Characterization Classification Long range ordering periodicity unit cell
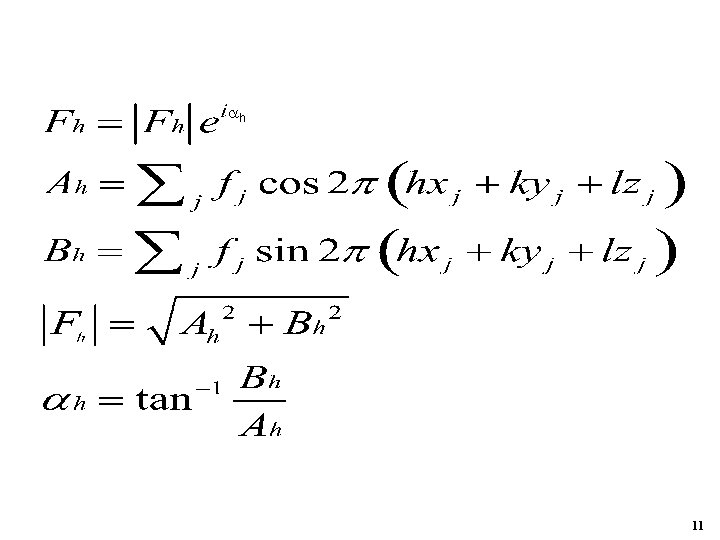

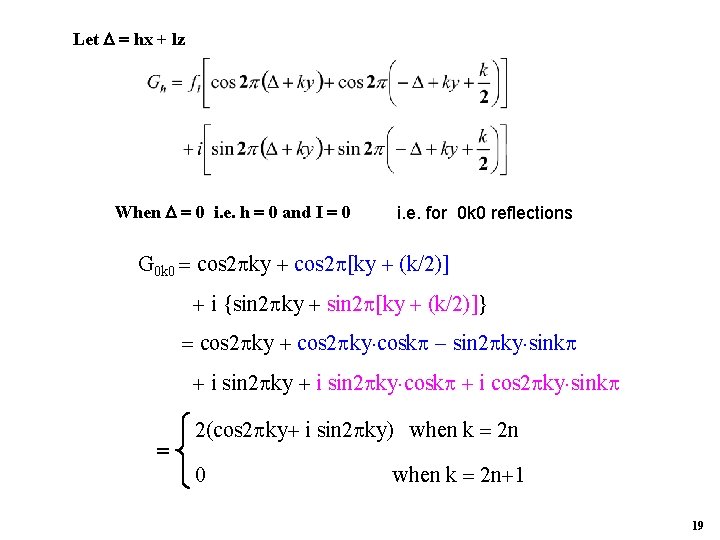


- Slides: 33

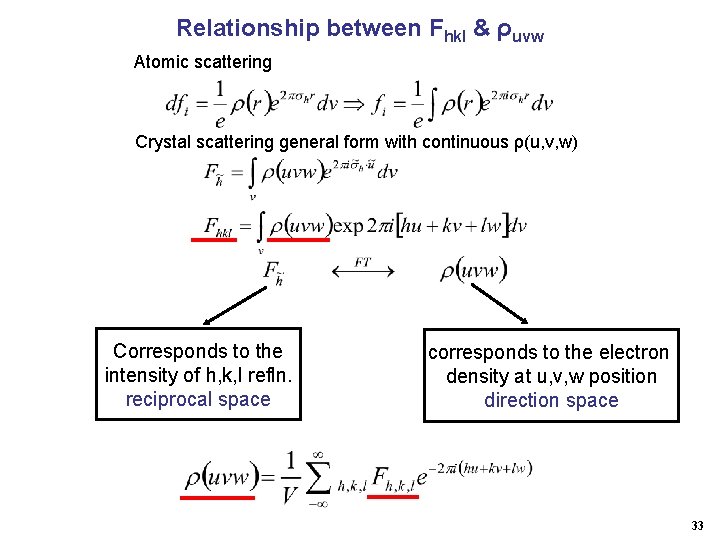
Characterization: Classification: Long range ordering periodicity unit cell Symmetry 7 crystal systems 230 space groups Structural information: Unit cell Miller indices (h, k, l) d spacing Relative intensity atomic positions 1

X-ray Crystallography Introduction Crystal Diffraction Structure · Structure Amplitudes, Fhkl · Atomic scattering factors · Fourier transfer Fhkl (x, y, z) · Least squares refinement · Structure properties --- Distance / angles ; packing etc. · Structure data base 2

Structural Analysis: Data measurement: h, k, l, dhkl , nhkl , Ihkl ( F 2 hkl ) Phase determination heavy atom method; direct method; multiple scattering Fourier transformation: F 2 (r); reciprocal real space Least squares refinement: (r) xi, yi, zi, ui Structural model: bond distances; bond angles; atomic thermal vibration etc. 3

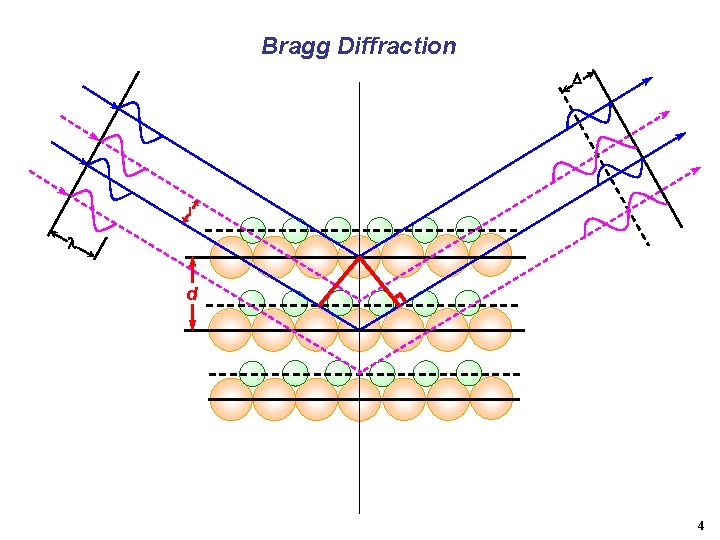
Bragg Diffraction l d 4

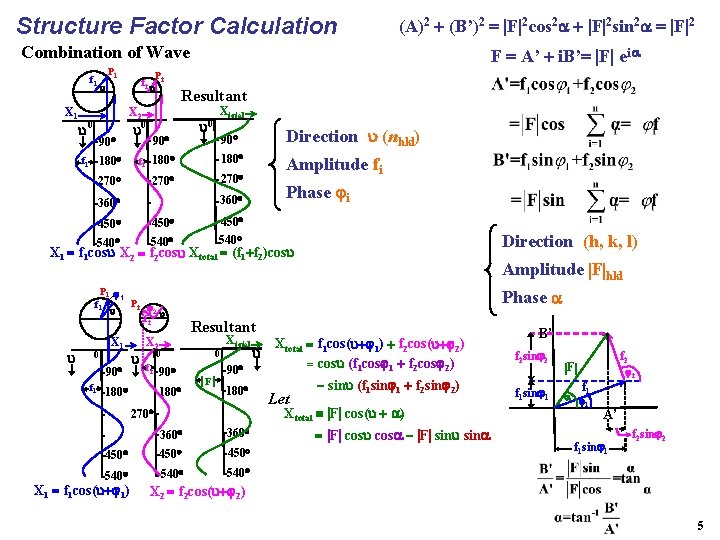
Structure Factor Calculation (A)2 (B’)2 F 2 cos 2 F 2 sin 2 F 2 Combination of Wave P 1 f 1 X 1 f 2 P 2 Resultant X 2 0 90 180 f 1 270 Xtotal 0 0 f 2 F A’ i. B’ F ei 90 180 270 Direction (nhkl) Amplitude fi Phase i 360 450 540 Direction (h, k, l) X 1 f 1 cos X 2 f 2 cos Xtotal (f 1 f 2)cos P 1 1 P 2 f 1 2 f 2 0 X 1 90 f 1 X 2 90 180 Phase Resultant 0 0 f 2 F Xtotal 90 180 Xtotal f 1 cos( 1) f 2 cos( 2) cos (f 1 cos 1 f 2 cos 2) Let sin (f 1 sin 1 f 2 sin 2) Xtotal F cos( ) 270 360 450 540 X 1 f 1 cos( 1) Amplitude F hkl F cos F sin B’ f 2 sin 2 f 1 sin 1 f 2 F 2 f 1 1 A’ f 1 sin 1 f 2 sin 2 X 2 f 2 cos( 2) 5

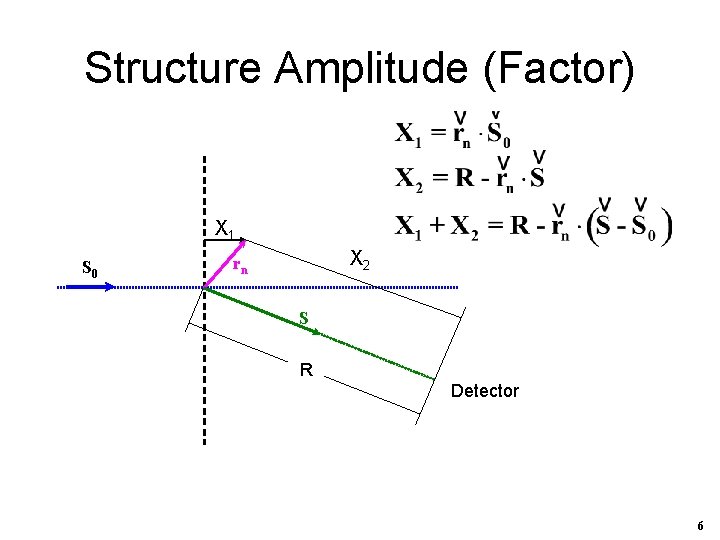
Structure Amplitude (Factor) X 1 S 0 X 2 rn S R Detector 6

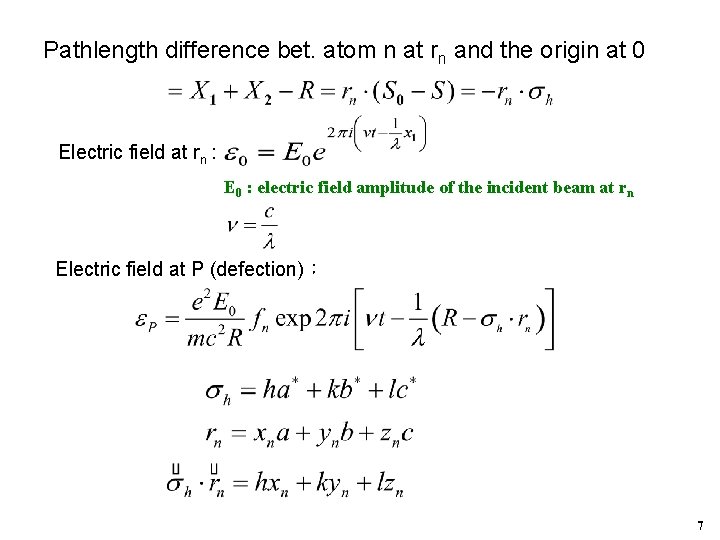
Pathlength difference bet. atom n at rn and the origin at 0 Electric field at rn : E 0 : electric field amplitude of the incident beam at rn Electric field at P (defection): 7

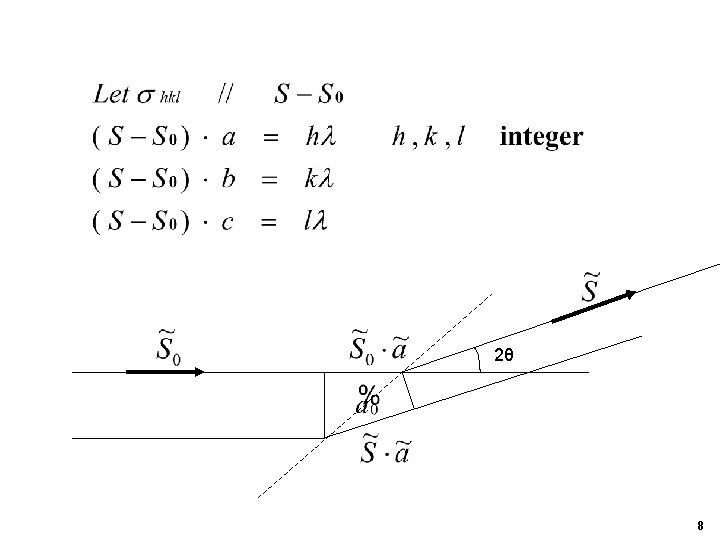
2θ 8

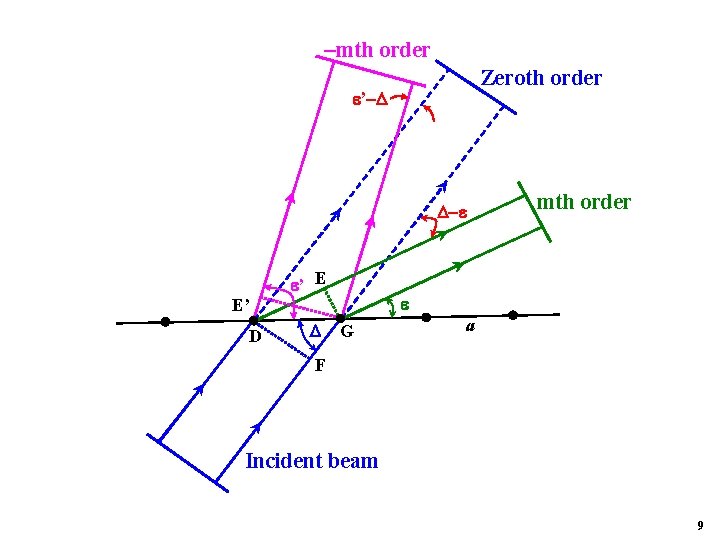
mth order Zeroth order ’ E E’ D mth order G a F Incident beam 9

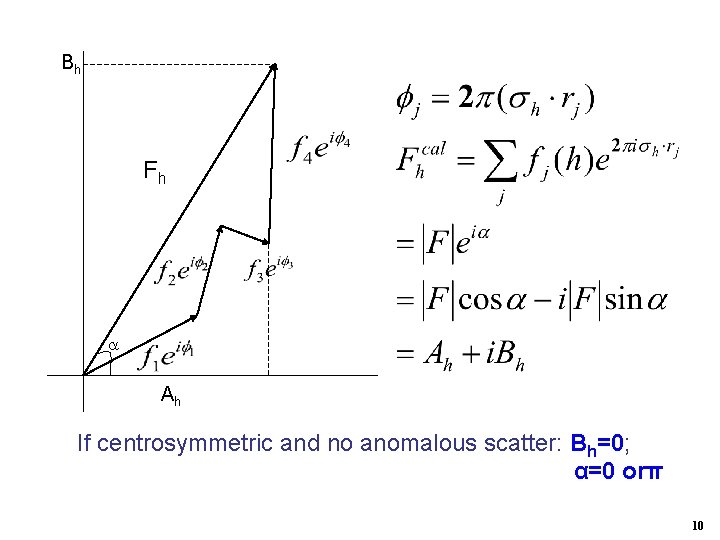
Bh Fh Ah If centrosymmetric and no anomalous scatter: Bh=0; α=0 orπ 10
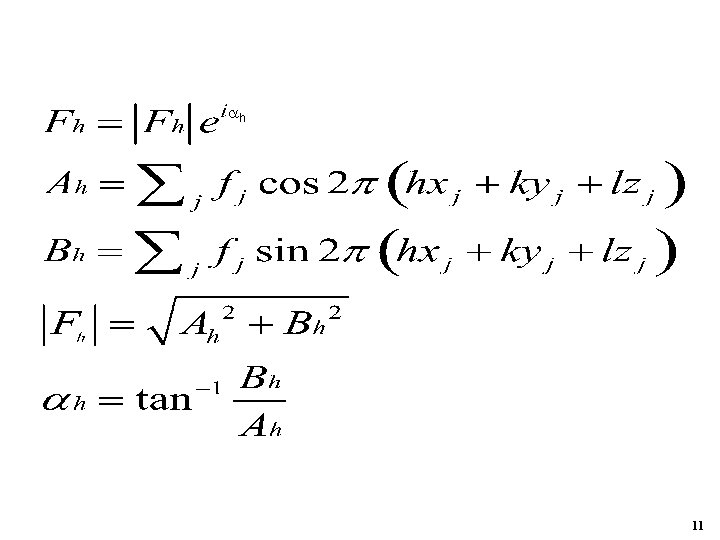
h 11

Centric case with non-anomalous scatterers Z pt. charge B=0 B>0 atomic sphere (fixed atom at ri) T=0 K with thermal vibration T as function of B : Thermal parameter 12

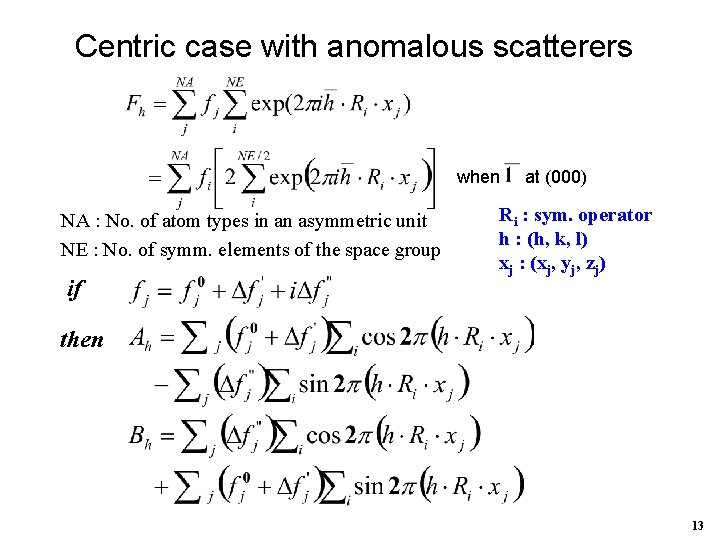
Centric case with anomalous scatterers when NA : No. of atom types in an asymmetric unit NE : No. of symm. elements of the space group if at (000) Ri : sym. operator h : (h, k, l) xj : (xj, yj, zj) then 13

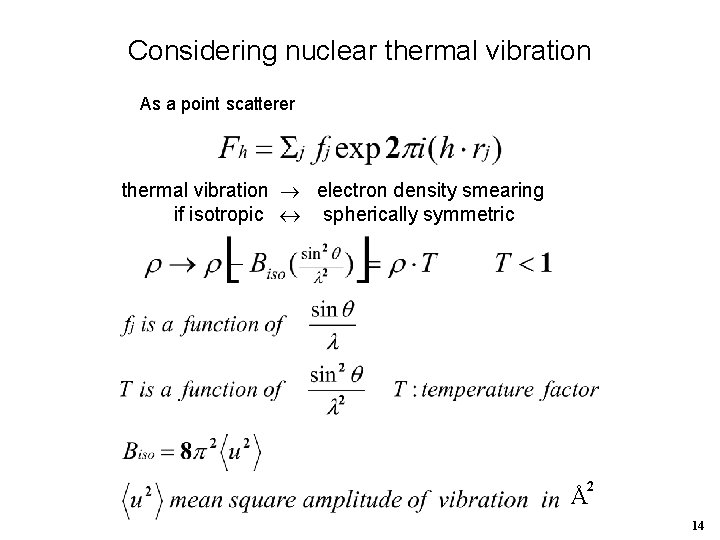
Considering nuclear thermal vibration As a point scatterer thermal vibration electron density smearing if isotropic spherically symmetric 2 Å 14

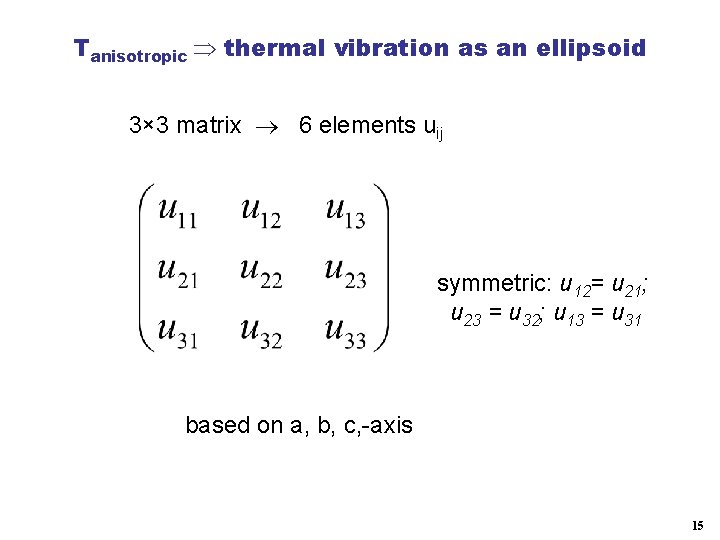
Tanisotropic thermal vibration as an ellipsoid 3× 3 matrix 6 elements uij symmetric: u 12= u 21; u 23 = u 32; u 13 = u 31 based on a, b, c, -axis 15

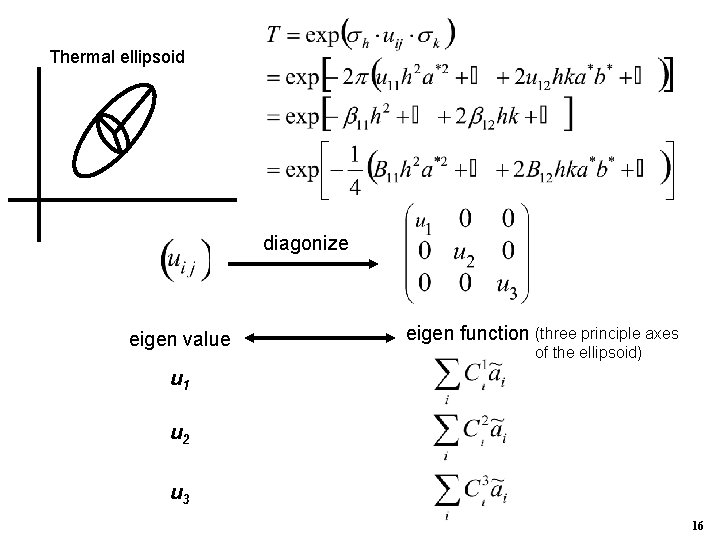
Thermal ellipsoid diagonize eigen value eigen function (three principle axes of the ellipsoid) u 1 u 2 u 3 16

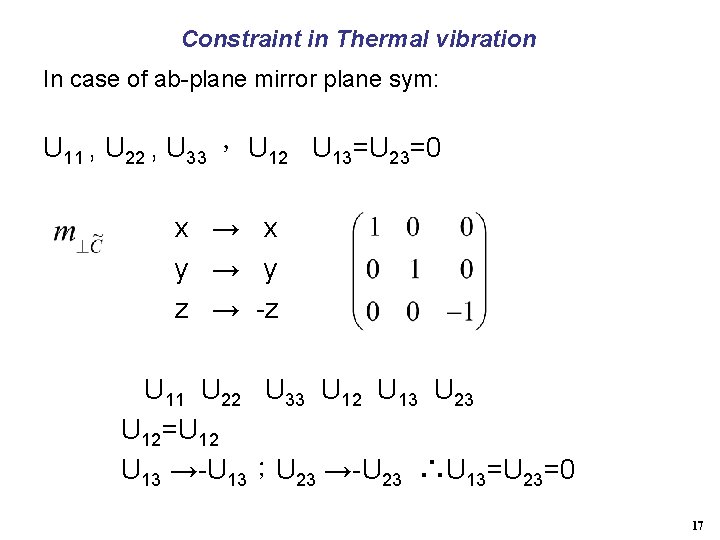
Constraint in Thermal vibration In case of ab-plane mirror plane sym: U 11 , U 22 , U 33 , U 12 U 13=U 23=0 x → x y → y z → -z U 11 U 22 U 33 U 12 U 13 U 23 U 12=U 12 U 13 →-U 13;U 23 →-U 23 ∴U 13=U 23=0 17

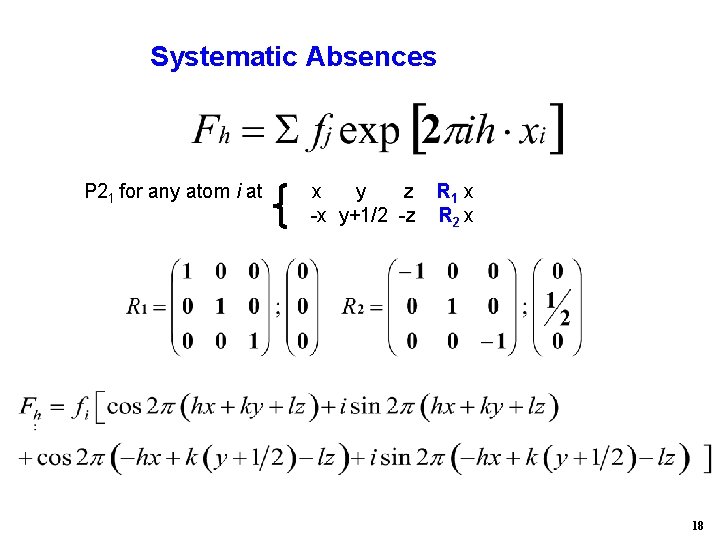
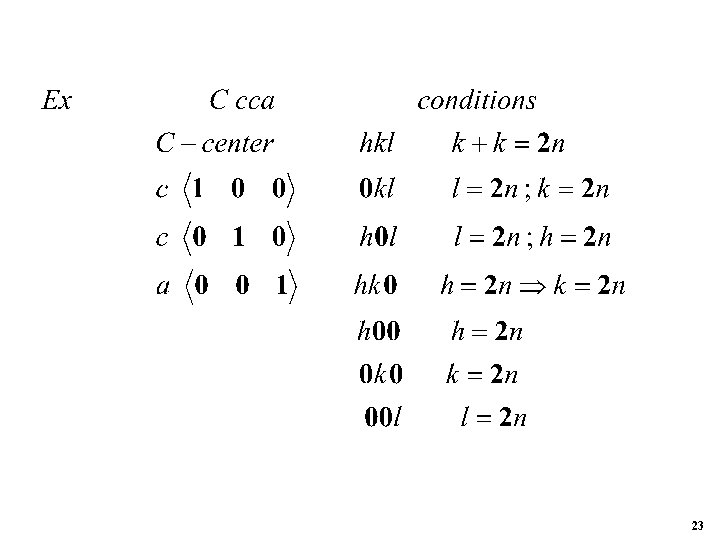
Systematic Absences P 21 for any atom i at x y z -x y+1/2 -z R 1 x R 2 x 18
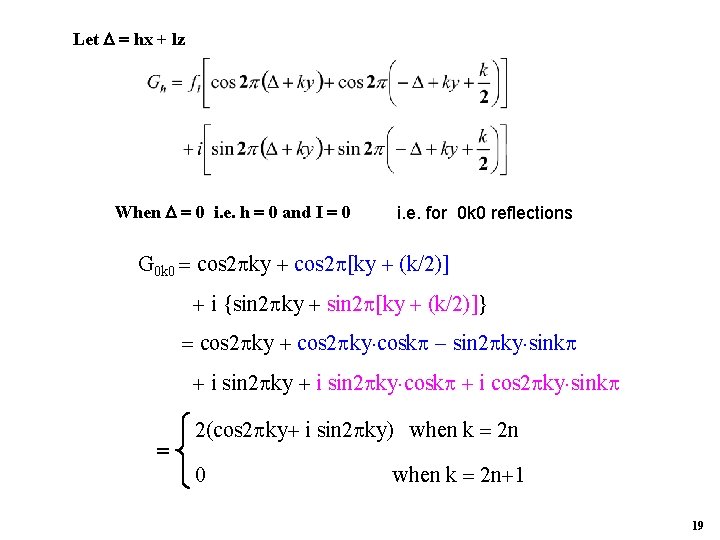
Let hx + lz When 0 i. e. h 0 and I 0 i. e. for 0 k 0 reflections G 0 k 0 cos 2 ky cos 2 [ky (k/2)] i {sin 2 ky sin 2 [ky (k/2)]} cos 2 ky cosk sin 2 ky sink i sin 2 ky cosk i cos 2 ky sink 2(cos 2 ky i sin 2 ky) when k 2 n 0 when k 2 n 1 19

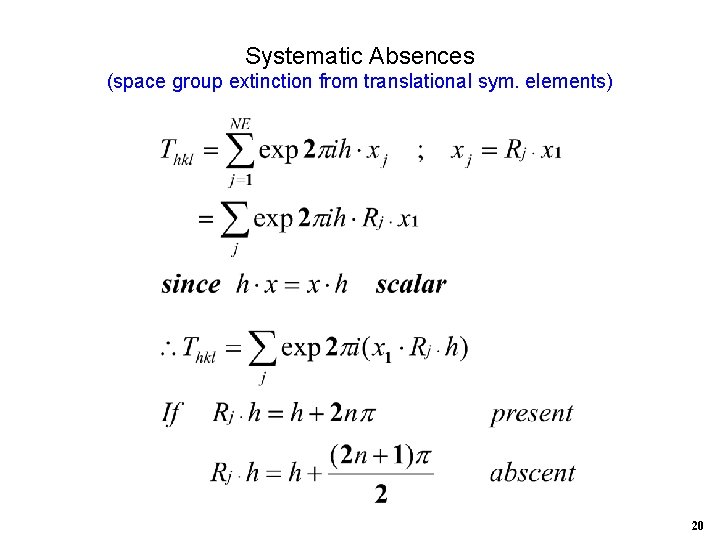
Systematic Absences (space group extinction from translational sym. elements) 20

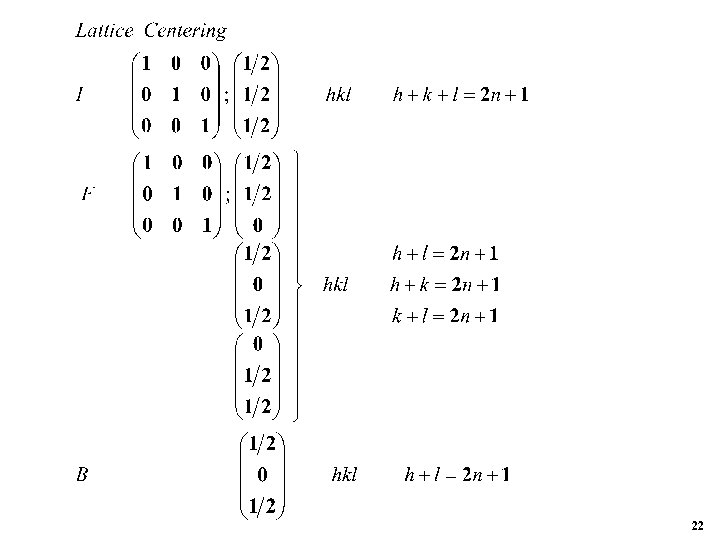
21

22

23

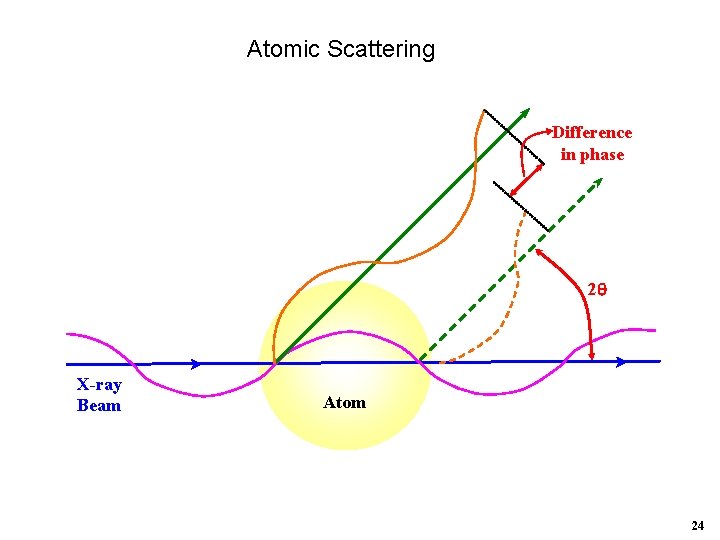
Atomic Scattering Difference in phase 2 X-ray Beam Atom 24

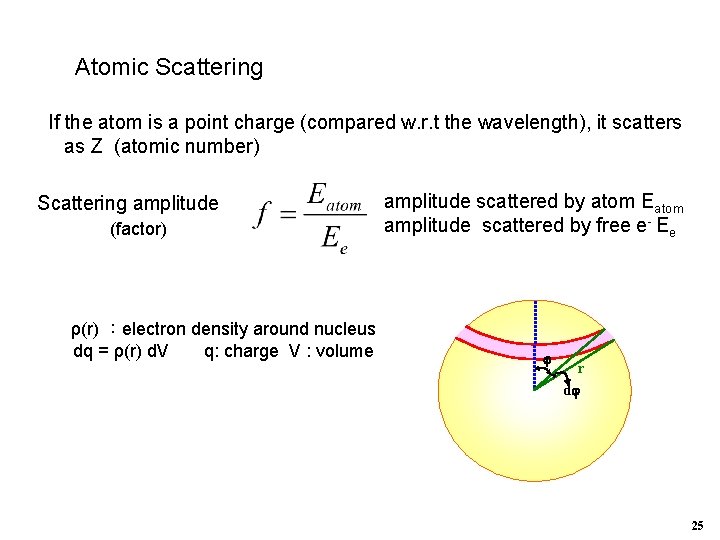
Atomic Scattering If the atom is a point charge (compared w. r. t the wavelength), it scatters as Z (atomic number) Scattering amplitude (factor) ρ(r) :electron density around nucleus dq = ρ(r) d. V q: charge V : volume amplitude scattered by atom Eatom amplitude scattered by free e- Ee r d 25

26

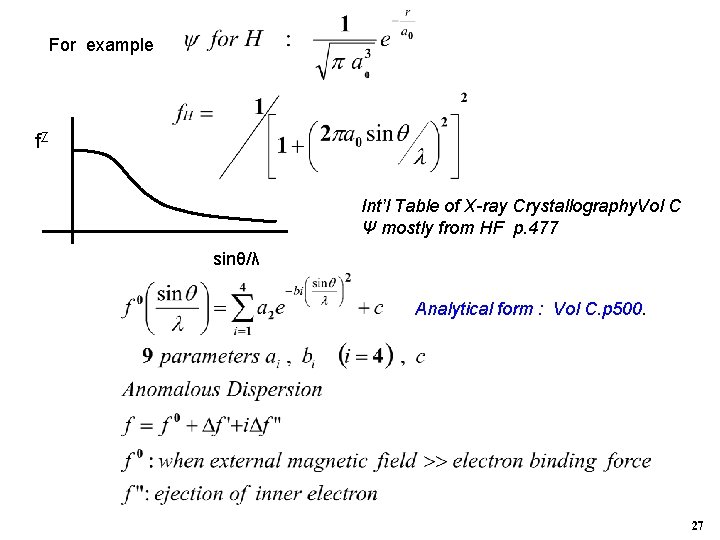
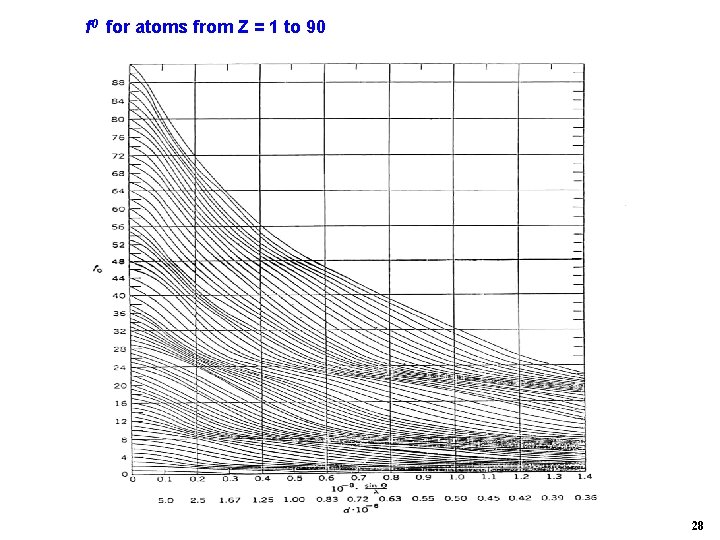
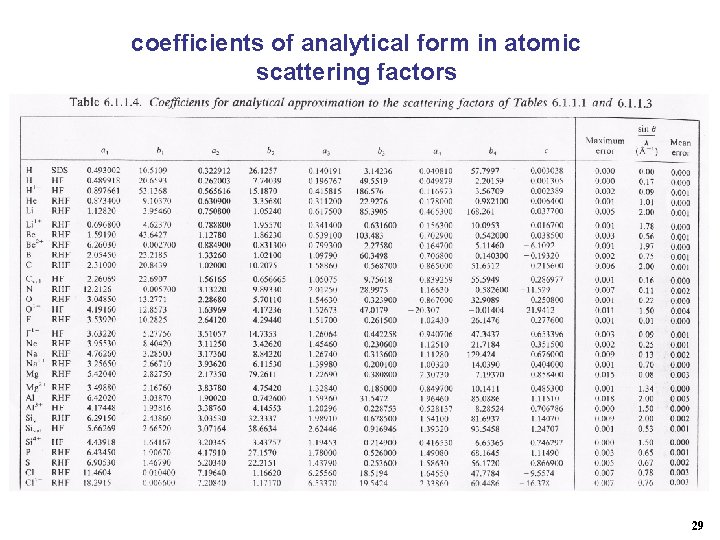
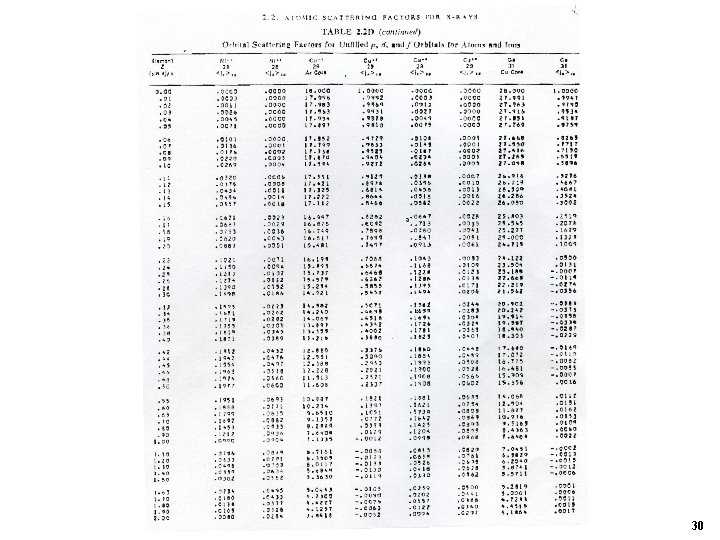
For example f. Z Int’l Table of X-ray Crystallography. Vol C Ψ mostly from HF p. 477 sinθ/λ Analytical form : Vol C. p 500. 27

f 0 for atoms from Z = 1 to 90 28

coefficients of analytical form in atomic scattering factors 29

30

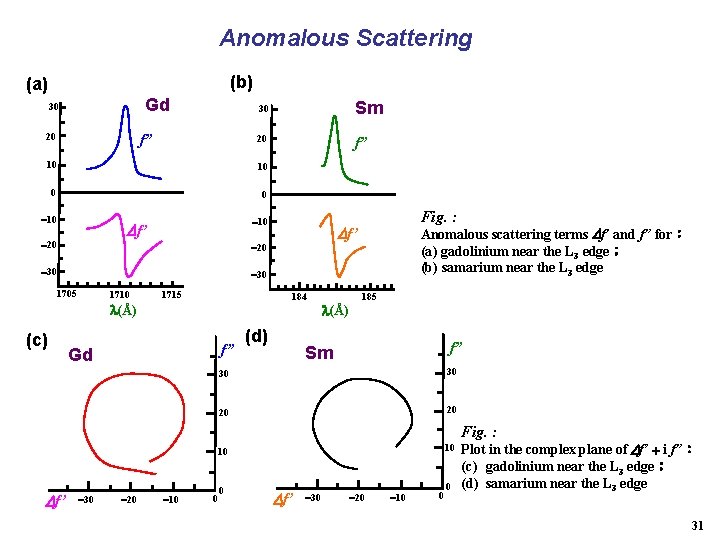
Anomalous Scattering (b) (a) Gd 30 20 f” 30 Sm 20 f” 10 10 0 0 10 f’ 20 30 Fig. : Anomalous scattering terms f’ and f” for: (a) gadolinium near the L 3 edge; (b) samarium near the L 3 edge 30 1705 1710 1715 184 (Å) (c) f” Gd (d) (Å) 185 f” Sm 30 30 20 20 10 10 Fig. : f’ 30 20 10 0 0 Plot in the complex plane of f’ i f”: (c) gadolinium near the L 3 edge; (d) samarium near the L 3 edge 31

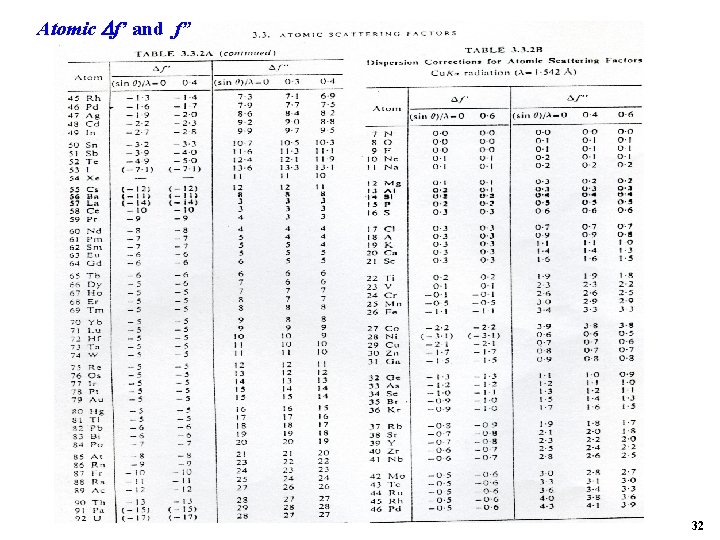
Atomic f’ and f” 32

Relationship between Fhkl & ρuvw Atomic scattering Crystal scattering general form with continuous ρ(u, v, w) Corresponds to the intensity of h, k, l refln. reciprocal space corresponds to the electron density at u, v, w position direction space 33