Redox Rxns Part II Common Redox Rxns Chapter














- Slides: 28

Redox Rxns: Part II: Common Redox Rxns Chapter 5 Sec 3, 4 & 5 of Jespersen 7 TH ed) Note: We skip Sec 5. 2 this semester. It is covered in Gen Chem II. Dr. C. Yau Spring 2015 1

Redox Rxns Review: Redox Rxns involve a transfer of electrons. Oxidation is INCREASE in oxidation number. e. g. 2 KCl Cl 2 Cl has lost one e- each. -1 0 Reduction is DECREASE in oxidation number e. g. Mn. O 2 Mn. SO 4 +4 +2 Mn has gained 2 e- each. 2

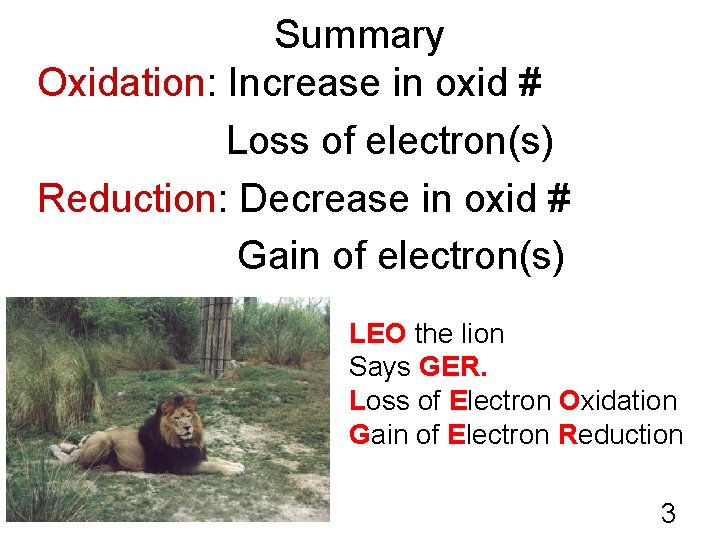
Summary Oxidation: Increase in oxid # Loss of electron(s) Reduction: Decrease in oxid # Gain of electron(s) LEO the lion Says GER. Loss of Electron Oxidation Gain of Electron Reduction 3

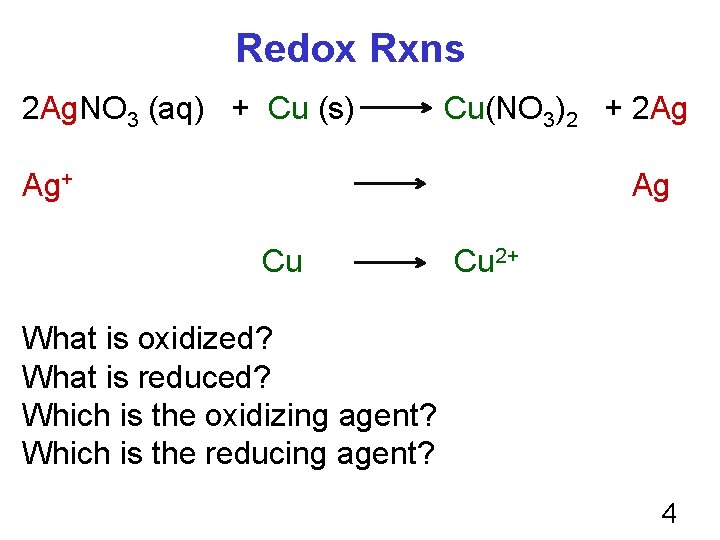
Redox Rxns 2 Ag. NO 3 (aq) + Cu (s) Cu(NO 3)2 + 2 Ag Ag+ Ag Cu Cu 2+ What is oxidized? What is reduced? Which is the oxidizing agent? Which is the reducing agent? 4

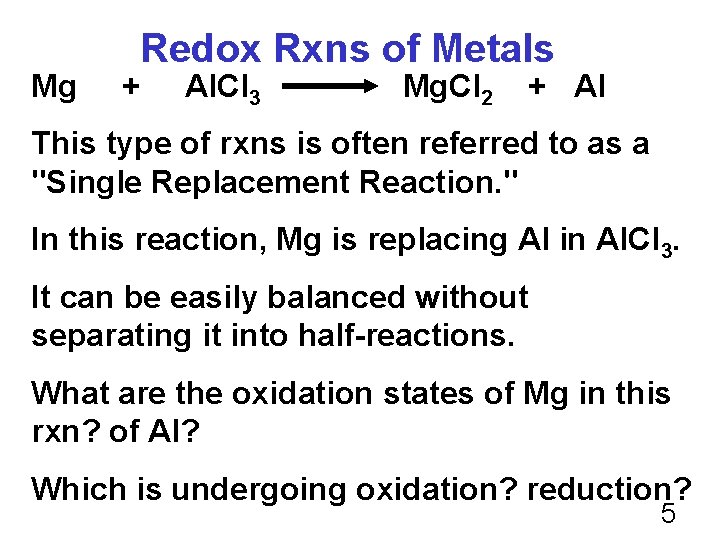
Mg Redox Rxns of Metals + Al. Cl 3 Mg. Cl 2 + Al This type of rxns is often referred to as a "Single Replacement Reaction. " In this reaction, Mg is replacing Al in Al. Cl 3. It can be easily balanced without separating it into half-reactions. What are the oxidation states of Mg in this rxn? of Al? Which is undergoing oxidation? reduction? 5

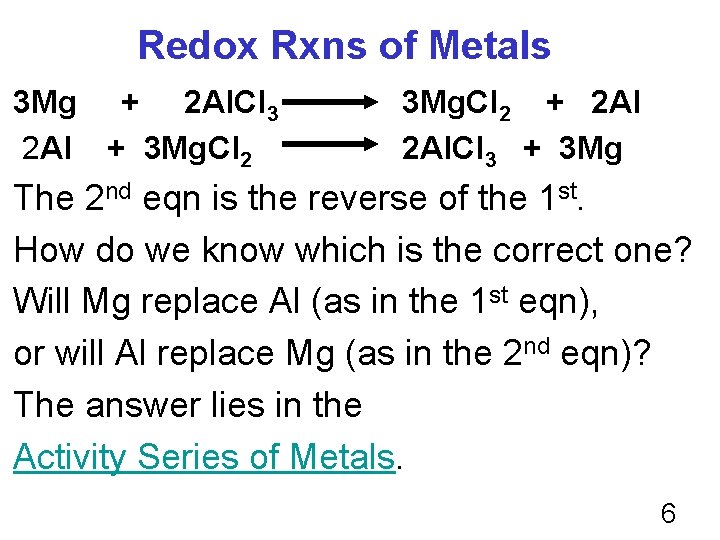
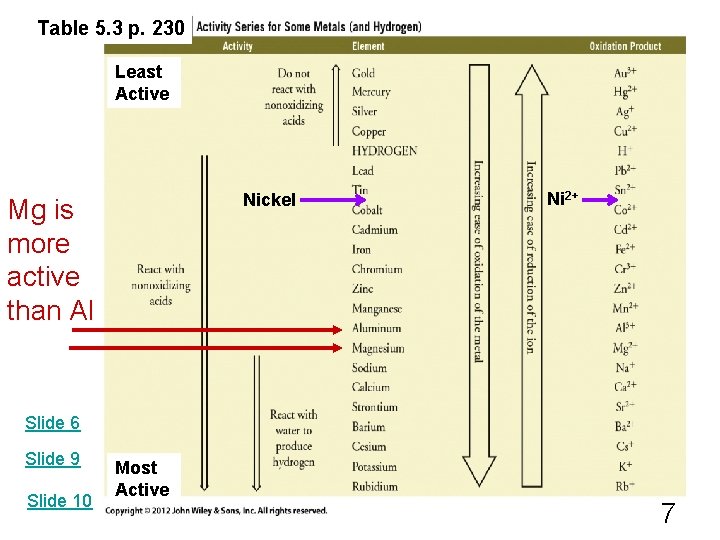
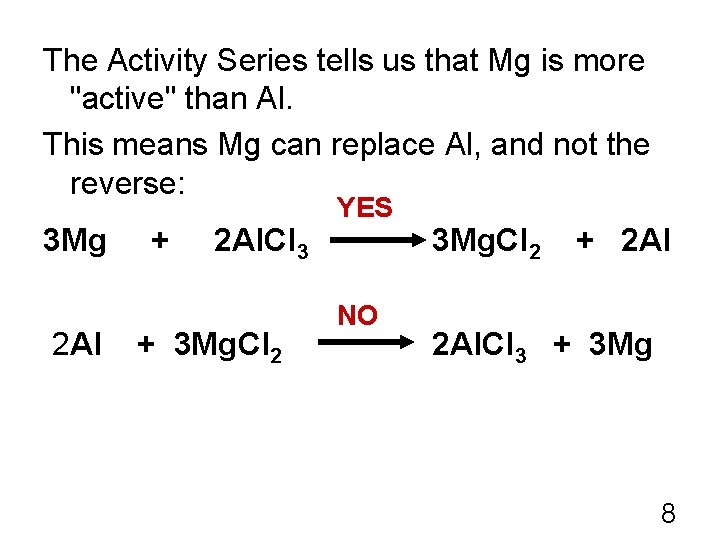
Redox Rxns of Metals 3 Mg + 2 Al. Cl 3 2 Al + 3 Mg. Cl 2 + 2 Al. Cl 3 + 3 Mg The 2 nd eqn is the reverse of the 1 st. How do we know which is the correct one? Will Mg replace Al (as in the 1 st eqn), or will Al replace Mg (as in the 2 nd eqn)? The answer lies in the Activity Series of Metals. 6

Table 5. 3 p. 230 Least Active Nickel Mg is more active than Al Ni 2+ Slide 6 Slide 9 Slide 10 Most Active 7

The Activity Series tells us that Mg is more "active" than Al. This means Mg can replace Al, and not the reverse: 3 Mg 2 Al + 2 Al. Cl 3 + 3 Mg. Cl 2 YES NO 3 Mg. Cl 2 + 2 Al. Cl 3 + 3 Mg 8

Example 5. 6 p. 231 What will happen if an iron nail is dipped into a solution containing copper(II) sulfate? What is the molecular equation? What happens if an iron nail is dipped into a solution of aluminum sulfate? What is the molecular equation? Do Pract Exer 13 & 14 on p. 233 Activity Series 9

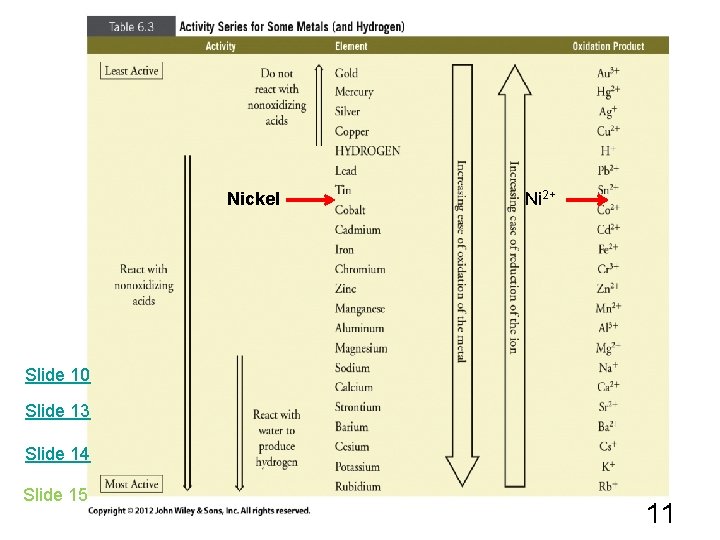
Redox Rxns of Metals with Water Note that in the Activity Series, H is included even though it is not a metal. H is used as a reference & is water is best viewed as HOH. The most active metals will react with HOH as liquid or steam, Na + HOH Na. OH + H 2 And the least active metals will not react with water. Al + H 2 O Pb + H 2 O Modified Activity Series ? ? 10

Nickel Ni 2+ Slide 10 Slide 13 Slide 14 Slide 15 11

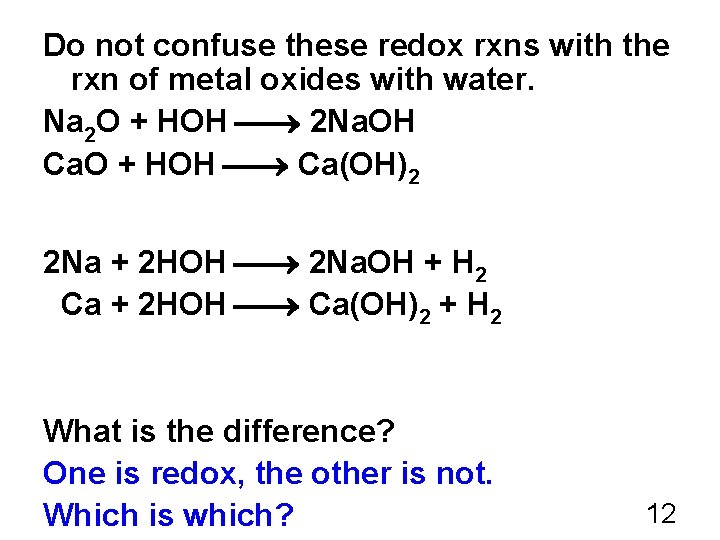
Do not confuse these redox rxns with the rxn of metal oxides with water. Na 2 O + HOH 2 Na. OH Ca. O + HOH Ca(OH)2 2 Na + 2 HOH 2 Na. OH + H 2 Ca + 2 HOH Ca(OH)2 + H 2 What is the difference? One is redox, the other is not. Which is which? 12

Redox Rxns of Metals with Acids The more reactive metals reduce H+ in acids to H 2. Again we need to consult the activity series. e. g. Zn + HCl Zn. Cl 2 + H 2 Cu + HCl ? Mn + HBr ? You will not be asked to memorize the activity series, but you are expected to know how to use it. Activity Series 13

Redox Rxns of Metals Write the equation for the reaction of aluminum with hydrobromic acid. Will it go? Is aluminum gaining or losing electrons? What is happening to the bromine in this reaction? Where are the electrons going to? Write the two balanced half-reactions. Which is the oxidizing agent in this rxn? Activity Series 14

Oxidizing and Nonoxidizing Acids (p. 226) Examples we have just gone through were of “nonoxidizing acids, ” where H+ is being reduced. HCl, HBr, HI, dilute H 2 SO 4 are such acids. Zn + H 2 SO 4 Zn. SO 4 + H 2 The H+ in all acids have the potential of oxidizing metals that are below H in the activity series. Oxidizing acids are acids with anions that also act as an oxidizing agent. Cu + 4 HNO 3 Cu(NO 3)2 + 2 NO 2 + 2 H 2 O Give the oxidation numbers of all the elements. 15

Oxidizing Acids Can React with Most Metals HNO 3: (TABLE 5. 2 p. 227) (conc) NO 3 - + 2 H+(aq) + e- → NO 2(g)+ H 2 O(l) (dil) NO 3 - (aq) + 4 H+(aq)+ 3 e- → NO(g) + 4 H 2 O(l) (v. dil): NO 3 - (aq) + 10 H+ + 8 e- → NH 4+ (aq) + 3 H 2 O(l) H 2 SO 4: (hot, conc. )SO 42 - + 4 H+(aq) + 3 e- → SO 2(g) +2 H 2 O(l) (hot, conc, with strong reducing agent) SO 42 - (aq) + 10 H+(aq) + 8 e- → H 2 S(g) + 4 H 2 O(l) You will not be asked to predict these reactions, but you should be able to recognize these are redox rxns by the change in oxidation numbers. 16

Molecular Oxygen as a Powerful Oxidizing Agent (Sec. 5. 5) You have previously learned that "burning" is always a rxn with O 2. These are redox rxns with O 2 as the oxidizing agent. CH 4 + 2 O 2 CO 2 + 2 H 2 O C 6 H 10 O 5 + O 2 CO 2 + H 2 O (C 6 H 10 O 5 =simplified formula for cellulose, the chief combustible ingredient in wood) Balance the equation. 17

Molecular Oxygen as a Powerful Oxidizing Agent Burning of organic compounds containing sulfur produces sulfur dioxide. 2 C 2 H 5 SH + 9 O 2 4 CO 2 + 6 H 2 O + 2 SO 2 is an air pollutant. Note that this is a "nonmetal oxide. " In the presence of rain, what does it become? Do Pract Exer 15 & 16 p. 236 18

O 2 as an Oxidizing Agent Many metals react with oxygen: Rusting of iron: 2 Fe + 3 O 2 (s) (g) Fe 2 O 3 (s) Fig. 5. 11 p. 237 Magnesium flares Mg (s) + O 2 (g) Do Pract Exer 17 & 18 p. 237 ? 19

Reaction with O 2 • O 2 is always the oxidizing agent. It becomes O 2 -. The products are the oxides of the each element in the reactant. • If the reaction is rapid and producing large amounts of heat, we call it combustion. • C 2 H 2 + O 2 CO 2 + H 2 O • Mg+ O 2 Mg. O + heat • If it is slow, it is usually considered “tarnishing” or “oxidation” of the metal. • Al + O 2 Al 2 O 3 20

What caused the explosion at the Fukushima nuclear power plant in Japan after the earthquake hit in 2011? It was NOT a nuclear explosion. . . luckily. 21

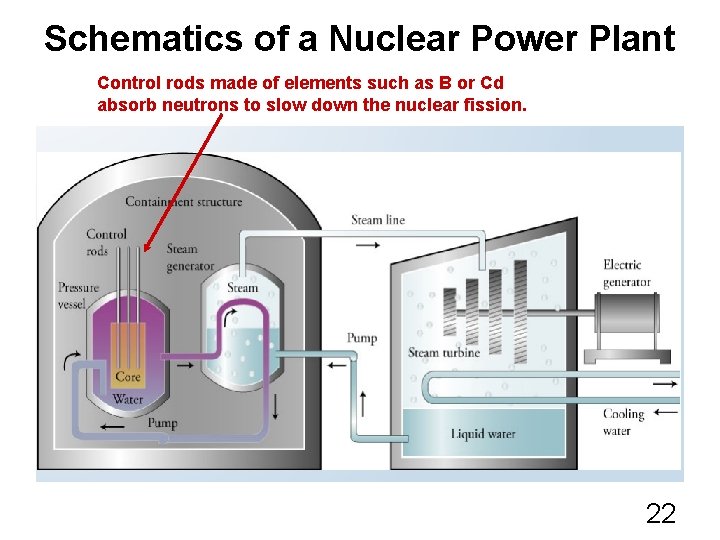
Schematics of a Nuclear Power Plant Control rods made of elements such as B or Cd absorb neutrons to slow down the nuclear fission. 22

What caused the explosion at the nuclear power plants in Japan? The earthquake did not damage the reactor containment structure, but the tsunami heavily damaged the cooling systems, all 15 backup cooling systems. Electricity is needed to pump in cooling water. Batteries lasted a few hours and new ones had to be brought in. Special pump used to pump in sea water ran out of gas. Sea water was no longer circulating and over heated. 2 H 2 O (g) 2 H 2 (g) + O 2 (g) 23

What kind of redox equations are you expected to be able to write and balance? Single replacement reactions such as: • Mn + Al. Cl 3 • Mn + H 2 O • Mn + HCl • In addition, you should be able to use the Activity Series to determine whether the above reactions will go. 24

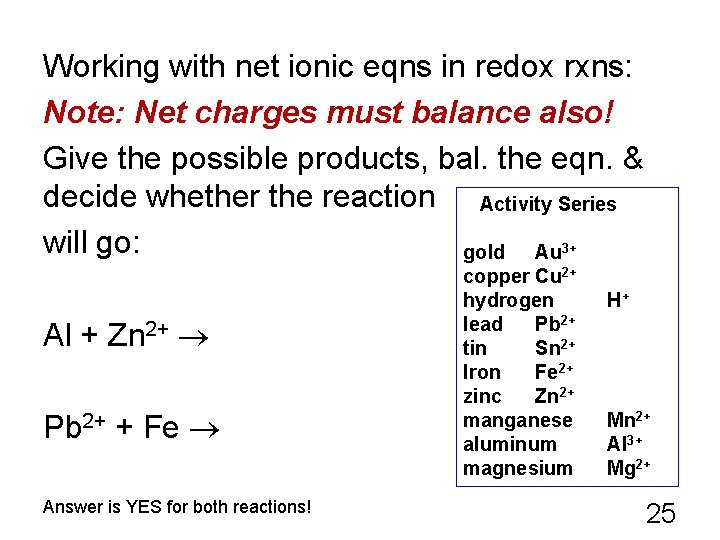
Working with net ionic eqns in redox rxns: Note: Net charges must balance also! Give the possible products, bal. the eqn. & decide whether the reaction Activity Series will go: gold Au 3+ Al + Zn 2+ Pb 2+ + Fe Answer is YES for both reactions! copper Cu 2+ hydrogen lead Pb 2+ tin Sn 2+ Iron Fe 2+ zinc Zn 2+ manganese aluminum magnesium H+ Mn 2+ Al 3+ Mg 2+ 25

Stoichiometry of Redox Rxns In a reaction, 45. 0 g of magnesium is to react with 500. 0 m. L of 0. 300 M HCl. At the end of the reaction, will there be any magnesium left? How much of the excess is left over? 26

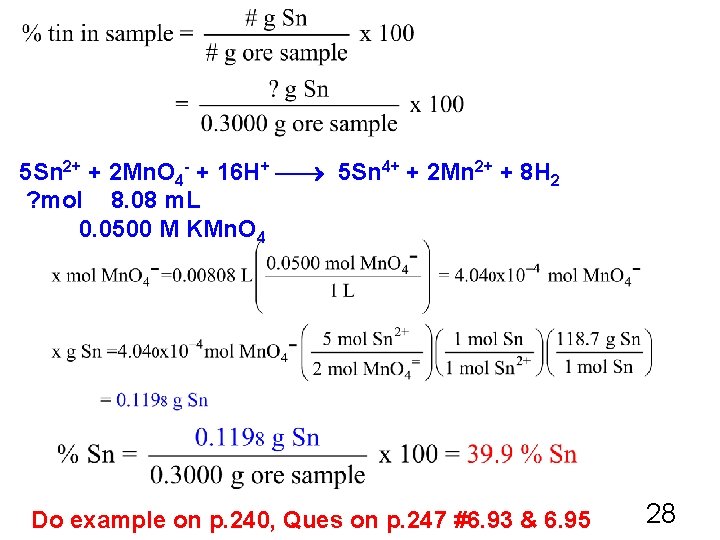
Ore Analysis #5. 122 p. 249 A 0. 3000 g sample of tin ore was dissolved in acid solution converting all the tin to tin(II). In a titration, 8. 08 m. L of 0. 0500 M KMn. O 4 was required to oxidize the tin(II) to tin(IV). The reaction is as follows: 5 Sn 2+ + 2 Mn. O 4 - + 16 H+ 5 Sn 4+ + 2 Mn 2+ + 8 H 2 O What was the percentage tin in the original sample? 27

5 Sn 2+ + 2 Mn. O 4 - + 16 H+ 5 Sn 4+ + 2 Mn 2+ + 8 H 2 ? mol 8. 08 m. L 0. 0500 M KMn. O 4 Do example on p. 240, Ques on p. 247 #6. 93 & 6. 95 28