CHAPTER 4 IMPERFECTIONS IN SOLIDS Deviations from perfect








- Slides: 24

CHAPTER 4: IMPERFECTIONS IN SOLIDS - Deviations from perfect structure Not always adverse, and many times deliberately introduced Can be classified according to dimensionality: 1. Point Defects (affect one or two atom positions) – “zero” dimension 2. Linear Defects (a. k. a. dislocations) – 1 -D 3. Interfacial or boundary defects – 2 -D 4. Volume Defects – 3 -D

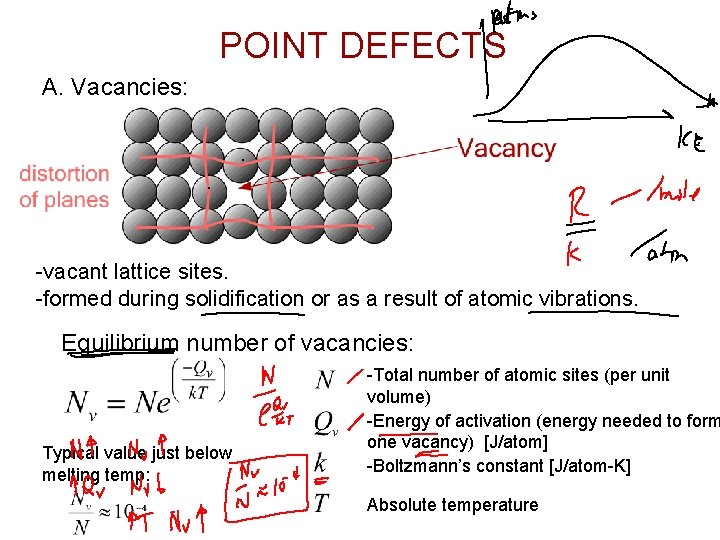
POINT DEFECTS A. Vacancies: -vacant lattice sites. -formed during solidification or as a result of atomic vibrations. Equilibrium number of vacancies: Typical value just below melting temp: -Total number of atomic sites (per unit volume) -Energy of activation (energy needed to form one vacancy) [J/atom] -Boltzmann’s constant [J/atom-K] Absolute temperature

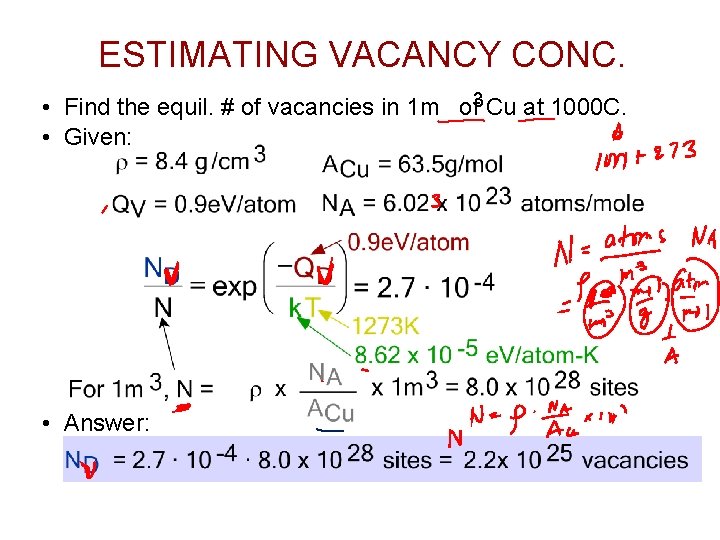
ESTIMATING VACANCY CONC. • Find the equil. # of vacancies in 1 m of 3 Cu at 1000 C. • Given: • Answer:

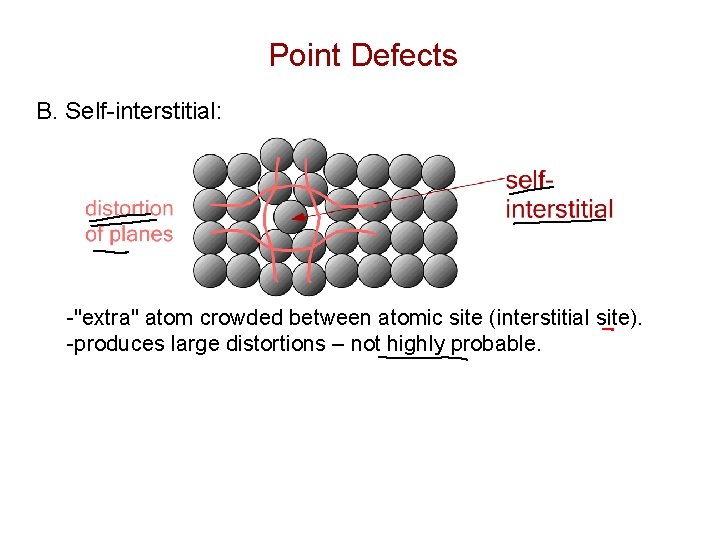
Point Defects B. Self-interstitial: -"extra" atom crowded between atomic site (interstitial site). -produces large distortions – not highly probable.

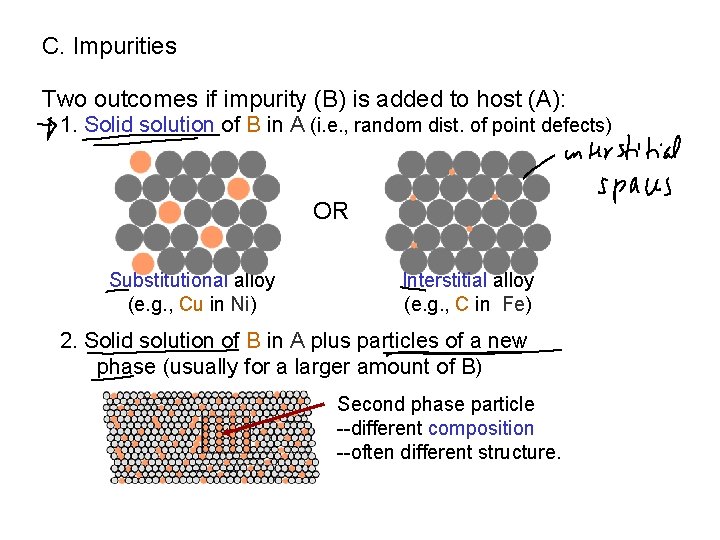
C. Impurities Two outcomes if impurity (B) is added to host (A): 1. Solid solution of B in A (i. e. , random dist. of point defects) OR Substitutional alloy (e. g. , Cu in Ni) Interstitial alloy (e. g. , C in Fe) 2. Solid solution of B in A plus particles of a new phase (usually for a larger amount of B) Second phase particle --different composition --often different structure.

Impurities Solid Solutions: Solute – minority atoms Solvent – majority atoms Crystal structure of solvent is maintained – solute atoms are randomly distributed. Types of Solid solutions: a) Substitutional – impurity atoms replace host atoms Atomic radii difference is < ± 15% Complete Solubility: i. Relative atomic radii ii. Same crystal structure iii. Electronegativities iv. Valence Ex. Cu & Ni (both FCC, RCu=0. 128 nm, RNi=0. 125 nm; Electroneg: 1. 9 & 1. 8) b) Interstitial – impurity atoms fill interstitial spaces Small impurity atom Ex: Carbon in iron, RC=0. 071, RFe=0. 124 nm.

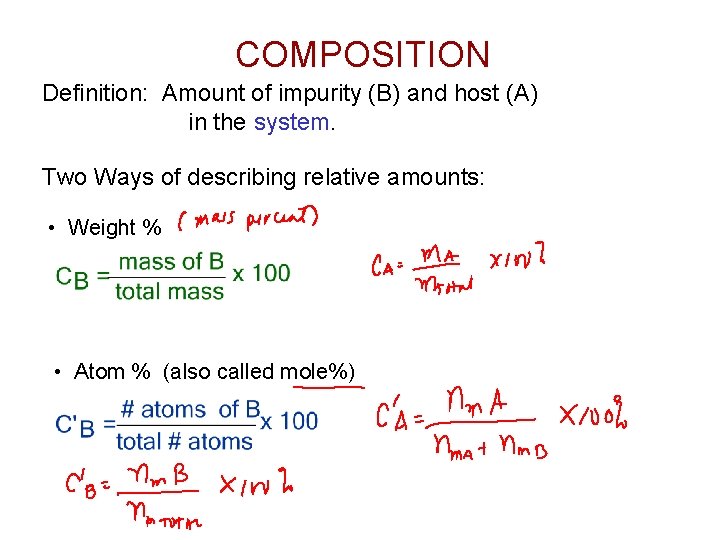
COMPOSITION Definition: Amount of impurity (B) and host (A) in the system. Two Ways of describing relative amounts: • Weight % • Atom % (also called mole%)

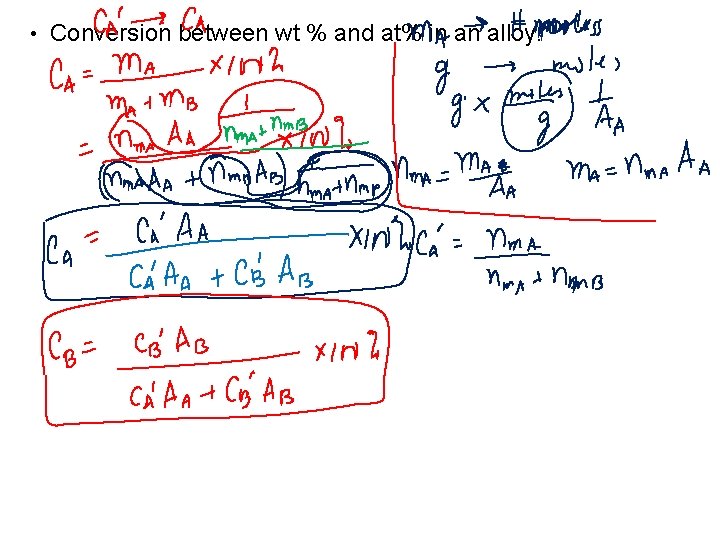
• Conversion between wt % and at% in an alloy:


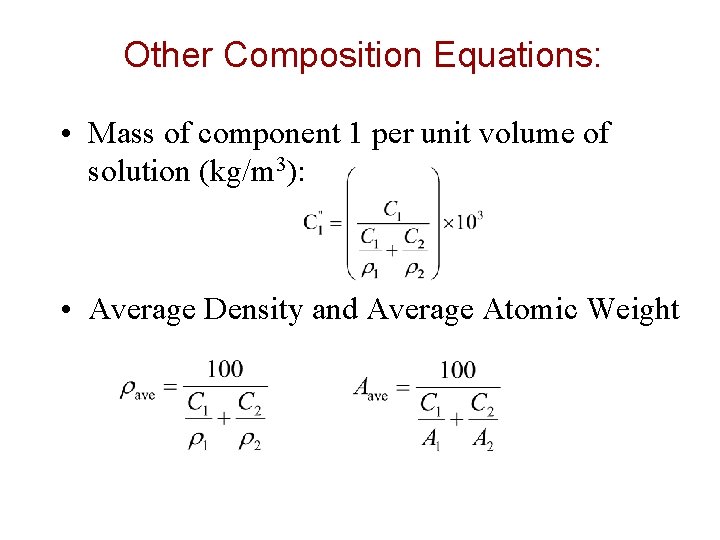
Other Composition Equations: • Mass of component 1 per unit volume of solution (kg/m 3): • Average Density and Average Atomic Weight

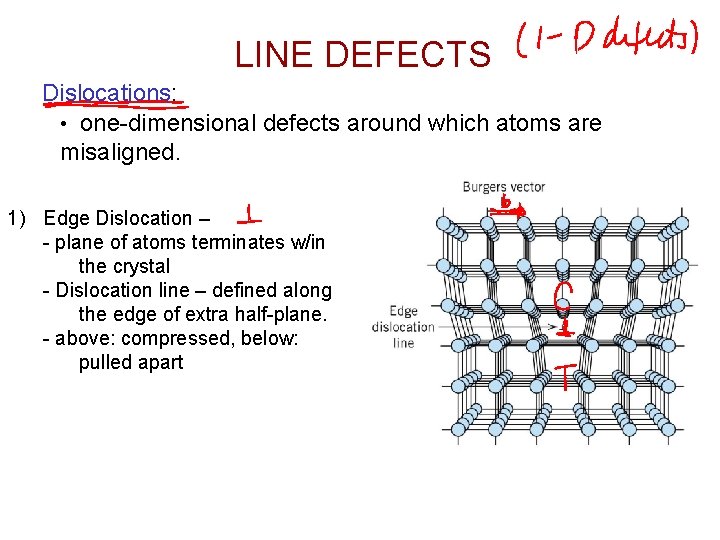
LINE DEFECTS Dislocations: • one-dimensional defects around which atoms are misaligned. 1) Edge Dislocation – - plane of atoms terminates w/in the crystal - Dislocation line – defined along the edge of extra half-plane. - above: compressed, below: pulled apart

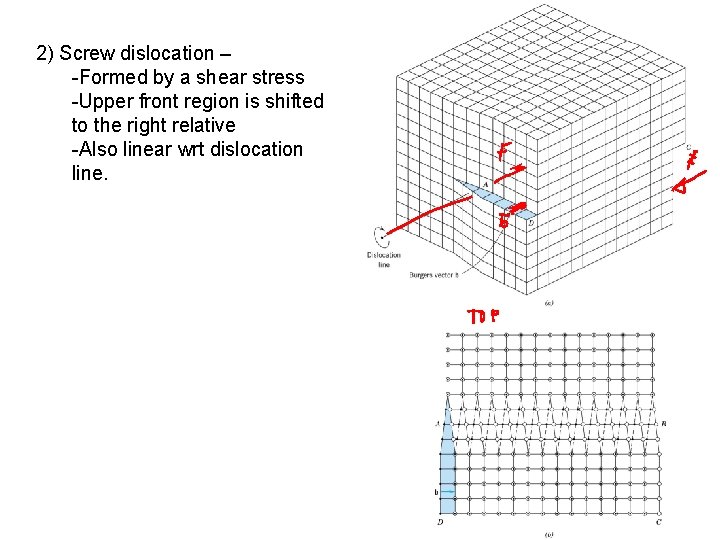
2) Screw dislocation – -Formed by a shear stress -Upper front region is shifted to the right relative -Also linear wrt dislocation line.

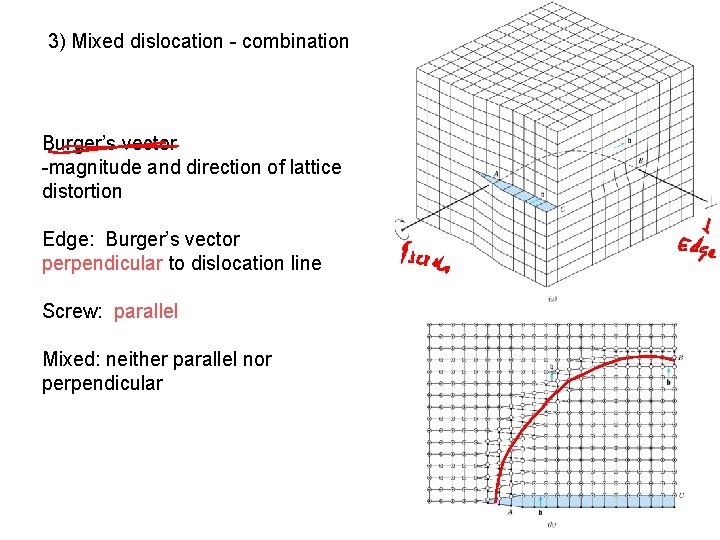
3) Mixed dislocation - combination Burger’s vector -magnitude and direction of lattice distortion Edge: Burger’s vector perpendicular to dislocation line Screw: parallel Mixed: neither parallel nor perpendicular

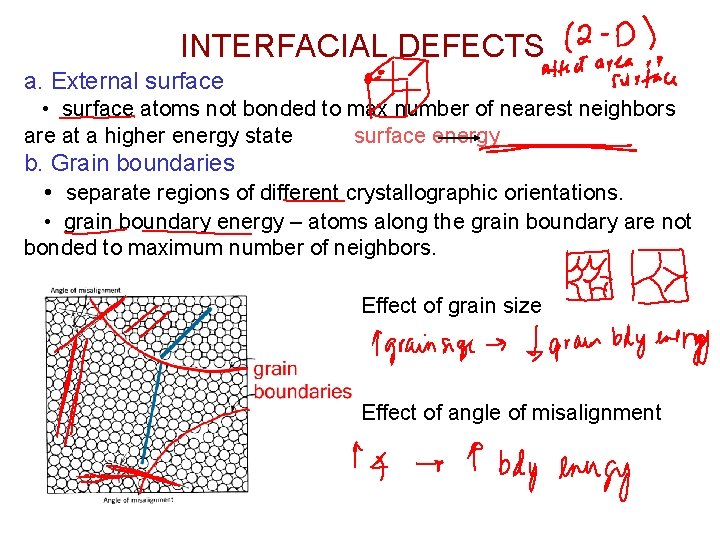
INTERFACIAL DEFECTS a. External surface • surface atoms not bonded to max number of nearest neighbors are at a higher energy state surface energy b. Grain boundaries • separate regions of different crystallographic orientations. • grain boundary energy – atoms along the grain boundary are not bonded to maximum number of neighbors. Effect of grain size Effect of angle of misalignment

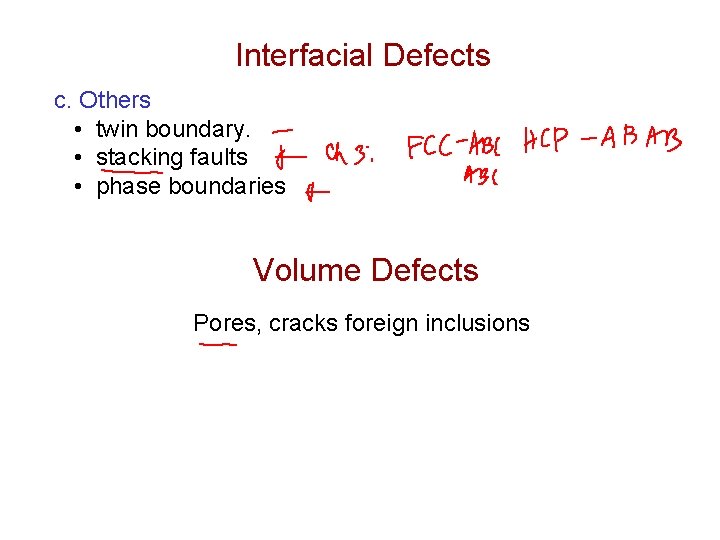
Interfacial Defects c. Others • twin boundary. • stacking faults • phase boundaries Volume Defects Pores, cracks foreign inclusions

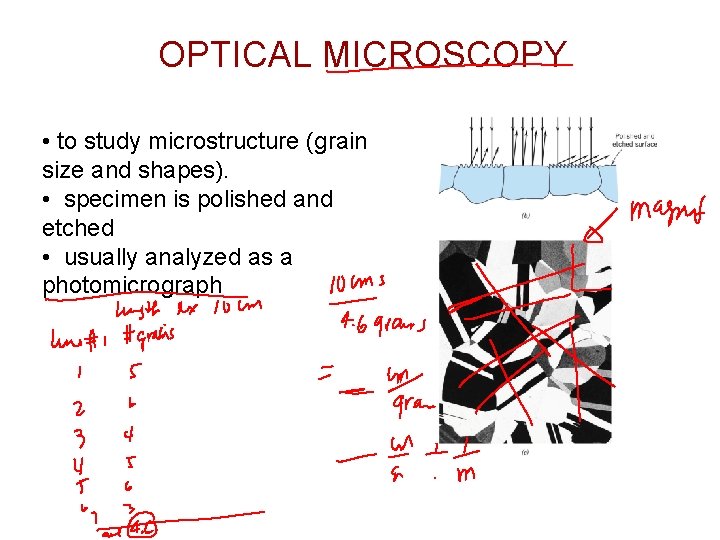
OPTICAL MICROSCOPY • to study microstructure (grain size and shapes). • specimen is polished and etched • usually analyzed as a photomicrograph

Grain Size Intercept Method: • Draw straight lines of same length and random orientation, • Count number of grains intersected by each line. • Line length ÷ # grains [m/grain]; average, then ÷ magnification ASTM Grain Size Number

1. A one-dimensional imperfection wherein the plane of atoms terminates within the crystal is called a(n) A. Dislocation. B. Vacancy. C. Edge dislocation. D. Screw dislocation.

2) Which of the following will increase the equilibrium number of vacancy for a given crystal? A. Higher temperature B. Higher activation energy for vacancy. C. Higher atomic weight. D. None of the above.

3) Which of the following will form an interstitial solid solution with Iron? A. Nitrogen B. Nickel C. Copper D. Aluminum.

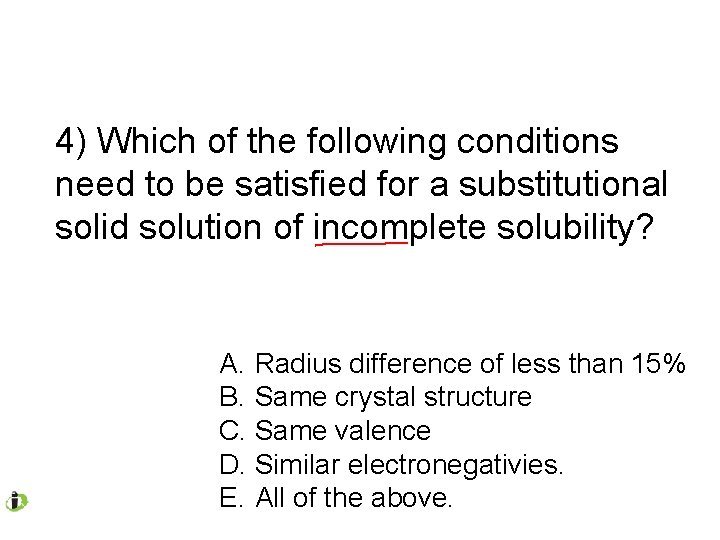
4) Which of the following conditions need to be satisfied for a substitutional solid solution of incomplete solubility? A. Radius difference of less than 15% B. Same crystal structure C. Same valence D. Similar electronegativies. E. All of the above.

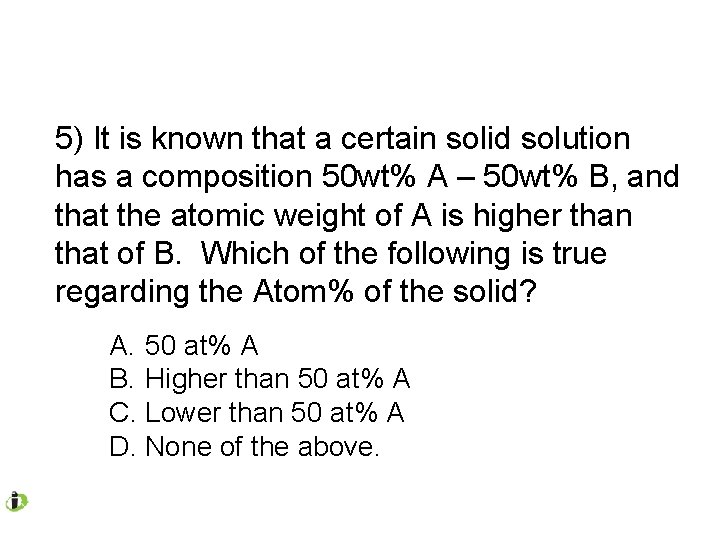
5) It is known that a certain solid solution has a composition 50 wt% A – 50 wt% B, and that the atomic weight of A is higher than that of B. Which of the following is true regarding the Atom% of the solid? A. 50 at% A B. Higher than 50 at% A C. Lower than 50 at% A D. None of the above.

6) Which of the following interfacial defects has the least amount of surface energy per unit volume? A. Small grains with large angles of misalignment. B. Large grains with small angles of misalignment. C. Small grains with small angles of misalignment. D. Large grains with large angles of misalignment.

7) For an FCC crystal, along which direction is the linear density the smallest? A. [100] B. [110] C. [111] D.