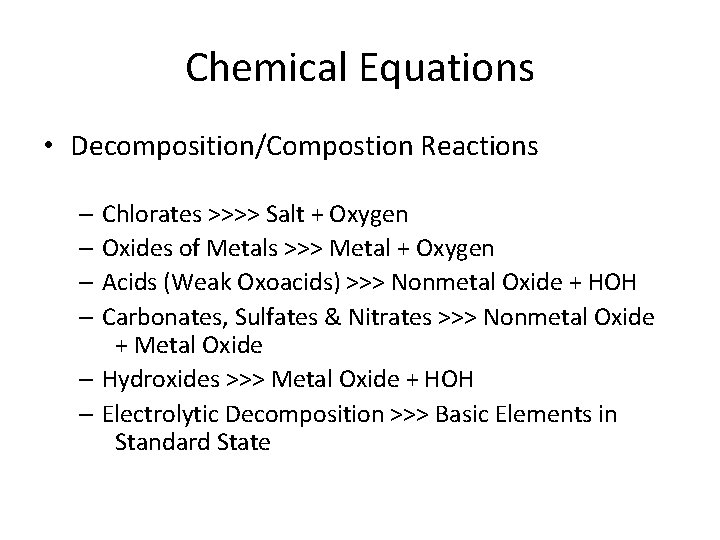
Chemical Equations • Decomposition/Compostion Reactions – Chlorates >>>> Salt + Oxygen – Oxides of Metals >>> Metal + Oxygen – Acids (Weak Oxoacids) >>> Nonmetal Oxide + HOH – Carbonates, Sulfates & Nitrates >>> Nonmetal Oxide + Metal Oxide – Hydroxides >>> Metal Oxide + HOH – Electrolytic Decomposition >>> Basic Elements in Standard State

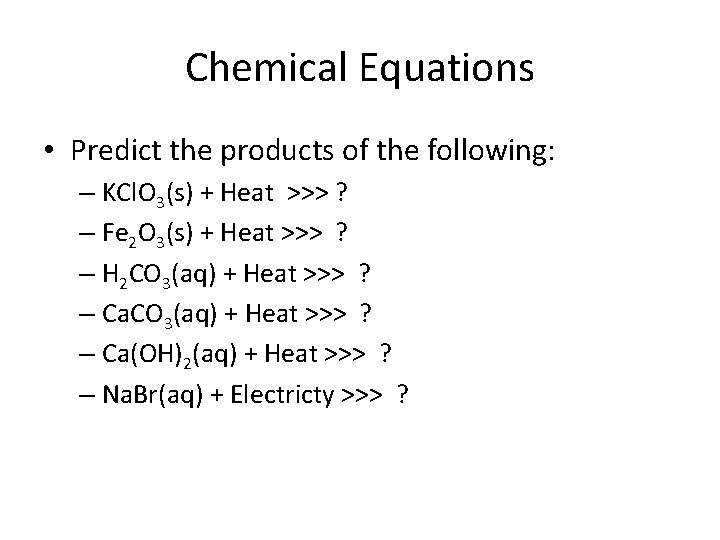
Chemical Equations • Predict the products of the following: – KCl. O 3(s) + Heat >>> ? – Fe 2 O 3(s) + Heat >>> ? – H 2 CO 3(aq) + Heat >>> ? – Ca(OH)2(aq) + Heat >>> ? – Na. Br(aq) + Electricty >>> ?

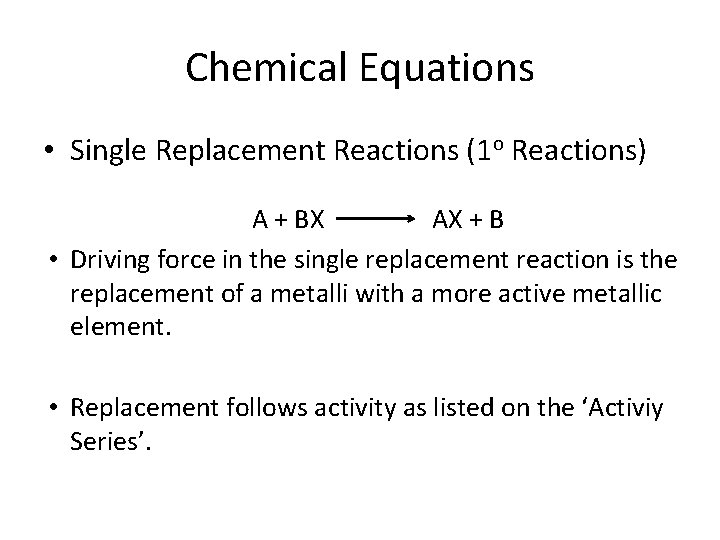
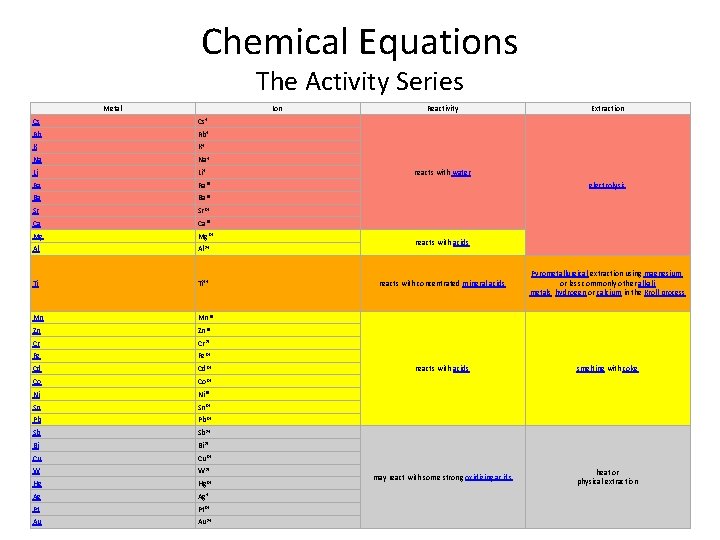
Chemical Equations • Single Replacement Reactions (1 o Reactions) A + BX AX + B • Driving force in the single replacement reaction is the replacement of a metalli with a more active metallic element. • Replacement follows activity as listed on the ‘Activiy Series’.

Chemical Equations The Activity Series Metal Ion Cs Cs+ Rb Rb+ K K+ Na Na+ Li Li+ Ra Ra 2+ Ba Ba 2+ Sr Sr 2+ Ca Ca 2+ Mg Mg 2+ Al Al 3+ Ti Ti 4+ Mn Mn 2+ Zn Zn 2+ Cr Cr 3+ Fe Fe 2+ Cd Cd 2+ Co Co 2+ Ni Ni 2+ Sn Sn 2+ Pb Pb 2+ Sb Sb 3+ Bi Bi 3+ Cu Cu 2+ W W 3+ Hg Hg 2+ Ag Ag+ Pt Pt 2+ Au Au 3+ Reactivity Extraction reacts with water electrolysis reacts with acids reacts with concentrated mineral acids Pyrometallurgical extraction using magnesium, or less commonly other alkali metals, hydrogen or calcium in the Kroll process reacts with acids smelting with coke may react with some strong oxidizing acids heat or physical extraction

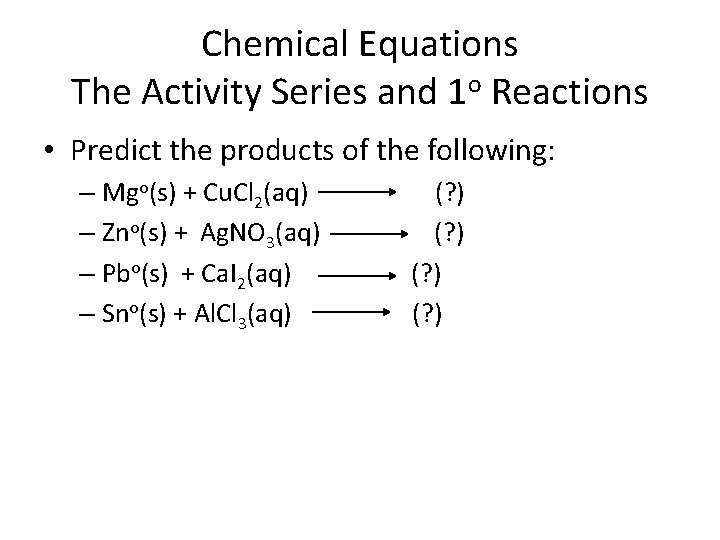
Chemical Equations The Activity Series and 1 o Reactions • Predict the products of the following: – Mgo(s) + Cu. Cl 2(aq) – Zno(s) + Ag. NO 3(aq) – Pbo(s) + Ca. I 2(aq) – Sno(s) + Al. Cl 3(aq) (? )

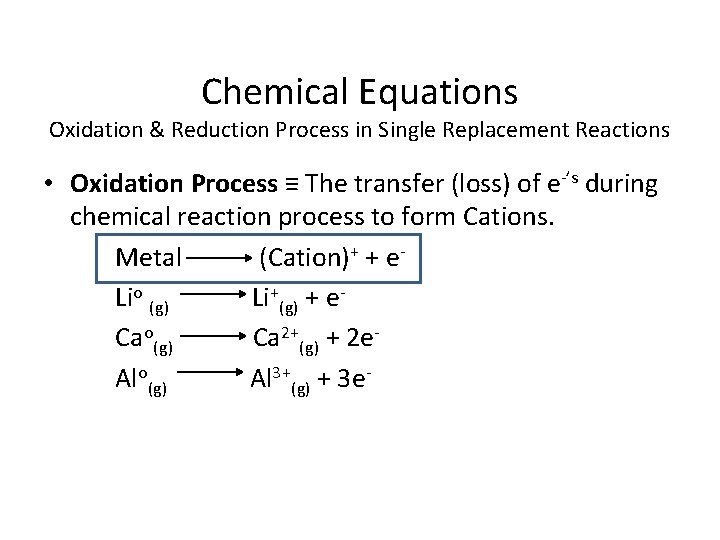
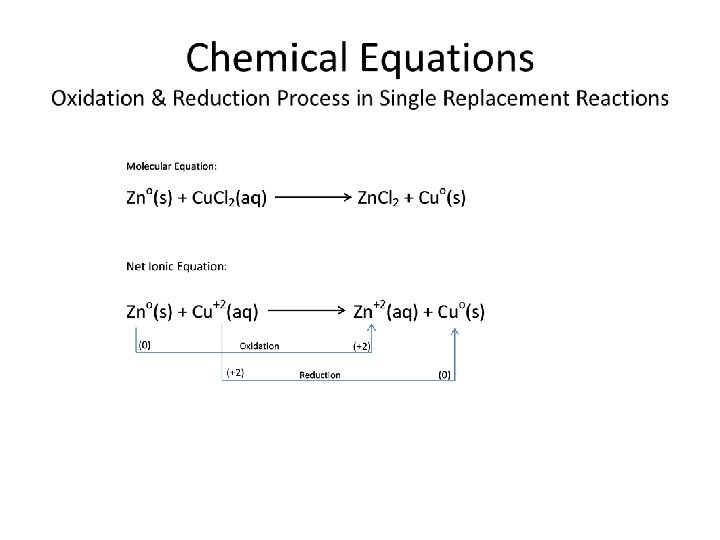
Chemical Equations Oxidation & Reduction Process in Single Replacement Reactions • Oxidation Process ≡ The transfer (loss) of e-’s during chemical reaction process to form Cations. Metal (Cation)+ + e. Lio (g) Li+(g) + e. Cao(g) Ca 2+(g) + 2 e. Alo(g) Al 3+(g) + 3 e-

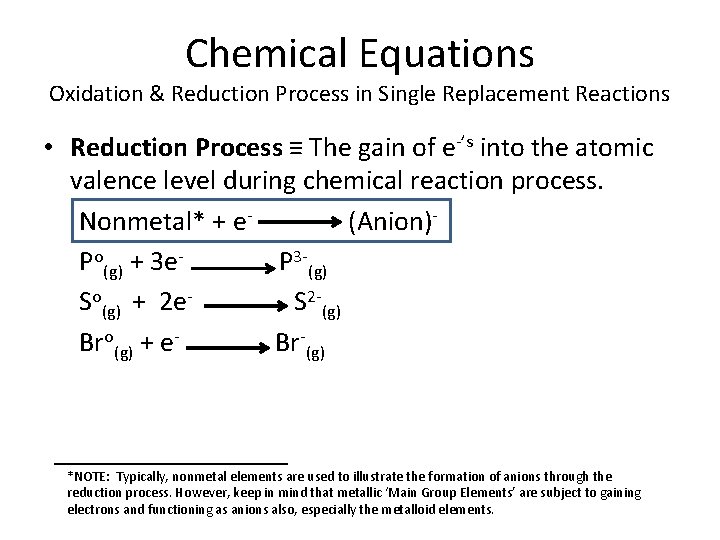
Chemical Equations Oxidation & Reduction Process in Single Replacement Reactions • Reduction Process ≡ The gain of e-’s into the atomic valence level during chemical reaction process. Nonmetal* + e(Anion)Po(g) + 3 e. P 3 -(g) So(g) + 2 e. S 2 -(g) Bro(g) + e. Br-(g) *NOTE: Typically, nonmetal elements are used to illustrate the formation of anions through the reduction process. However, keep in mind that metallic ‘Main Group Elements’ are subject to gaining electrons and functioning as anions also, especially the metalloid elements.


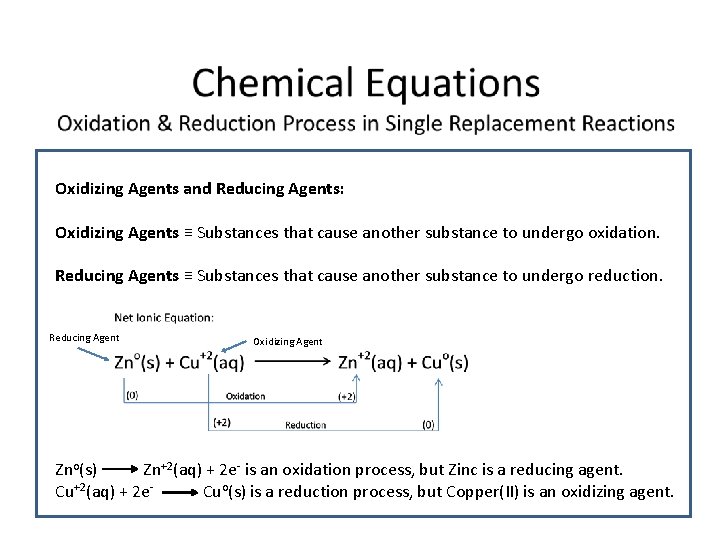
Oxidizing Agents and Reducing Agents: Oxidizing Agents ≡ Substances that cause another substance to undergo oxidation. Reducing Agents ≡ Substances that cause another substance to undergo reduction. Reducing Agent Oxidizing Agent Zno(s) Zn+2(aq) + 2 e- is an oxidation process, but Zinc is a reducing agent. Cu+2(aq) + 2 e. Cuo(s) is a reduction process, but Copper(II) is an oxidizing agent.

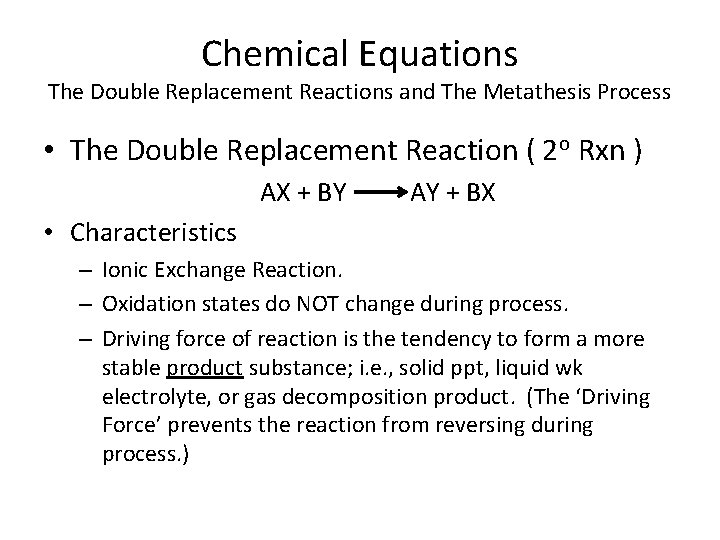
Chemical Equations The Double Replacement Reactions and The Metathesis Process • The Double Replacement Reaction ( 2 o Rxn ) AX + BY AY + BX • Characteristics – Ionic Exchange Reaction. – Oxidation states do NOT change during process. – Driving force of reaction is the tendency to form a more stable product substance; i. e. , solid ppt, liquid wk electrolyte, or gas decomposition product. (The ‘Driving Force’ prevents the reaction from reversing during process. )

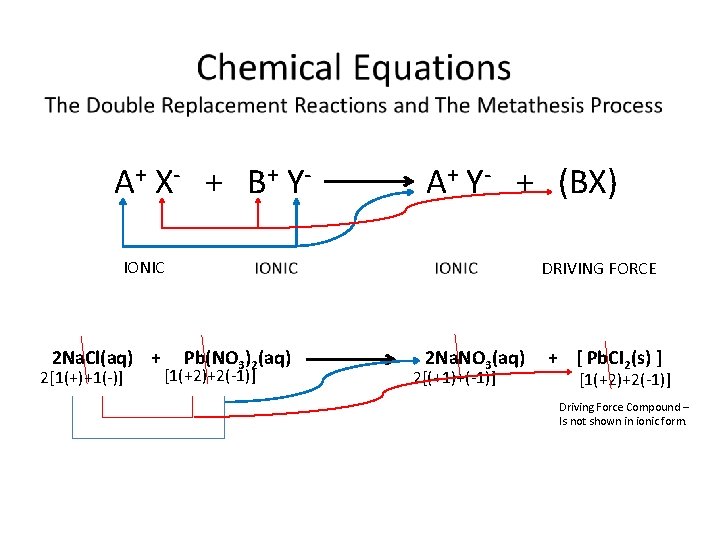
A + X- + B + Y - A+ Y- + (BX) IONIC 2 Na. Cl(aq) + 2[1(+)+1(-)] DRIVING FORCE Pb(NO 3)2(aq) [1(+2)+2(-1)] 2 Na. NO 3(aq) 2[(+1)+(-1)] + [ Pb. CI 2(s) ] [1(+2)+2(-1)] Driving Force Compound – Is not shown in ionic form.

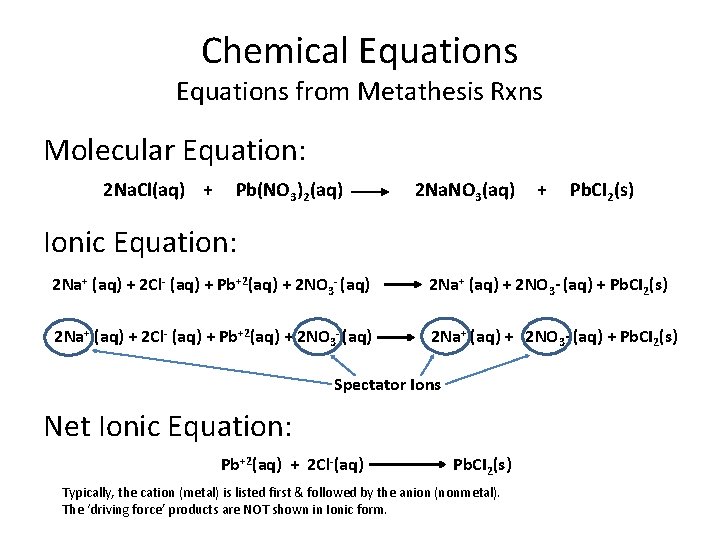
Chemical Equations from Metathesis Rxns Molecular Equation: 2 Na. Cl(aq) + Pb(NO 3)2(aq) 2 Na. NO 3(aq) + Pb. CI 2(s) Ionic Equation: 2 Na+ (aq) + 2 Cl- (aq) + Pb+2(aq) + 2 NO 3 - (aq) 2 Na+ (aq) + 2 NO 3 - (aq) + Pb. CI 2(s) Spectator Ions Net Ionic Equation: Pb+2(aq) + 2 Cl-(aq) Pb. CI 2(s) Typically, the cation (metal) is listed first & followed by the anion (nonmetal). The ‘driving force’ products are NOT shown in Ionic form.

• Predict the products resulting from mixing the following ionic compounds. Write and balance – The molecular equation – The ionic equation – The net ionic equation • Sodium Phosphate + Calcium Nitrate >>>> ?
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review I intro
I intro Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Section 1 chemical changes
Section 1 chemical changes Toxic reactions chemical equations lesson 68 answers
Toxic reactions chemical equations lesson 68 answers Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Toxic reactions chemical equations
Toxic reactions chemical equations How to write a balanced redox reaction
How to write a balanced redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
Si unit of newton's first law Reactants and products
Reactants and products Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Limiting reactant lab report answers
Limiting reactant lab report answers What is released or absorbed whenever chemical
What is released or absorbed whenever chemical