LECTURE4 POINT IMPERFECTIONS LINE IMPERFECTIONS SURFACE IMPERFECTIONS VOLUME



























- Slides: 46

LECTURE-4 POINT IMPERFECTIONS LINE IMPERFECTIONS SURFACE IMPERFECTIONS VOLUME IMPERFECTIONS 1

CRYSTAL DEFECTS AND IMPERFECTIONS An ideal crystal is a perfect crystal in which each atom has identical surroundings. Real crystals are not perfect. A real crystal always has a large number of imperfections in the lattice. One can reduce crystal defects considerably, but can never eliminate them entirely. 2


Crystal defects Defects can affect F Strength ﺍﻟﻘﻮﺓ F Conductivity ﺍﻟﺘﻮﺻﻠﻴﺔ F Deformation style ﺗﺸﻮﻩ ﺍﻟﻨﻤﻂ F Color ﺍﻟﻠﻮﻥ 4

CRYSTAL DEFECTS AND IMPERFECTIONS The study of imperfections has a two fold purpose, namely, A better understanding of crystals and how they affect the properties of metals. Exploration of possibilities of minimizing or eliminating these defects. The term “defect” or “imperfection” is generally used to describe any deviation from the perfect periodic array of atoms in the crystal. 5


CRYSTAL DEFECTS AND IMPERFECTIONS Crystal imperfections can be classified on the basis of their geometry as, Point Imperfections, Line imperfections Surface (or) plane imperfections and Volume imperfections 7


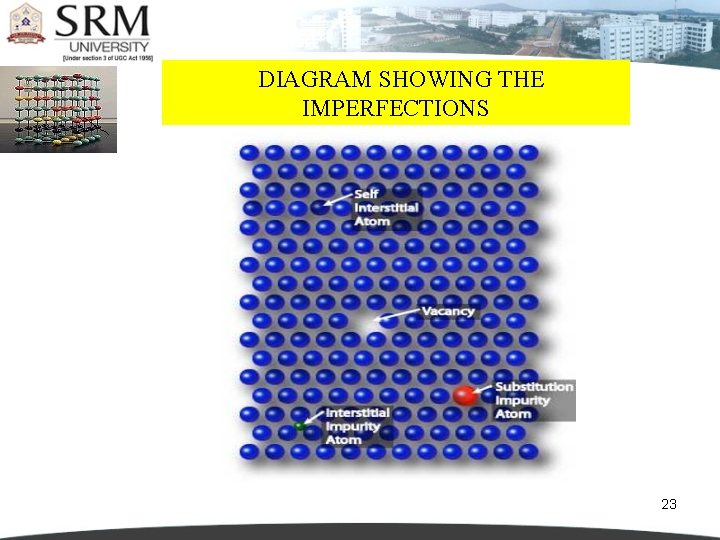
POINT IMPERFECTIONS They are imperfect point- like regions, one or two atomic diameters in size and hence referred to as ‘zero dimensional imperfections’. There are different kinds of point imperfections. VACANCIES If an atom is missing from its normal site in the matrix, the defect is called a vacancy defect. It may be a single vacancy, divacancy or a trivacancy. 9

POINT DEFECT-VACANCY 10


POINT IMPERFECTIONS In metals vacancies and created by thermal excitation. When the temperature is sufficiently high, as the atoms vibrate around their regular positions, some acquire enough energy to leave the site completely. When the regular atom leaves, a vacancy is created. A pair of one cation and one anion can be missed from an ionic crystal. Such a pair of vacant ion sites is called Schottky imperfection. This type of defect is dominant in alkali halides. 12


SCHOTTKY IMPERFECTIONS 14

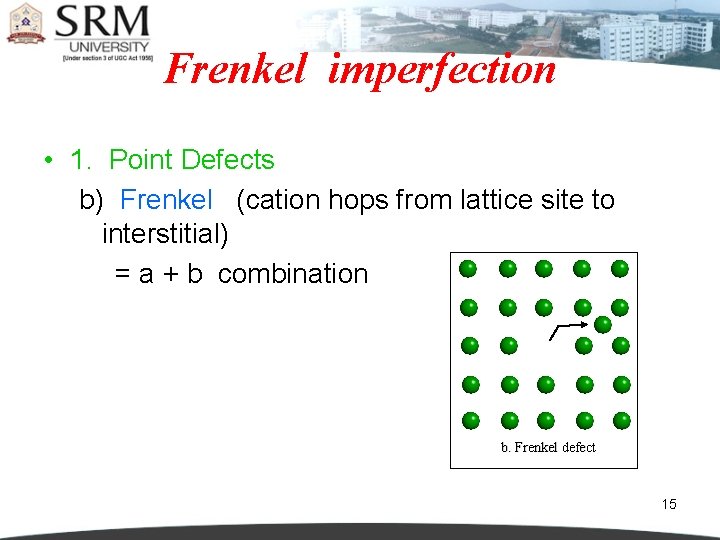
Frenkel imperfection • 1. Point Defects b) Frenkel (cation hops from lattice site to interstitial) = a + b combination b. Frenkel defect 15


SUBSTITUTIONAL IMPURITY It refers to a foreign atom that substitutes for or replaces a parent atom in the crystal. Pentavalent or trivalent impurity atoms doped in Silicon or Germanium are also substitutional impurities in the crystal. 17


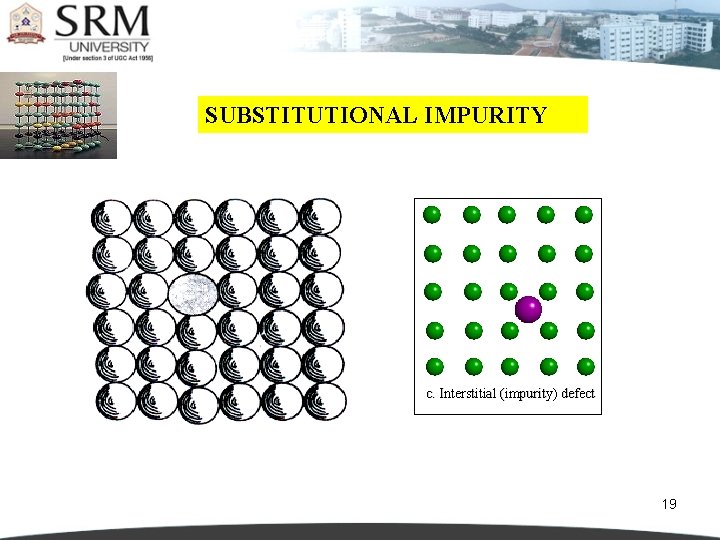
SUBSTITUTIONAL IMPURITY c. Interstitial (impurity) defect 19

INTERSTITIAL IMPURITY An interstitial defect arises when an atom occupies a definite position in the lattice that is not normally occupied in the perfect crystal. If a small sized atom occupies the void space in the parent crystal without disturbing the parent atoms from their regular sites, then it is called as ‘interstitial impurity’. 20


INTERSTITIAL IMPURITY 22

DIAGRAM SHOWING THE IMPERFECTIONS 23

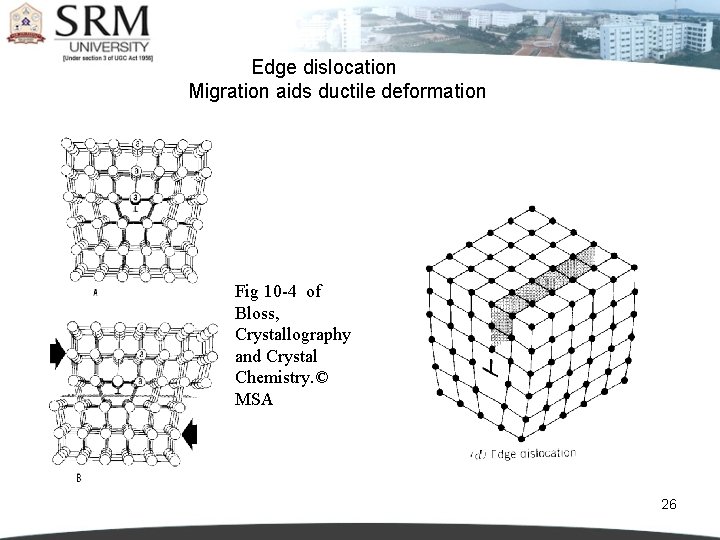
2 - LINE IMPERFECTIONS The defects, which take place due to dislocation or distortion of atoms along a line, in some direction are called as ‘line defects’. Line defects are also called dislocations. In the geometic sense, they may be called as ‘one dimensional defects’. A dislocation may be defined as a disturbed region between two substantially perfect parts of a crystal. It is responsible for the phenomenon of slip by which most metals deform plastically. 24


Edge dislocation Migration aids ductile deformation Fig 10 -4 of Bloss, Crystallography and Crystal Chemistry. © MSA 26

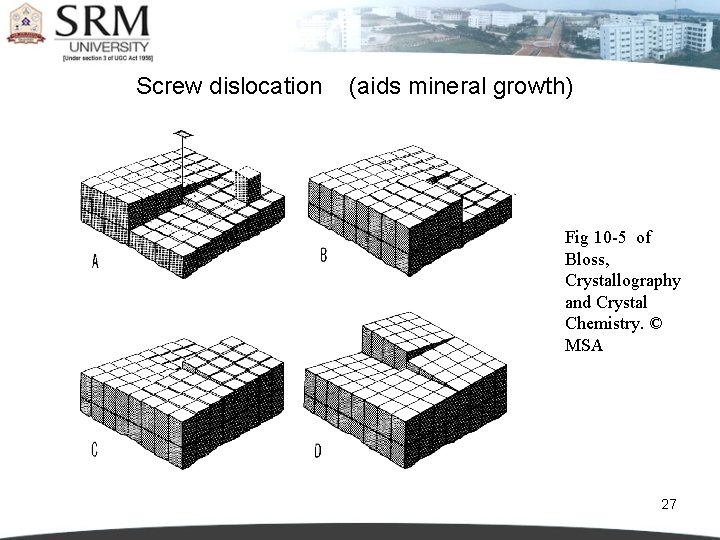
Screw dislocation (aids mineral growth) Fig 10 -5 of Bloss, Crystallography and Crystal Chemistry. © MSA 27

3 -SURFACE IMPERFECTIONS Surface imperfections arise from a change in the stacking of atomic planes on or across a boundary. The change may be one of the orientations or of the stacking sequence of atomic planes. In geometric concept, surface imperfections are twodimensional. They are of two types external and internal surface imperfections. 28


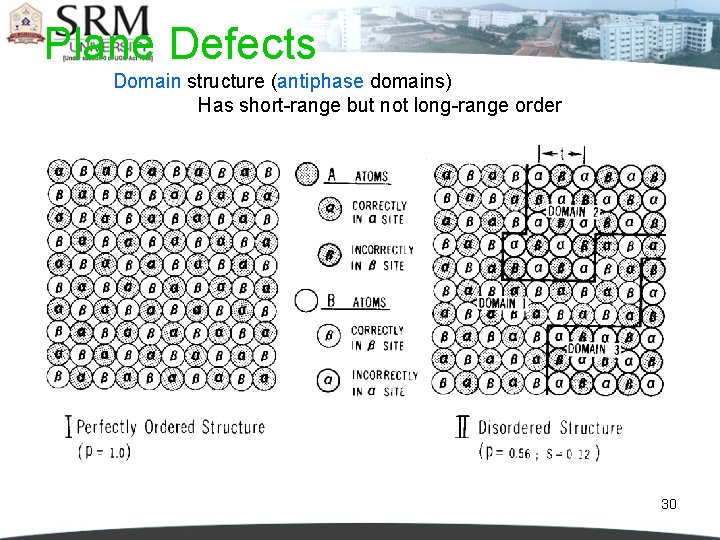
Plane Defects Domain structure (antiphase domains) Has short-range but not long-range order 30

• 3. Plane Defects Stacking faults Common in clays and low-T disequilibrium A - B - C layers may be various clay types (illite, smectite, etc. ) ABCABCABCABC AAAAAABAAAAAAA ABABABCABABAB 31

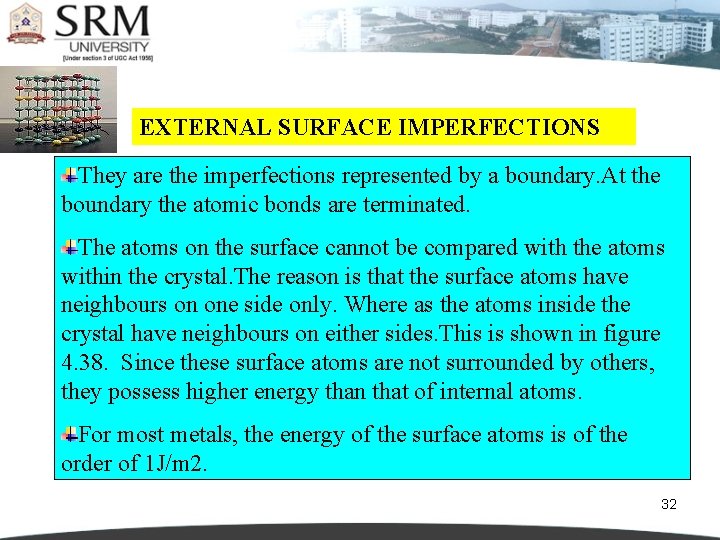
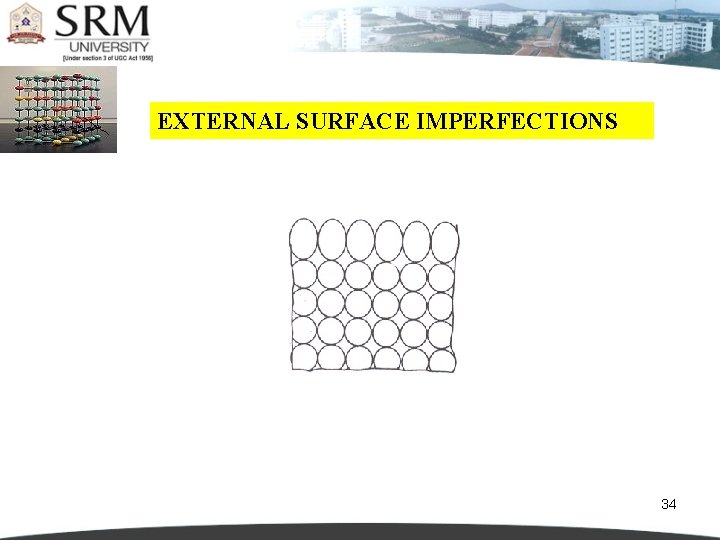
EXTERNAL SURFACE IMPERFECTIONS They are the imperfections represented by a boundary. At the boundary the atomic bonds are terminated. The atoms on the surface cannot be compared with the atoms within the crystal. The reason is that the surface atoms have neighbours on one side only. Where as the atoms inside the crystal have neighbours on either sides. This is shown in figure 4. 38. Since these surface atoms are not surrounded by others, they possess higher energy than that of internal atoms. For most metals, the energy of the surface atoms is of the order of 1 J/m 2. 32


EXTERNAL SURFACE IMPERFECTIONS 34

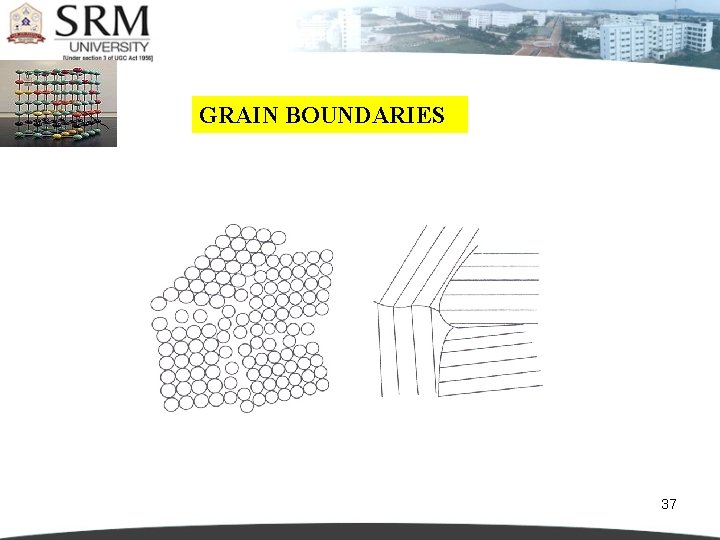
INTERNAL SURFACE IMPERFECTIONS Internal surface imperfections are the imperfections which occurred inside a crystal. It is caused by the defects such as, grain boundaries. tilt boundaries, twin boundaries and stacking faults. 35


GRAIN BOUNDARIES 37

4 -VOLUME IMPERFECTIONS Volume defects such as cracks may arise in crystals when there is only small electrostatic dissimilarity between the stacking sequences of close packed planes in metals. Presence of a large vacancy or void space, when cluster of atoms are missed is also considered as a volume imperfection. 38


Crystal Defects • There are principally two kinds of defects that occur in crystalline substances. Chemical impurities, such as in rubies, where the crystal is mainly aluminum oxide with an occasional Al 3+ ion replaced with Cr 3+, which gives a red color. Defects in the formation of the lattice. Crystal planes may be misaligned, or sites in the crystal lattice may remain vacant.

Calculations Involving Unit Cell Dimensions • X-ray diffraction is a method for determining the structure and dimensions Once the dimensions and structure are known, the volume and mass of a unit cell in a crystalline compound. single atom in the crystal can be calculated. The determination of the mass of a single atom gave us one of the first accurate determinations of Avogadro’s number.

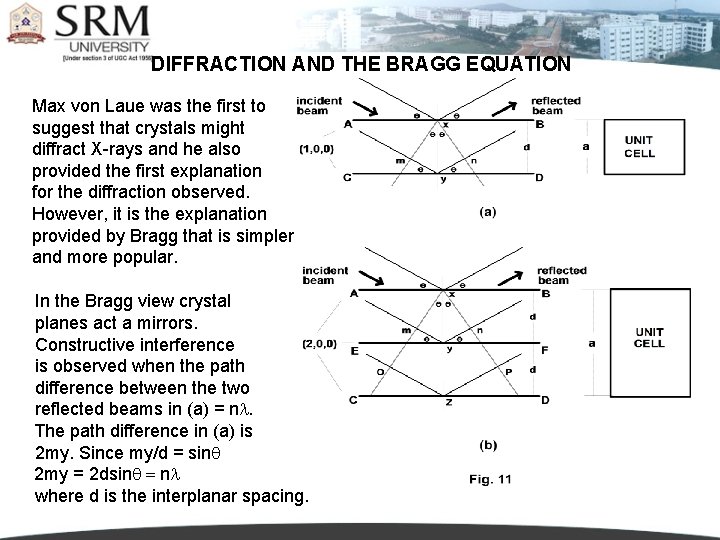
DIFFRACTION AND THE BRAGG EQUATION Max von Laue was the first to suggest that crystals might diffract X-rays and he also provided the first explanation for the diffraction observed. However, it is the explanation provided by Bragg that is simpler and more popular. In the Bragg view crystal planes act a mirrors. Constructive interference is observed when the path difference between the two reflected beams in (a) = nl. The path difference in (a) is 2 my. Since my/d = sin 2 my = 2 dsin = nl where d is the interplanar spacing.

Bragg’s Law animated In (a) above it is clear that the planes are the (1, 0, 0) set of planes. If the path difference is simply one wavelength the Bragg condition can be stated as This is a first order reflection. If the path difference is two wave lengths the Bragg condition becomes and the reflection is a second order reflection.

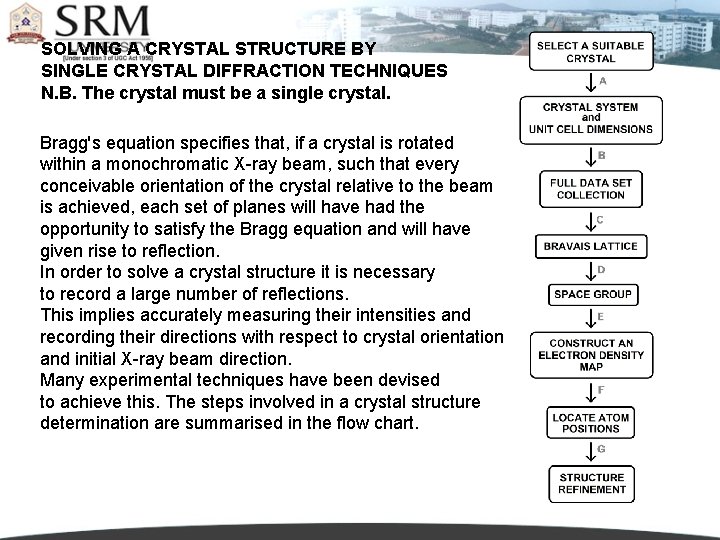
SOLVING A CRYSTAL STRUCTURE BY SINGLE CRYSTAL DIFFRACTION TECHNIQUES N. B. The crystal must be a single crystal. Bragg's equation specifies that, if a crystal is rotated within a monochromatic X-ray beam, such that every conceivable orientation of the crystal relative to the beam is achieved, each set of planes will have had the opportunity to satisfy the Bragg equation and will have given rise to reflection. In order to solve a crystal structure it is necessary to record a large number of reflections. This implies accurately measuring their intensities and recording their directions with respect to crystal orientation and initial X-ray beam direction. Many experimental techniques have been devised to achieve this. The steps involved in a crystal structure determination are summarised in the flow chart.

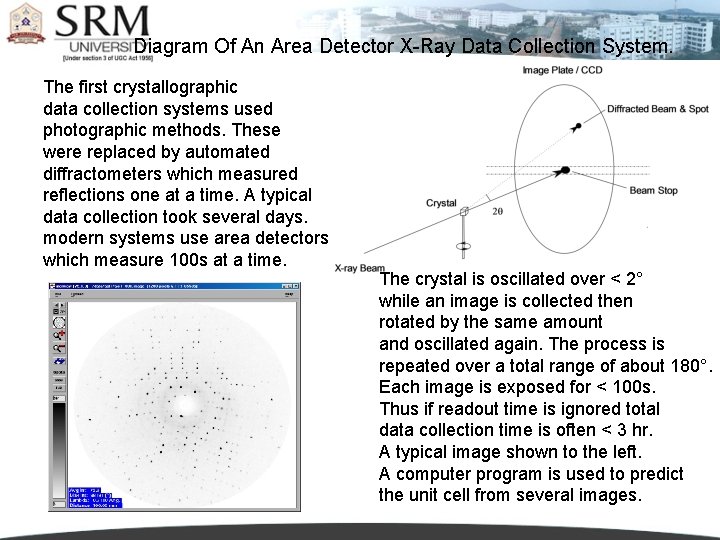
Diagram Of An Area Detector X-Ray Data Collection System. The first crystallographic data collection systems used photographic methods. These were replaced by automated diffractometers which measured reflections one at a time. A typical data collection took several days. modern systems use area detectors which measure 100 s at a time. The crystal is oscillated over < 2° while an image is collected then rotated by the same amount and oscillated again. The process is repeated over a total range of about 180°. Each image is exposed for < 100 s. Thus if readout time is ignored total data collection time is often < 3 hr. A typical image shown to the left. A computer program is used to predict the unit cell from several images.

Determination of the Lattice type and Space Group High symmetry can lead to reflections being systematically absent from the data set. Absent reflections have no measurable intensity. There are two types of absences, General Absences and Special Absences. The general absences determine the lattice type; Primitive (P) has no general absences and no restrictions on h, k or l. End Cantered (C) h+k=2 n+1 are all absent. Face Cantered (F) only h, k, l, all even or all odd are observed. Body Cantered (I) h+k+l=2 n+1 are all absent. The special absences refer to specific sets of reflections and are used to detect the presence of glide planes and screw axes. Some Space Groups are uniquely determined by special absences but in many cases several Space Groups will have to be considered. Computer programs are able to lay out the data in tables with absences indicated and possible Space Groups can be suggested however the choice of Space Group will often need much thought.