Chapter 9 RXNS EQNS Chemical Rxns Representing Chemical


















- Slides: 26

Chapter 9 RXNS & EQNS

Chemical Rxns

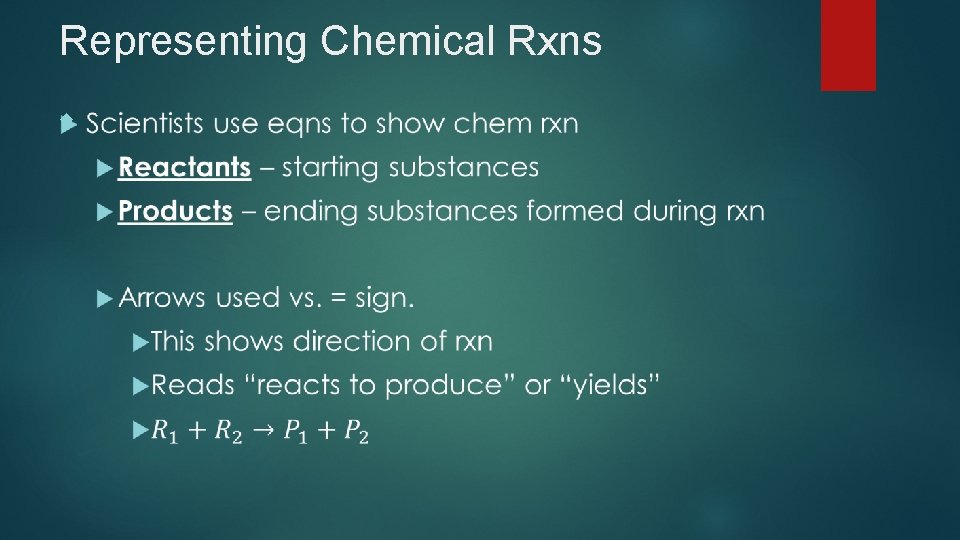
Representing Chemical Rxns

Representing Chemical Rxns Symbols used to show physical states (s) – solid (l) – liquid (g) – gas (aq) - aqueous When dissolved in water, said to be aqueous **Very important to show physical states of rxns reactants and products. These give clues about how rxn occurs!

Representing Chemical Rxns

Representing Chemical Rxns

Balancing Chem Eqns To balance eqns, must have correct coefficients in skeleton eqn Coefficients must be whole #’s describe lowest whole # ratio of amounts of all reactants and products

Balancing Chem Eqns Steps for balancing eqns: 1. Write skeleton eqn 2. Counts atoms in reactants 3. Count atoms in products 4. Change coefficient to make # atoms = 5. Write coefficients in lowest ratio 6. Double check work!!!

Example 1

Example 2

9. 2 – Classifying Chem Rxns

Types of Chem Rxns Classify chem rxns to organize the different rxns that occur daily Helps 4 give a better understanding of what is going on Types: Synthesis Combustion Decomposition Replacement

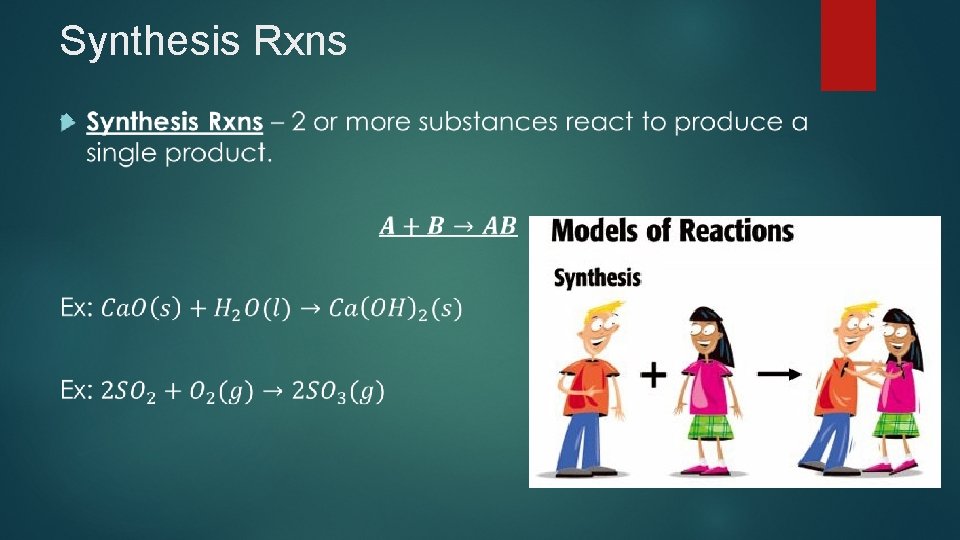
Synthesis Rxns

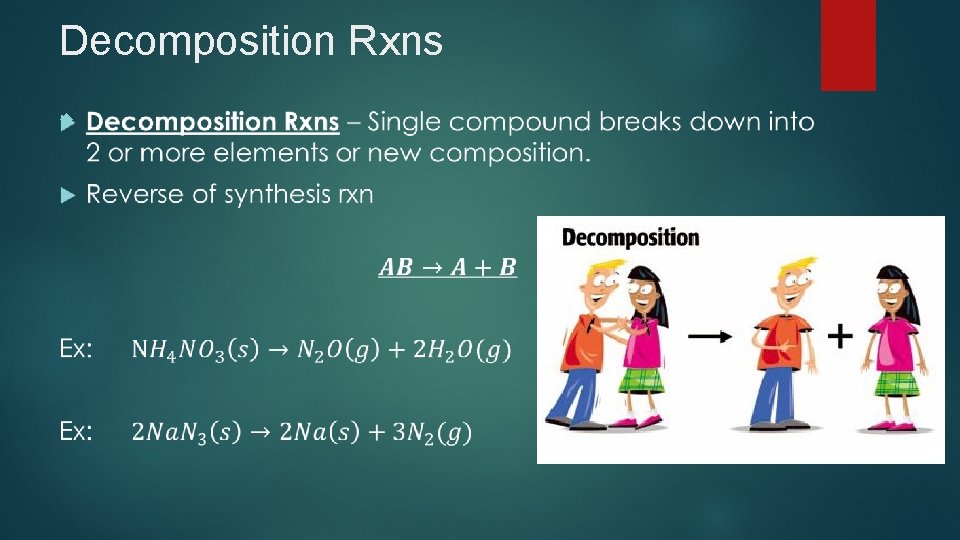
Decomposition Rxns

Combustion Rxns

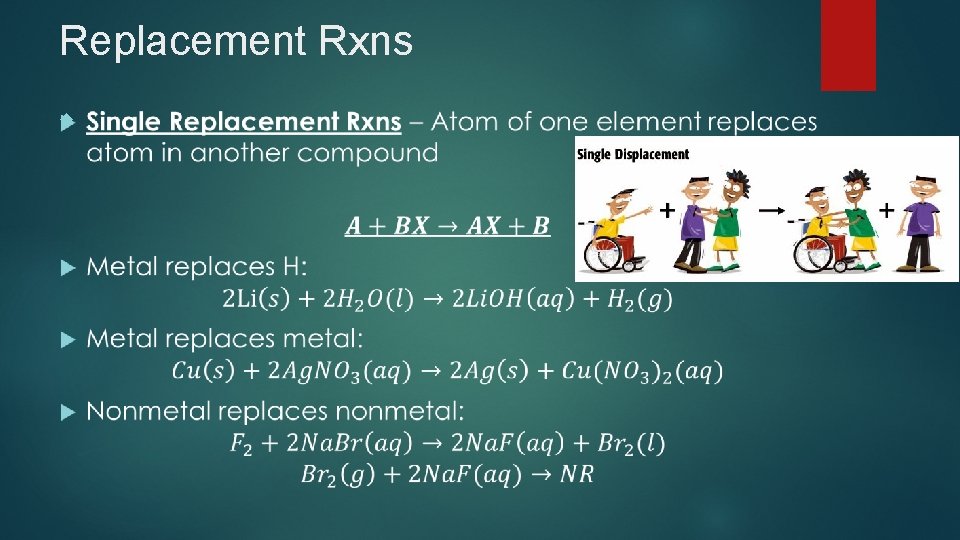
Replacement Rxns Replacement 2 Rxns – Replaces element(s) in compound Types Replacement Rxns: Single Replacement Double Replacement

Replacement Rxns

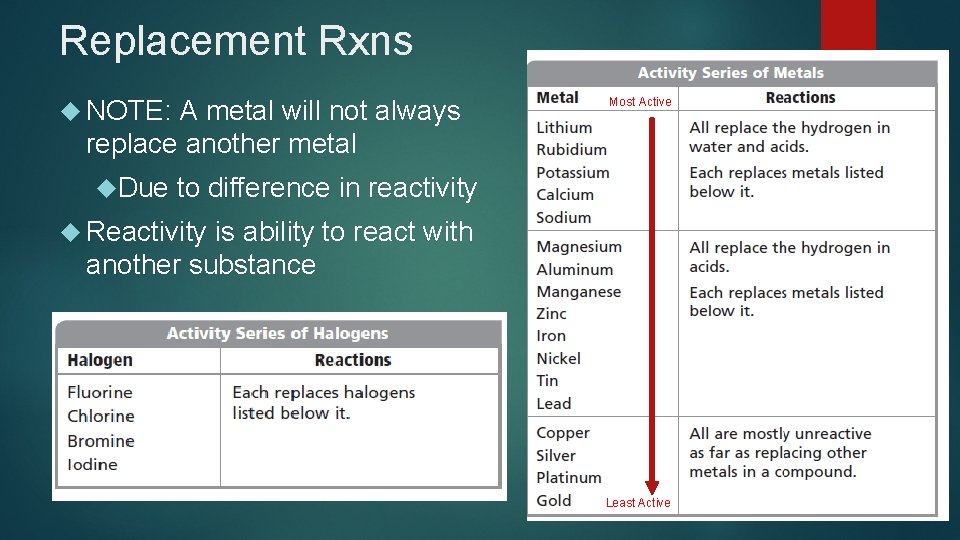
Replacement Rxns NOTE: A metal will not always replace another metal Due Most Active to difference in reactivity Reactivity is ability to react with another substance Least Active

Example

Single Replacement Example https: //youtu. be/PNa. Zqn. Fw. IIQ

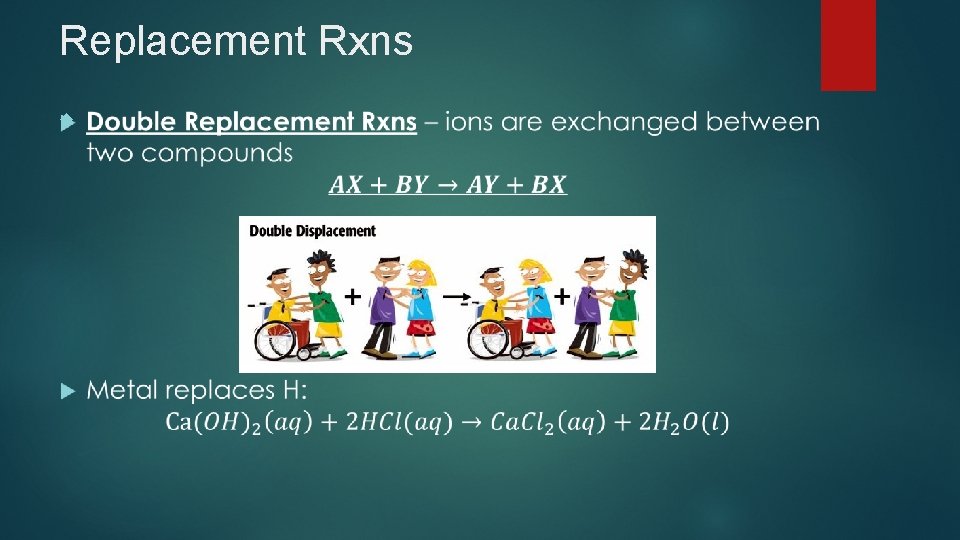
Replacement Rxns

Replacement Rxns

Writing Double Replacement Rxns

Writing Double Replacement Rxns

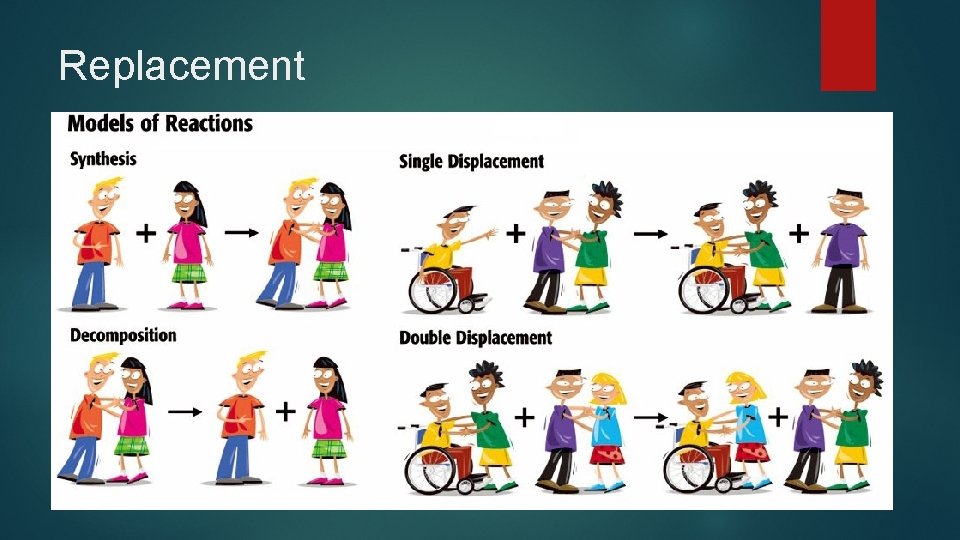
Replacement

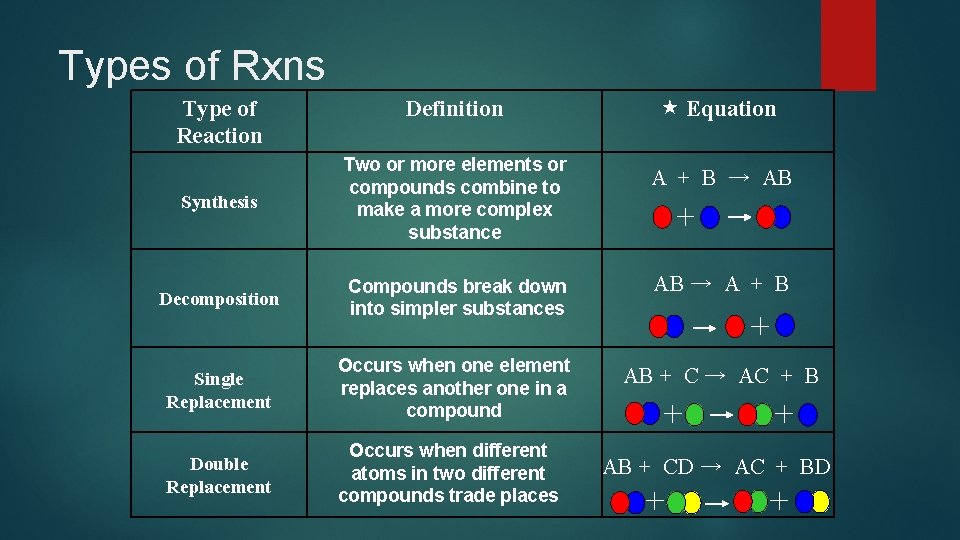
Types of Rxns Type of Reaction Definition Equation Synthesis Two or more elements or compounds combine to make a more complex substance Decomposition Compounds break down into simpler substances AB → A + B Single Replacement Occurs when one element replaces another one in a compound AB + C → AC + B Double Replacement Occurs when different atoms in two different compounds trade places A + B → AB AB + CD → AC + BD
 Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry Rxns chemistry
Rxns chemistry Rxns
Rxns Chapter 2 assessment physics answers
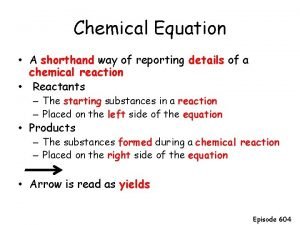
Chapter 2 assessment physics answers A chemist shorthand way of representing chemical reaction
A chemist shorthand way of representing chemical reaction A shorthand way to represent a molecule
A shorthand way to represent a molecule Chemist shorthand way of representing chemical reaction.
Chemist shorthand way of representing chemical reaction. Chapter 2 representing motion
Chapter 2 representing motion What is the purpose of drawing a motion diagram
What is the purpose of drawing a motion diagram Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Trinitrogen monosulfide formula
Trinitrogen monosulfide formula Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of speech style
Types of speech style Statistic vs parameter example
Statistic vs parameter example Representing identity in information security
Representing identity in information security Representing comparing and ordering decimals
Representing comparing and ordering decimals Vector arrows representing the vx and vy
Vector arrows representing the vx and vy Hsieh ho merged six cannons into japanese floral design
Hsieh ho merged six cannons into japanese floral design Lesson 6.1 representing functions answer key
Lesson 6.1 representing functions answer key Animal that symbolizes chaos
Animal that symbolizes chaos Representing motion
Representing motion Representing knowledge using rules
Representing knowledge using rules Symmetric relation
Symmetric relation Lesson 3 representing proportional relationships
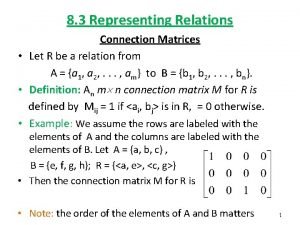
Lesson 3 representing proportional relationships Representing relations using digraphs
Representing relations using digraphs A function can be represented by
A function can be represented by Representing knowledge in an uncertain domain
Representing knowledge in an uncertain domain