Chapter 3 Scientific Measurement Jessica Contino Scientific Notation



























- Slides: 75

Chapter 3: Scientific Measurement Jessica Contino

Scientific Notation a way of expressing really big numbers or really small numbers. Separates measurement digits from placeholder zeros Helps in evaluating precision

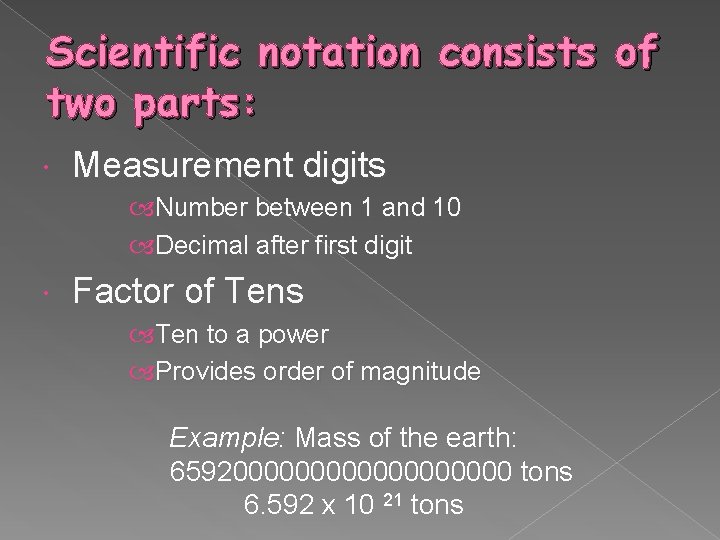
Scientific notation consists of two parts: Measurement digits Number between 1 and 10 Decimal after first digit Factor of Tens Ten to a power Provides order of magnitude Example: Mass of the earth: 6592000000000 tons 6. 592 x 10 21 tons

To change standard form to scientific notation… Place the decimal point so that there is one nonzero digit to the left of the decimal point. Count the number of decimal places the decimal point has “moved” from the original number. This will be the exponent on the 10. If the original number was less than 1, then the exponent is negative. If the original number was greater than 1, then the exponent is positive.

Examples Given: 289, 800, 000 Use: 2. 898 (moved 8 places) Answer: 2. 898 x 10 8 Given: 0. 000567 Use: 5. 67 (moved 4 places) Answer: 5. 67 x 10 - 4

To change scientific notation to standard form… Simply move the decimal point to the right for positive exponent 10. Move the decimal point to the left for negative exponent 10. (Use zeros to fill in places. )

Example Given: 5. 093 x 106 5, 093, 000 Answer: (moved 6 places to the right) Given: 1. 976 x 10 -4 Answer: 0. 0001976 (moved 4 places to the left)

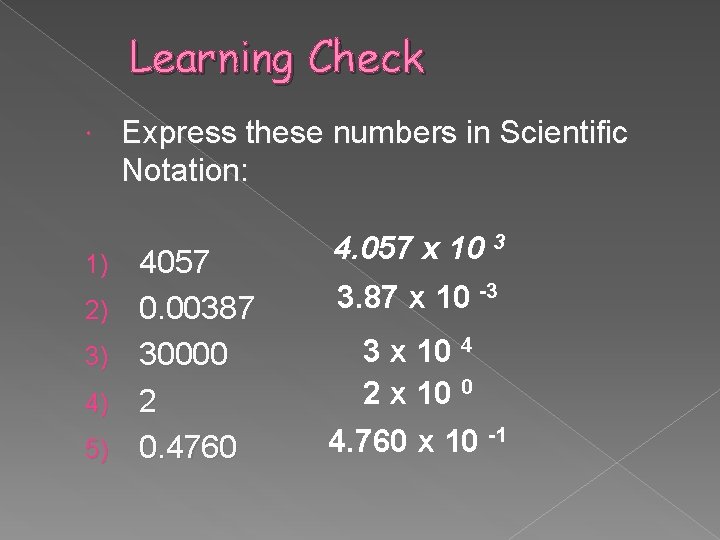
Learning Check 1) 2) 3) 4) 5) Express these numbers in Scientific Notation: 4057 0. 00387 30000 2 0. 4760 4. 057 x 10 3 3. 87 x 10 -3 3 x 10 4 2 x 10 0 4. 760 x 10 -1

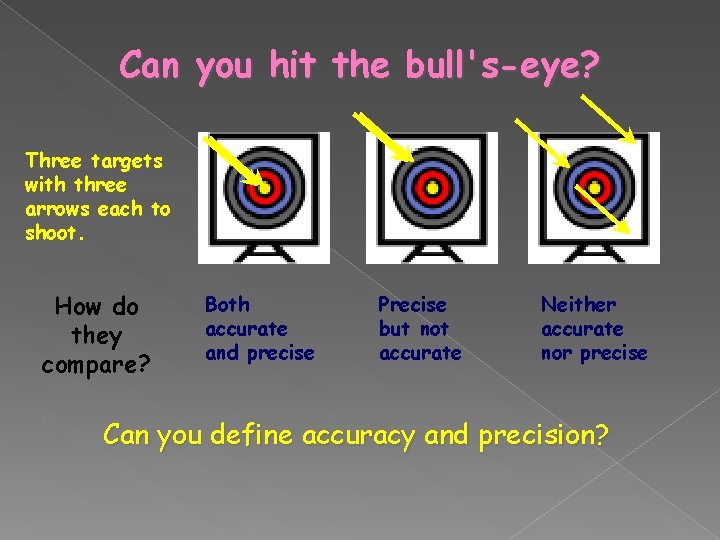
Can you hit the bull's-eye? Three targets with three arrows each to shoot. How do they compare? Both accurate and precise Precise but not accurate Neither accurate nor precise Can you define accuracy and precision?

Accuracy and percent error Absolute error – how far a measured value is compared to an accepted value Percentage – value per 100 units Percentage error: % error = measured value – accepted value Accepted value x 100

A student calculates the density of mercury to be 13. 2 g/cm 3. If the accepted value is 13. 6 g/cm 3, what is the percentage error? % error = measured value – accepted value Accepted value % error = 13. 2 - 13. 6 x 100 = - 2. 94 PERCENT NEGATIVE ERROR = MEASURED VALUE BELOW ACCEPTED!

Significant Figures v. The numbers reported in a measurement are limited by the measuring tool v. Significant figures in a measurement include the known digits plus one estimated digit

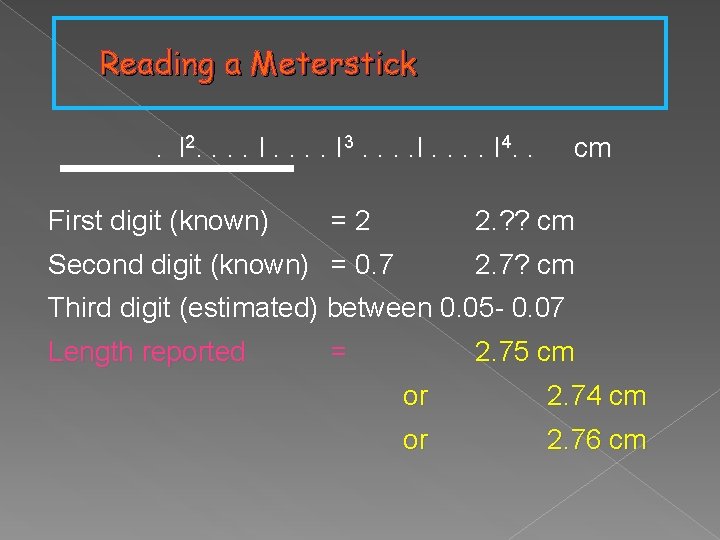
Reading a Meterstick. l 2. . . . I 3. . . . I 4. . First digit (known) =2 cm 2. ? ? cm Second digit (known) = 0. 7 2. 7? cm Third digit (estimated) between 0. 05 - 0. 07 Length reported = 2. 75 cm or 2. 74 cm or 2. 76 cm

Known + Estimated Digits In 2. 76 cm… • Known digits 2 and 7 are 100% certain • The third digit 6 is estimated (uncertain) • In the reported length, all three digits (2. 76 cm) are significant including the estimated one

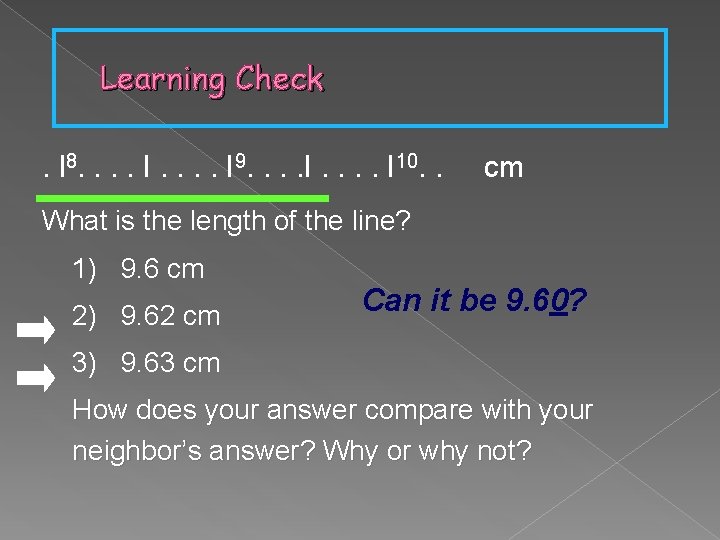
Learning Check. l 8. . . . I 9. . . . I 10. . cm What is the length of the line? 1) 9. 6 cm 2) 9. 62 cm Can it be 9. 60? 3) 9. 63 cm How does your answer compare with your neighbor’s answer? Why or why not?

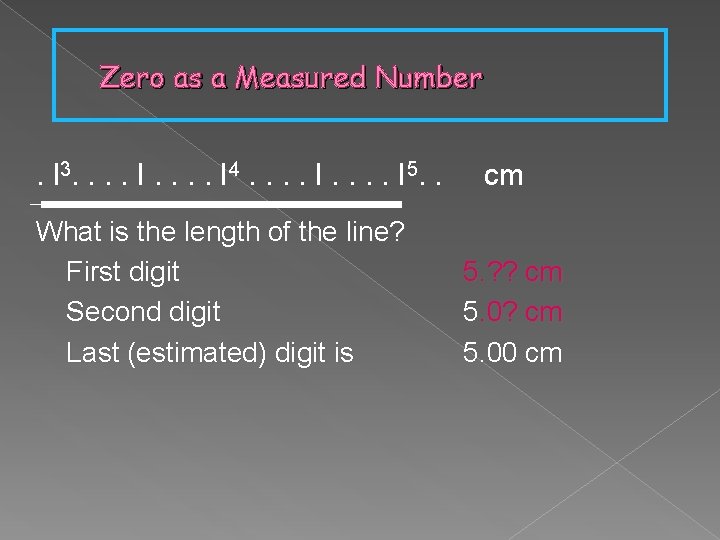
Zero as a Measured Number . l 3. . . . I 4. . . . I 5. . What is the length of the line? First digit Second digit Last (estimated) digit is cm 5. ? ? cm 5. 00 cm

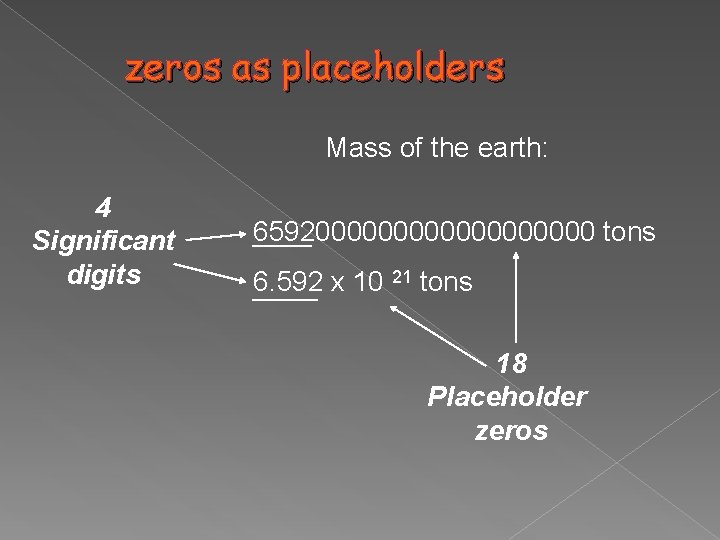
zeros as placeholders Mass of the earth: 4 Significant digits 6592000000000 tons 6. 592 x 10 21 tons 18 Placeholder zeros

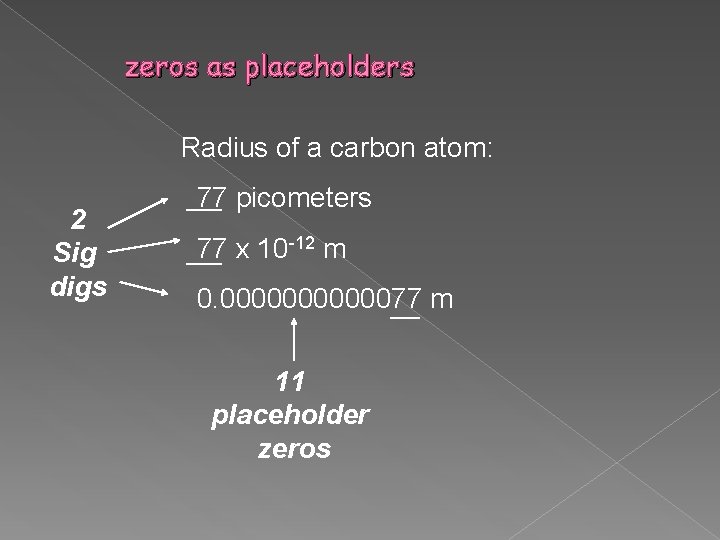
zeros as placeholders Radius of a carbon atom: 2 Sig digs 77 picometers 77 x 10 -12 m 0. 00000077 m 11 placeholder zeros

zeros as placeholders Placeholders left after rounding: Ex: 734567 Rounded to nearest thousand: 735000 3 sig digs 3 placeholder zeros Note: the zeros left after rounding are only placeholders They drop out when not needed – as in scientific notation 7. 35 x 10 5

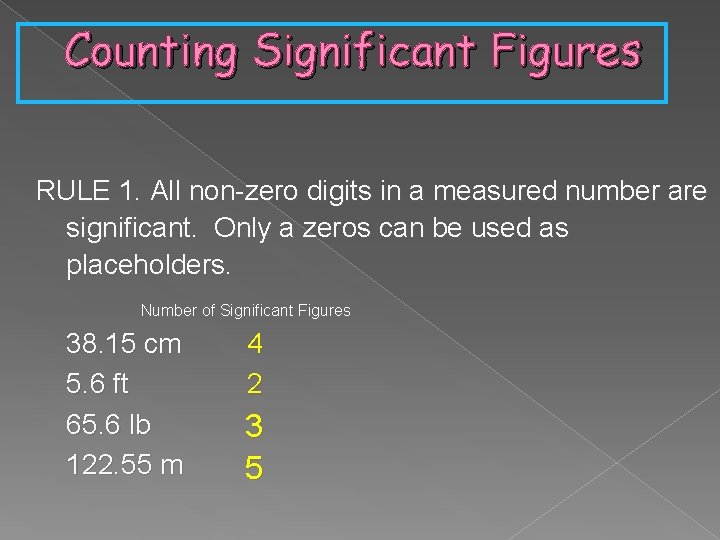
Counting Significant Figures RULE 1. All non-zero digits in a measured number are significant. Only a zeros can be used as placeholders. Number of Significant Figures 38. 15 cm 5. 6 ft 65. 6 lb 122. 55 m 4 2 3 5

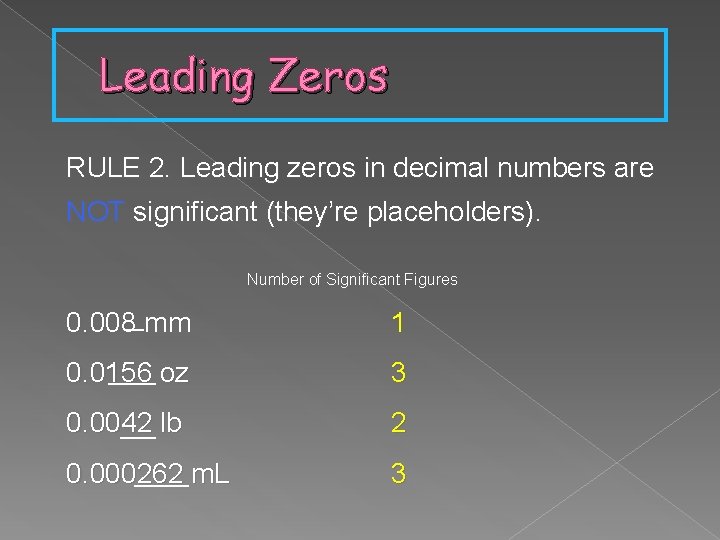
Leading Zeros RULE 2. Leading zeros in decimal numbers are NOT significant (they’re placeholders). Number of Significant Figures 0. 008 mm 1 0. 0156 oz 3 0. 0042 lb 2 0. 000262 m. L 3

Sandwiched Zeros RULE 3. Zeros between nonzero numbers are significant. (They can not be rounded or dropped, unless they are on an end of a number. ) Number of Significant Figures 50. 8 mm 2001 min 3 4 0. 702 lb 3 0. 00405 m 3

Trailing Zeros RULE 4. Trailing zeros in numbers without decimals are NOT significant. They are only serving as place holders (left after rounding). Number of Significant Figures 25, 000 in. 2 200. yr 3 48, 600 gal 3 25, 000 g 5 rounded to thousands decimal means not rounded

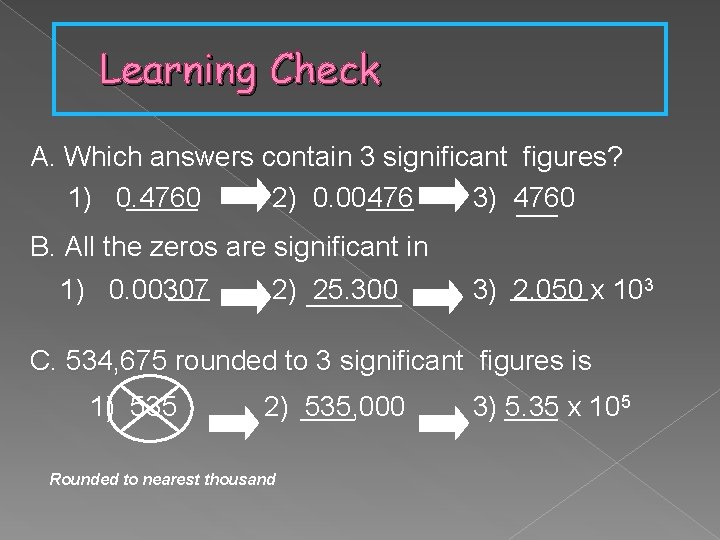
Learning Check A. Which answers contain 3 significant figures? 1) 0. 4760 2) 0. 00476 3) 4760 B. All the zeros are significant in 1) 0. 00307 2) 25. 300 3) 2. 050 x 103 C. 534, 675 rounded to 3 significant figures is 1) 535 2) 535, 000 Rounded to nearest thousand 3) 5. 35 x 105

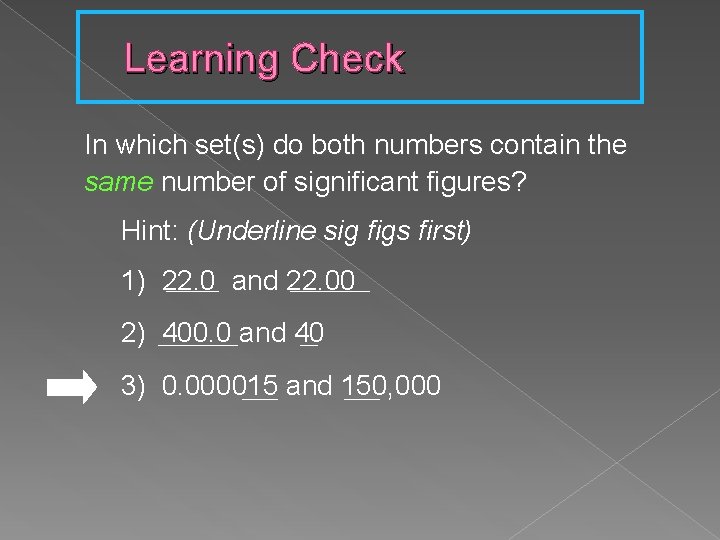
Learning Check In which set(s) do both numbers contain the same number of significant figures? Hint: (Underline sig figs first) ______ and 22. 00 _____ 1) 22. 0 2) _____ 400. 0 and 40 __ 3) 0. 000015 ____ and 150, 000 ____

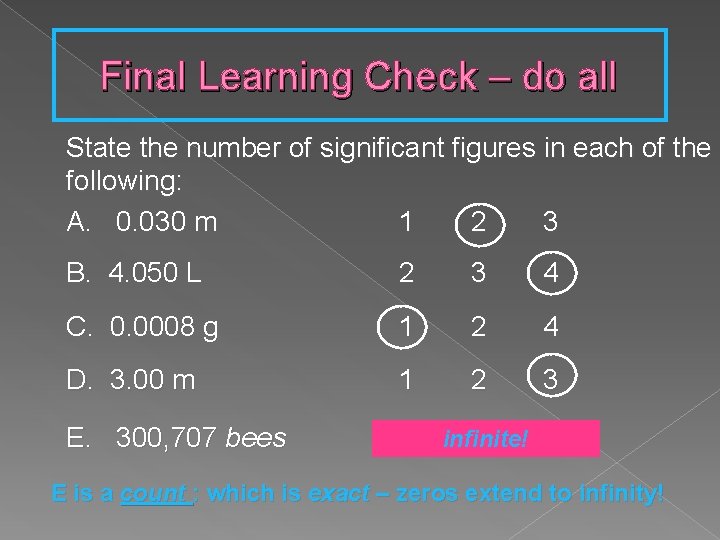
Final Learning Check – do all State the number of significant figures in each of the following: A. 0. 030 m 1 2 3 B. 4. 050 L 2 3 4 C. 0. 0008 g 1 2 4 D. 3. 00 m 1 2 3 E. 300, 707 bees 1 infinite! 3 6 E is a count : which is exact – zeros extend to infinity!

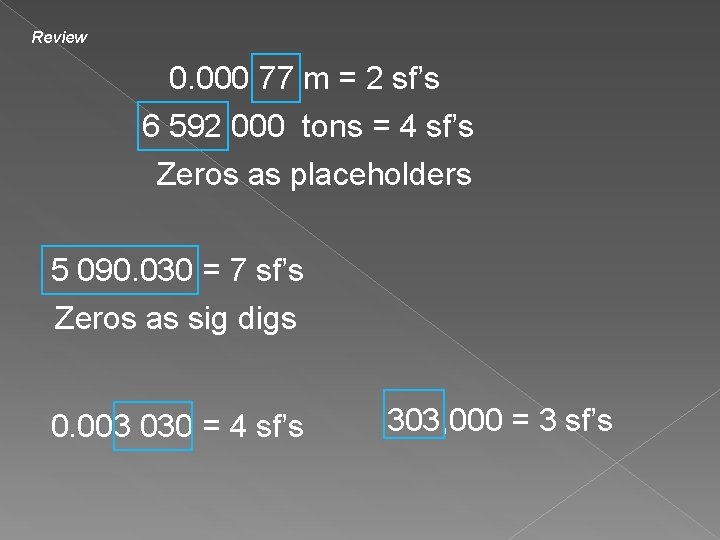
Review 0. 000 77 m = 2 sf’s 6 592 000 tons = 4 sf’s Zeros as placeholders 5 090. 030 = 7 sf’s Zeros as sig digs 0. 003 030 = 4 sf’s 303, 000 = 3 sf’s

Significant Numbers in Calculations A calculated answer cannot be more precise than the measuring tool. n A calculated answer must match the least precise measurement used in the calculation. n Significant figures are needed for final answers from 1) adding or subtracting 2) multiplying or dividing n

Adding and Subtracting The answer has the same number of “decimal places” as the measurement with the fewest decimal places. 25. 2? one decimal place + 1. 35 two decimal places 26. 55 answer 26. 6 one decimal place

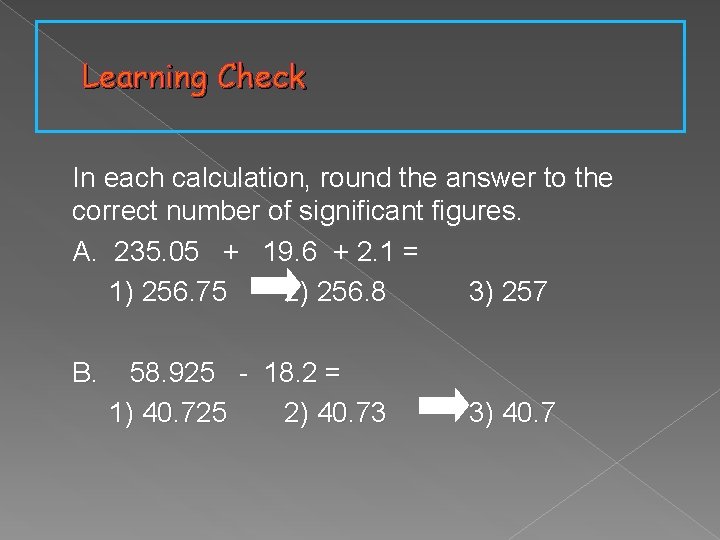
Learning Check In each calculation, round the answer to the correct number of significant figures. A. 235. 05 + 19. 6 + 2. 1 = 1) 256. 75 2) 256. 8 3) 257 B. 58. 925 - 18. 2 = 1) 40. 725 2) 40. 73 3) 40. 7

Multiplying and Dividing Round (or add zeros to) the calculated answer until you have the same number of significant figures as the measurement with the fewest significant figures.

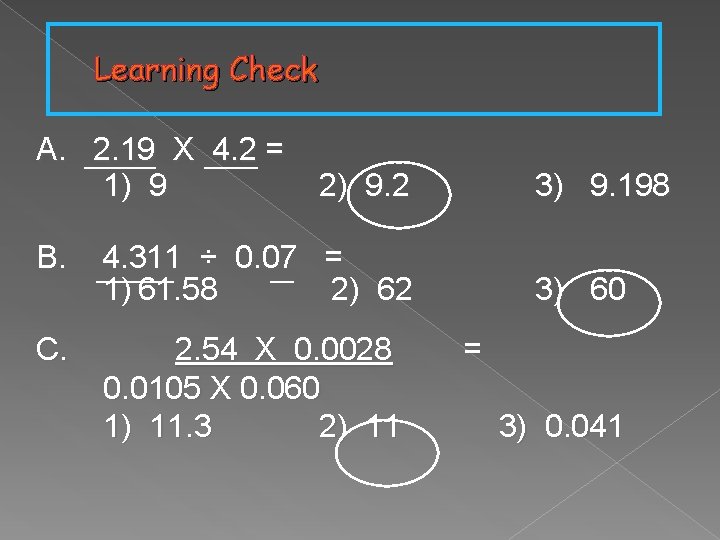
Learning Check A. 2. 19 X 4. 2 = 1) 9 2) 9. 2 B. 4. 311 ÷ 0. 07 = 1) 61. 58 2) 62 C. 2. 54 X 0. 0028 0. 0105 X 0. 060 1) 11. 3 2) 11 3) 9. 198 3) 60 = 3) 0. 041

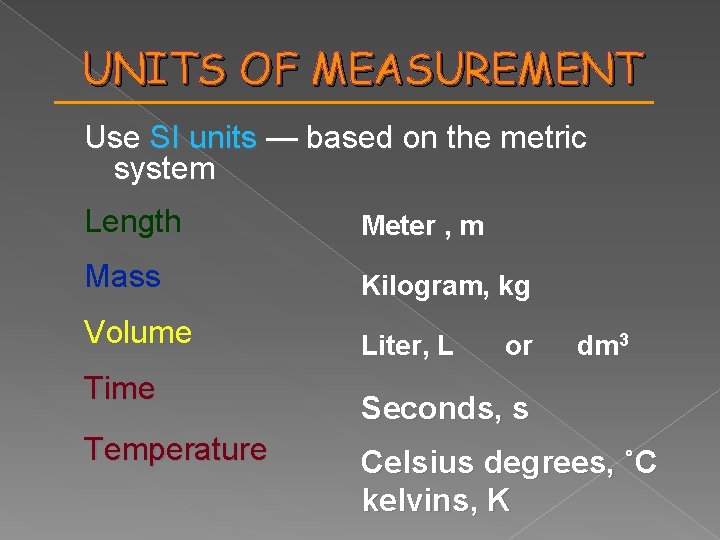
UNITS OF MEASUREMENT Use SI units — based on the metric system Length Meter , m Mass Kilogram, kg Volume Liter, L Time Temperature or dm 3 Seconds, s Celsius degrees, ˚C kelvins, K

Mass vs. Weight Mass: Amount of Matter (grams, measured with a BALANCE) Weight: Force exerted by the mass, only present with gravity (pounds, measured with a SCALE) Can you hear me now?

Metric Prefixes Kilo- means 1000 of that unit ex: 1 kilometer (km) = 1000 meters (m) 1 kilo-unit = 1000 units Centi- means 1/100 of that unit ex: 1 meter (m) = 100 centimeters (cm) 1 unit = 100 centi-units Milli- means 1/1000 of that unit ex: 1 Liter (L) = 1000 milliliters (m. L) 1 unit = 1000 milli-units

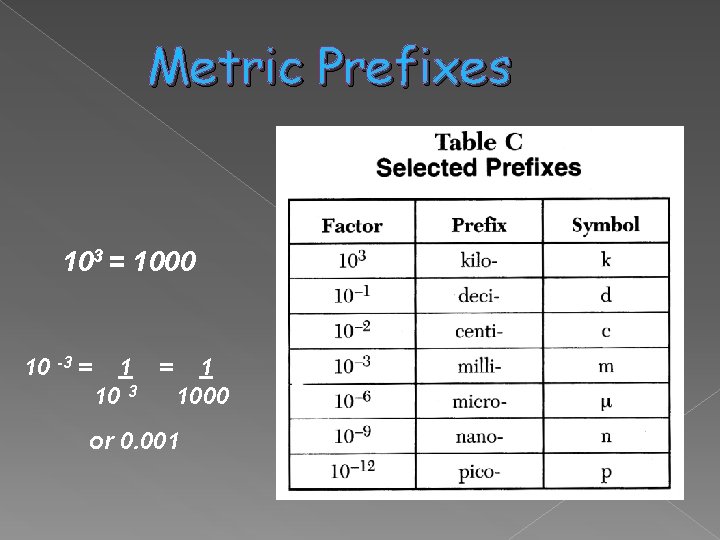
Metric Prefixes 103 = 1000 10 -3 = 1 10 3 1000 or 0. 001

Metric Prefixes in action A unit of Computer information is a byte Older computers could work with 64, 000 bytes at one time 64 thousand bytes 64 kilobytes OR 64 kb Later computers could work with 64, 000 bytes at one time 64 million 64 megabytes 64 mb The newest computers can work with Billions of bytes = gigabytes Trillions of bytes = terabytes A 1 Terabyte hard disk drive holds 1 trillion bytes of info = 1, 000, 000

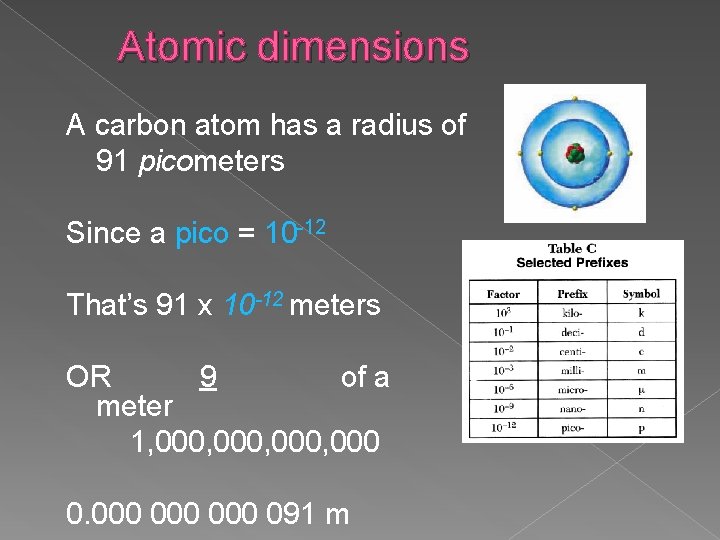
Atomic dimensions A carbon atom has a radius of 91 picometers Since a pico = 10 -12 That’s 91 x 10 -12 meters OR 9 of a meter 1, 000, 000 000 091 m

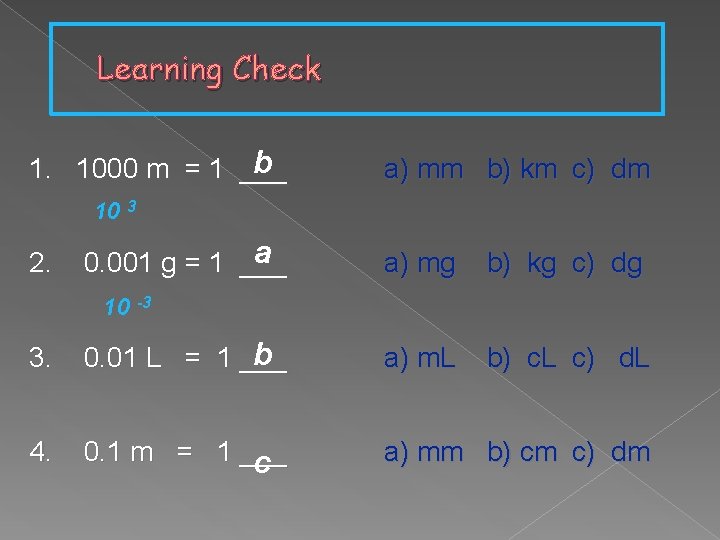
Learning Check b 1. 1000 m = 1 ___ a) mm b) km c) dm 10 3 2. a 0. 001 g = 1 ___ a) mg b) kg c) dg b) c. L c) d. L 10 -3 3. b 0. 01 L = 1 ___ a) m. L 4. 0. 1 m = 1 ___ c a) mm b) cm c) dm

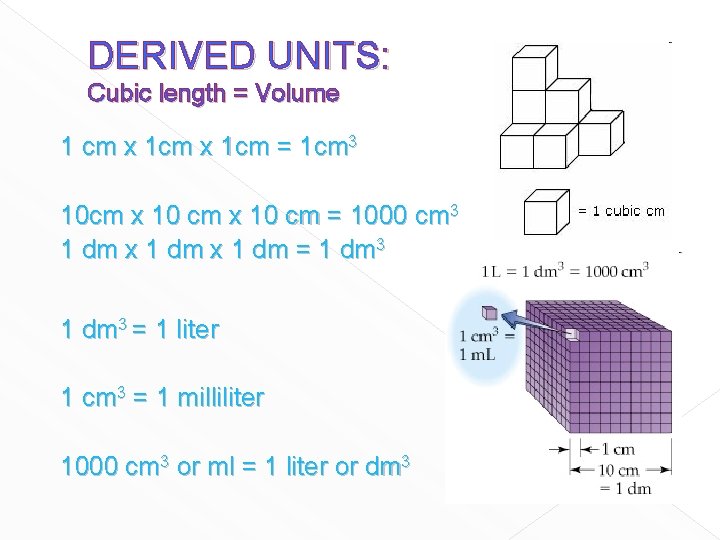
DERIVED UNITS: Cubic length = Volume 1 cm x 1 cm = 1 cm 3 10 cm x 10 cm = 1000 cm 3 1 dm x 1 dm = 1 dm 3 = 1 liter 1 cm 3 = 1 milliliter 1000 cm 3 or ml = 1 liter or dm 3

Converting between units Uses the factor – label method Also called dimensional analysis Involves conversion factors (fractions) Attention is paid to the labels (units) Must know equalities between units

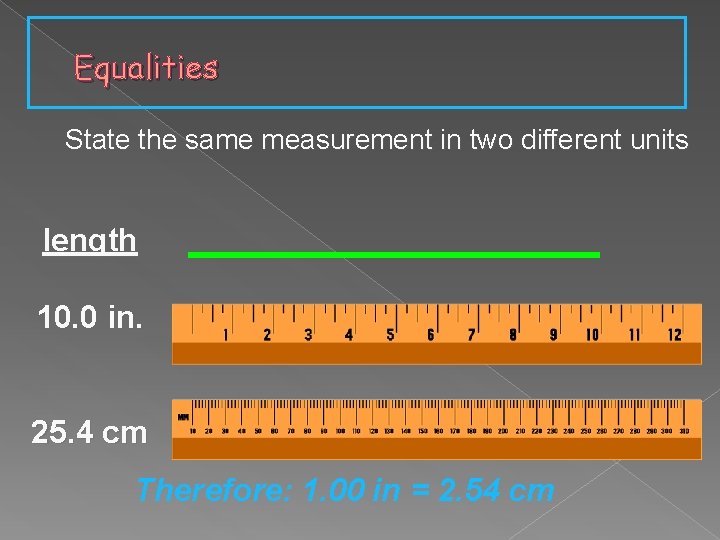
Equalities State the same measurement in two different units length 10. 0 in. 25. 4 cm Therefore: 1. 00 in = 2. 54 cm

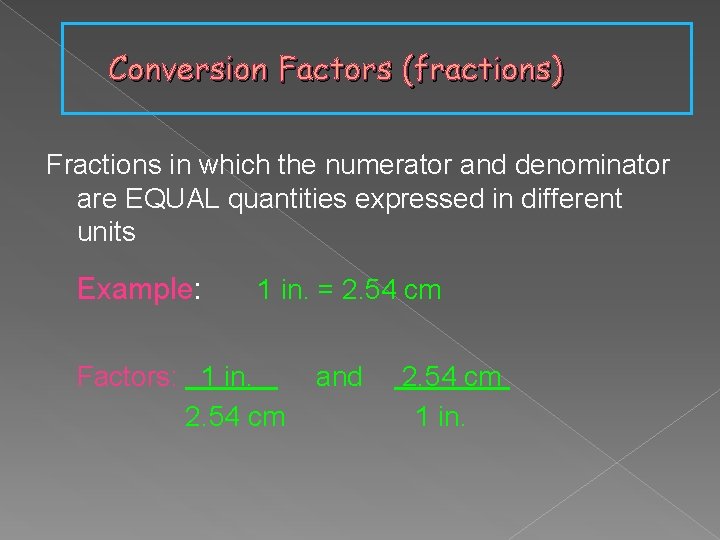
Conversion Factors (fractions) Fractions in which the numerator and denominator are EQUAL quantities expressed in different units Example: 1 in. = 2. 54 cm Factors: 1 in. 2. 54 cm and 2. 54 cm 1 in.

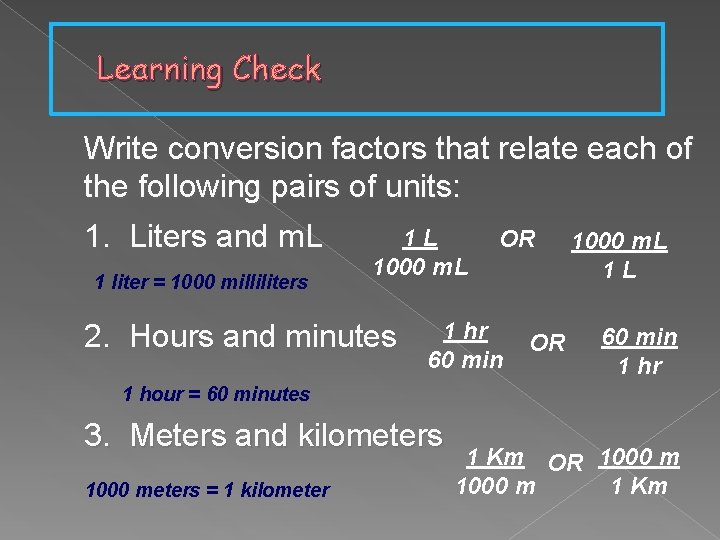
Learning Check Write conversion factors that relate each of the following pairs of units: 1 L 1. Liters and m. L OR 1000 m. L 1 liter = 1000 milliliters 1000 m. L 2. Hours and minutes 1 hr 60 min 1 L OR 60 min 1 hr 1 hour = 60 minutes 3. Meters and kilometers 1000 meters = 1 kilometer 1 Km OR 1000 m 1 Km

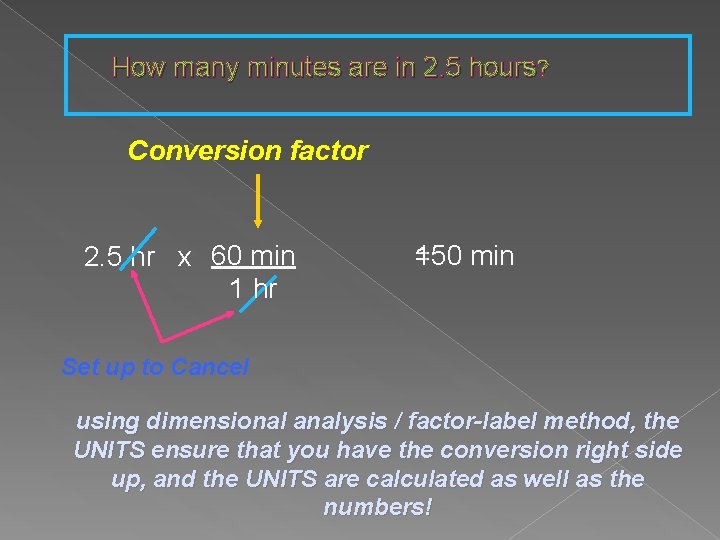
Setting up conversions How many minutes are in 2. 5 hours? Identify the starting and ending units: hours to minutes Recognize the equality between the unit: 1 hour = 60 minutes Build conversion factor with ending unit over starting unit: 60 min 1 hour Ending: ? minutes Starting: 2. 5 hours

How many minutes are in 2. 5 hours? Conversion factor 2. 5 hr x 60 min 1 hr 150 min = Set up to Cancel using dimensional analysis / factor-label method, the UNITS ensure that you have the conversion right side up, and the UNITS are calculated as well as the numbers!

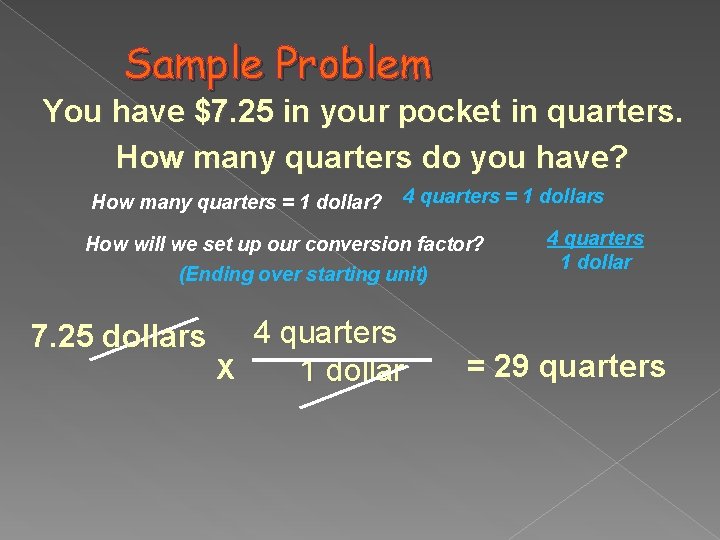
Sample Problem You have $7. 25 in your pocket in quarters. How many quarters do you have? How many quarters = 1 dollar? 4 quarters = 1 dollars How will we set up our conversion factor? (Ending over starting unit) 7. 25 dollars 4 quarters X 1 dollar 4 quarters 1 dollar = 29 quarters

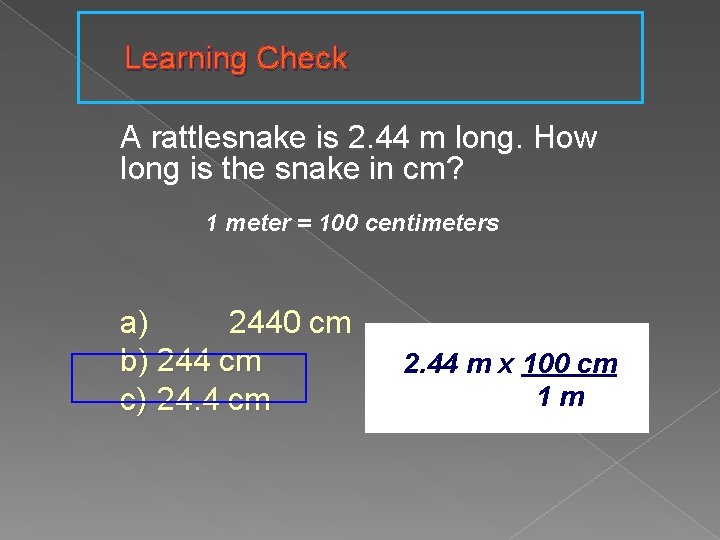
Learning Check A rattlesnake is 2. 44 m long. How long is the snake in cm? 1 meter = 100 centimeters a) 2440 cm b) 244 cm c) 24. 4 cm 2. 44 m x 100 cm 1 m

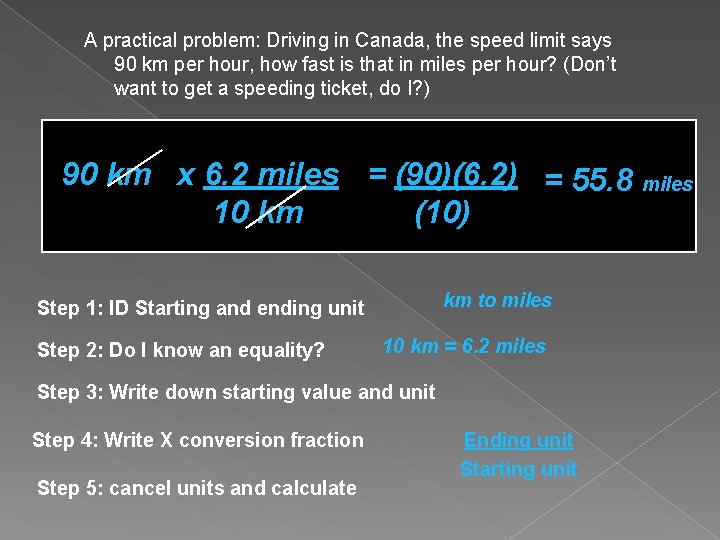
A practical problem: Driving in Canada, the speed limit says 90 km per hour, how fast is that in miles per hour? (Don’t want to get a speeding ticket, do I? ) 90 km x 6. 2 miles = (90)(6. 2) = 55. 8 miles 10 km (10) km to miles Step 1: ID Starting and ending unit Step 2: Do I know an equality? 10 km = 6. 2 miles Step 3: Write down starting value and unit Step 4: Write X conversion fraction Step 5: cancel units and calculate Ending unit Starting unit

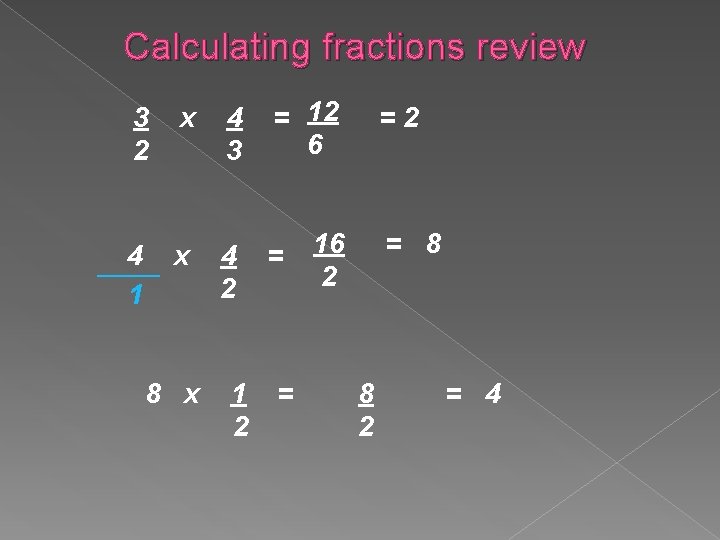
Calculating fractions review x 4 3 = 12 6 4 x ____ 1 4 2 = 3 2 8 x 1 2 = =2 16 2 = 8 8 2 = 4

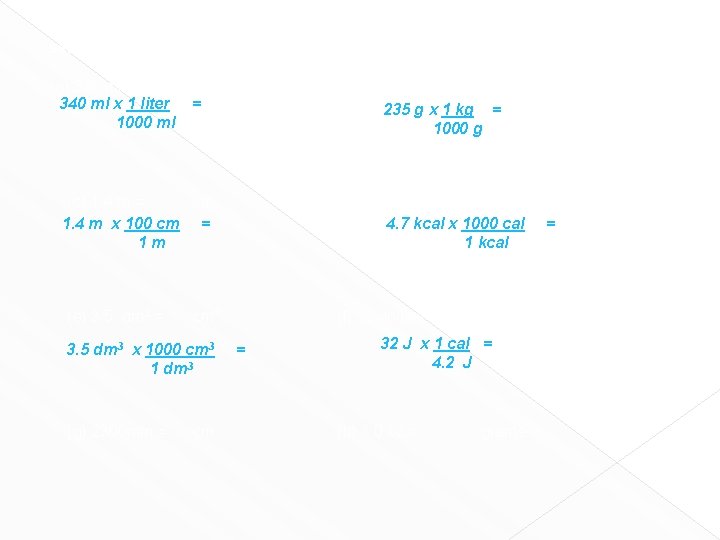
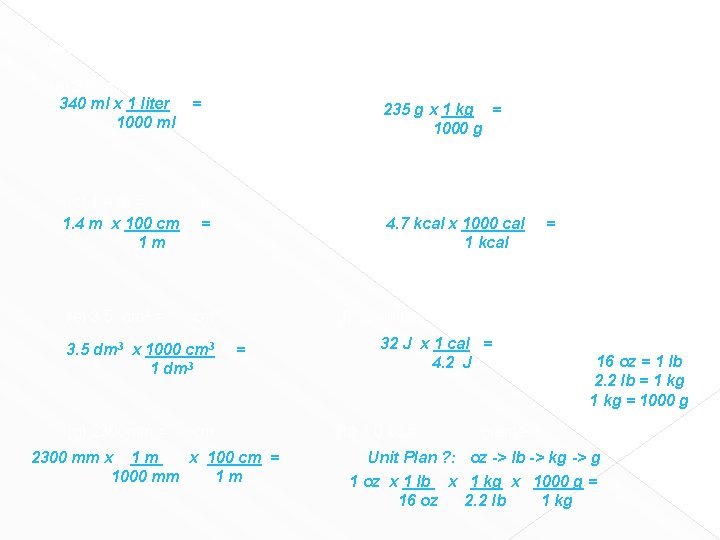
Worksheet # 8 Convert the following into new units. Show labeled factor label setup. (a) 340 ml = 340 ml x 1 liter 1000 ml L = (b) 235 g = Kg 235 g x 1 kg = 1000 g (c) 1. 4 m = cm 1. 4 m x 100 cm = 1 m (e) 3. 5 dm 3 = cm 3 3. 5 dm 3 x 1000 cm 3 1 dm 3 (g) 2300 mm = (d) 4. 7 Kilocalories = 4. 7 kcal x 1000 cal 1 kcal cm (f) 32 Joules = = calories 32 J x 1 cal = 4. 2 J (h) 1. 0 oz = grams calories =

Sample Problem – multi-step problems How many seconds are in 1. 4 days? Unit plan: days hr min seconds 1. 4 days x 24 hr 1 day x 60 min x 60 sec = 1 hr 1 min = 120, 000 sec Check ending unit

Sample Problem – multi-step problems How many feet is a meter? Unit plan: 1 m cm in feet 1 meter x 100 cm x 1 inch x 1 m 2. 5 cm 1 foot = 12 inches How do we plug this into a calculator? 1 x 100 ÷ 2. 5 ÷ 12 = 3. 3 ft Multiply tops, divide by bottoms Check ending unit

Using Table C 45 nm = _____pm 45 nm X 10 -9 m 1 nm X 1 pm 10 -12 m -3 = 45 10 -3 0. 001 = 45000 pm

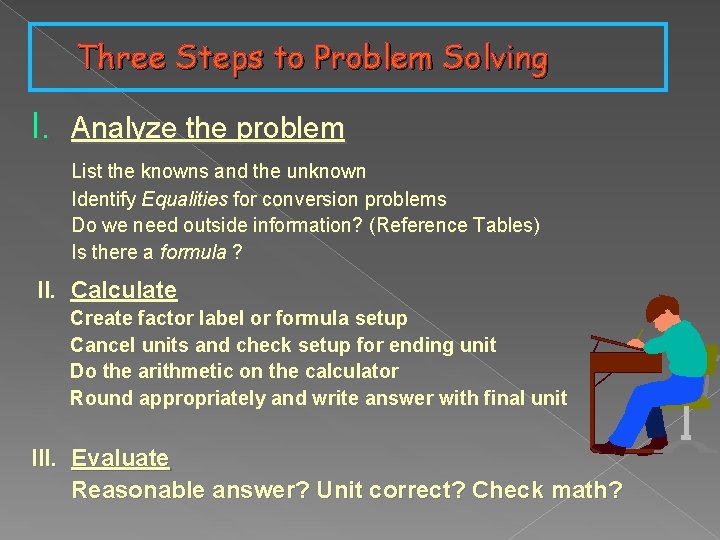
Three Steps to Problem Solving I. Analyze the problem List the knowns and the unknown Identify Equalities for conversion problems Do we need outside information? (Reference Tables) Is there a formula ? II. Calculate Create factor label or formula setup Cancel units and check setup for ending unit Do the arithmetic on the calculator Round appropriately and write answer with final unit III. Evaluate Reasonable answer? Unit correct? Check math?

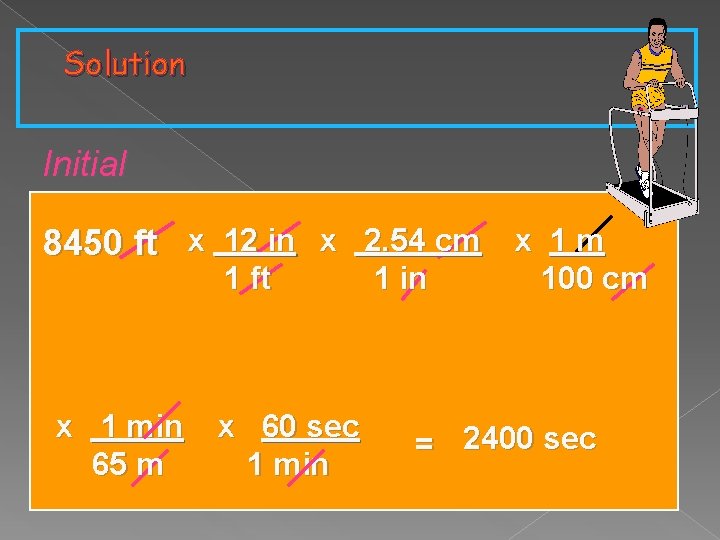
Dealing with Two Units If your pace on a treadmill is 65 meters per minute, how many seconds will it take for you to walk a distance of 8450 feet?

If your pace on a treadmill is 65 meters per minute, how many seconds will it take for you to walk a distance of 8450 feet? Knowns Starting: distance 8450 ft Rate (equality): 65 m / 1 min Unit plan: Unknown Ending unit : seconds ft inches cm meters Since rate is 65 m per minute Then 65 m = 1 minute Meters minutes seconds

Solution Initial 8450 ft x 12 in x 2. 54 cm x 1 m 1 ft x 1 min 65 m x 60 sec 1 min 100 cm = 2400 sec

Worksheet # 8 Convert the following into new units. Show labeled factor label setup. (a) 340 ml = 340 ml x 1 liter 1000 ml L = (b) 235 g = Kg 235 g x 1 kg = 1000 g (c) 1. 4 m = cm 1. 4 m x 100 cm = 1 m (e) 3. 5 dm 3 = cm 3 3. 5 dm 3 x 1000 cm 3 1 dm 3 (g) 2300 mm = (d) 4. 7 Kilocalories = 4. 7 kcal x 1000 cal 1 kcal (f) 32 Joules = = cm 2300 mm x 100 cm = 1000 mm 1 m = calories 32 J x 1 cal = 4. 2 J (h) 1. 0 oz = calories 16 oz = 1 lb 2. 2 lb = 1 kg = 1000 g grams Unit Plan ? : oz -> lb -> kg -> g 1 oz x 1 lb x 1 kg x 1000 g = 16 oz 2. 2 lb 1 kg

What about Square and Cubic units? Use the conversion factors you already know, but when you square or cube the unit, don’t forget to cube the number also! Best way: Square or cube the ENTIRE conversion factor Example: Convert 4. 3 cm 3 to mm 3 ( 3 = ) 4. 3 cm 3 10 mm 1 cm 4. 3 cm 3 1000 mm 3 1 cm 3 = 4300 mm 3

Temperature Scales Fahrenheit Celsius Kelvin Anders Celsius 1701 -1744 Lord Kelvin (William Thomson) 1824 -1907

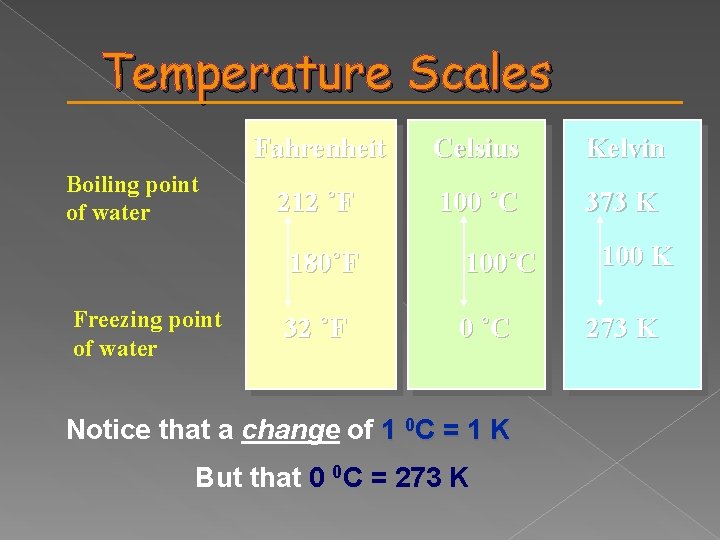
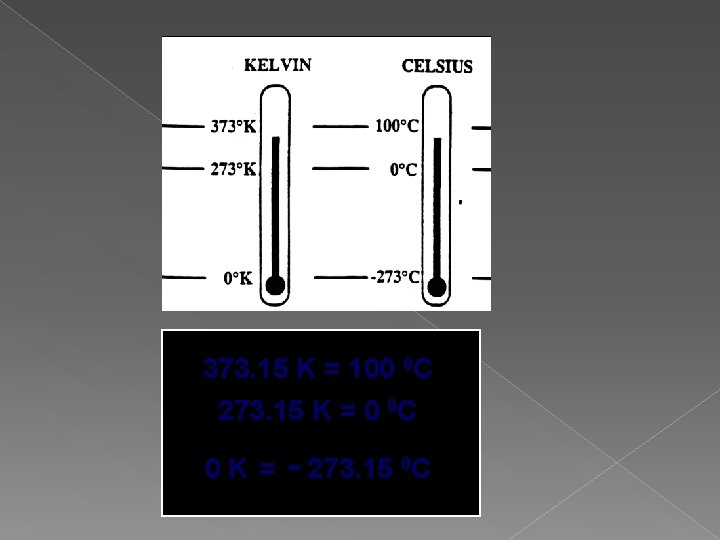
Temperature Scales Boiling point of water Freezing point of water Fahrenheit Celsius Kelvin 212 ˚F 100 ˚C 373 K 180˚F 100˚C 32 ˚F 0 ˚C Notice that a change of 1 0 C = 1 K But that 0 0 C = 273 K 100 K 273 K

Celsius is based on water Is a relative scale 0 0 C = normal melting point 100 0 C = normal boiling point Kelvin is based on molecular speed energy Is an absolute scale 0 K – absolute zero - molecules stop moving

373. 15 K = 100 0 C 273. 15 K = 0 0 C 0 K = - 273. 15 0 C

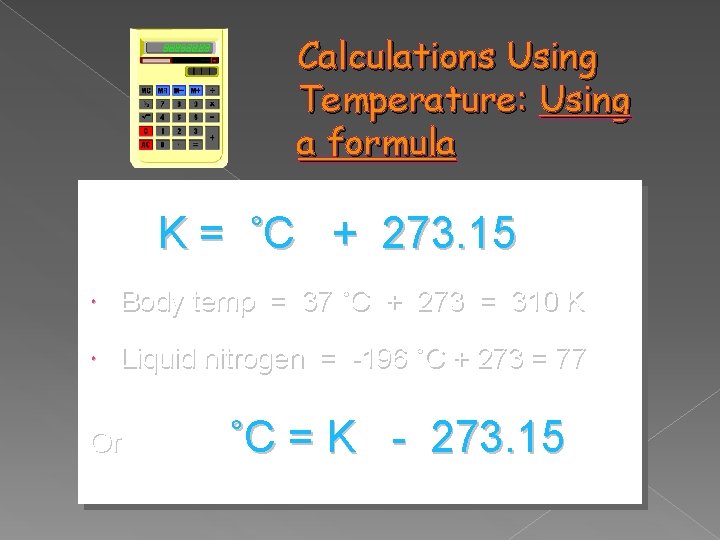
Calculations Using Temperature: Using a formula K = ˚C + 273. 15 Body temp = 37 ˚C + 273 = 310 K Liquid nitrogen = -196 ˚C + 273 = 77 Or ˚C = K - 273. 15

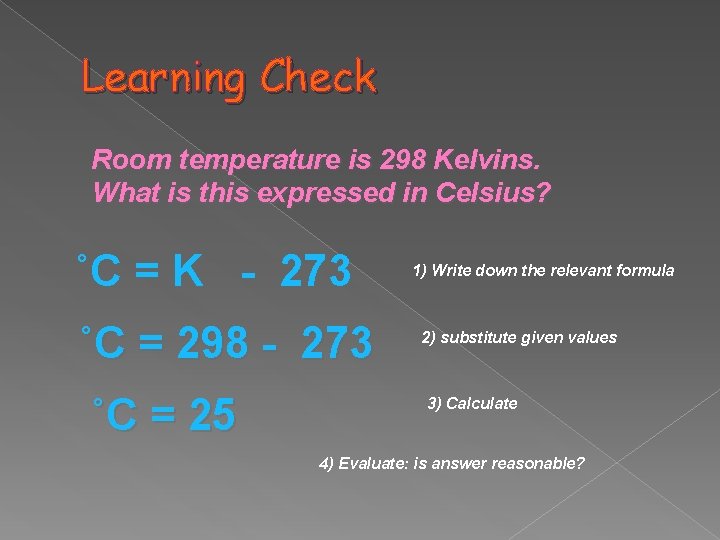
Learning Check Room temperature is 298 Kelvins. What is this expressed in Celsius? ˚C = K - 273 ˚C = 298 - 273 ˚C = 25 1) Write down the relevant formula 2) substitute given values 3) Calculate 4) Evaluate: is answer reasonable?

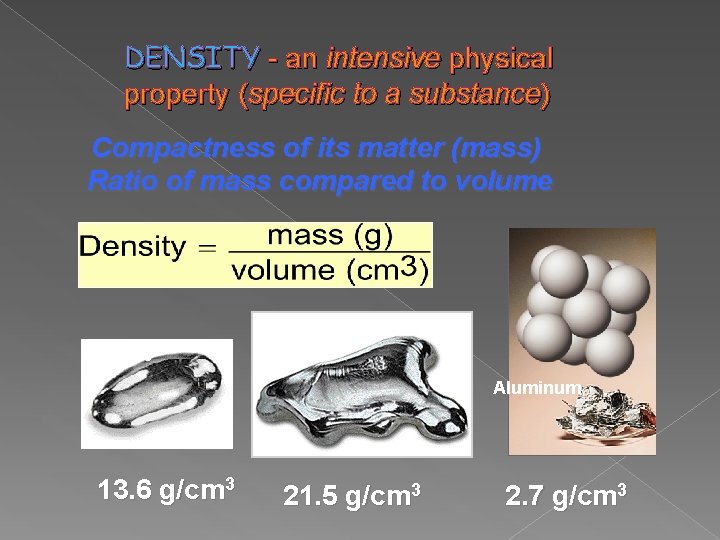
DENSITY - an intensive physical property (specific to a substance) Compactness of its matter (mass) Ratio of mass compared to volume Mercury Platinum Aluminum 13. 6 g/cm 3 21. 5 g/cm 3 2. 7 g/cm 3

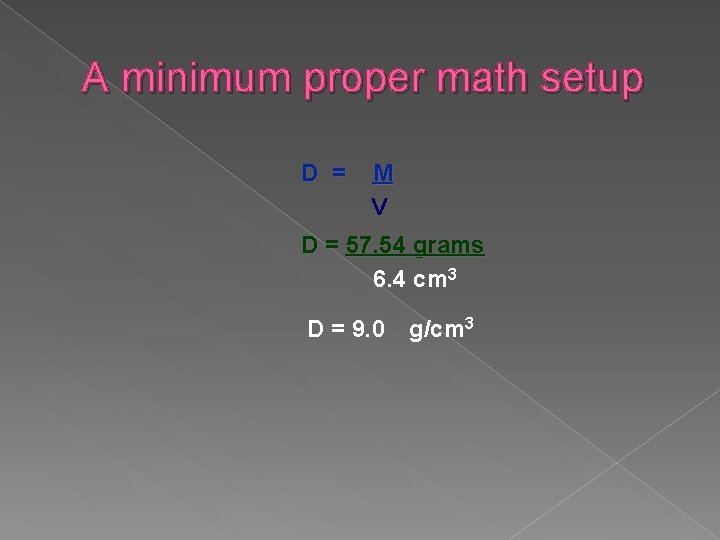
Problem A piece of copper has a mass of 57. 54 g. It displaces 6. 4 cm 3. Calculate its density (g/cm 3).

Using a formula 1. Analyze: List knowns and unknowns: Known: Mass = 57. 54 grams Volume = 6. 4 cm 3 Unknown: Density D = M write the formula: V 2. Setup problem by substituting values Solve, Round, And label 3. D = 57. 54 grams 6. 4 cm 3 D = 8. 9953125 D = 9. 0 g/cm 3 Evaluate answer: is it reasonable? what should be the mass of 1 of the 6 cm 3’s?

A minimum proper math setup D = M V D = 57. 54 grams 6. 4 cm 3 D = 9. 0 g/cm 3

PROBLEM: Mercury (Hg) has a density of 13. 6 g/cm 3. What is the mass of 95 m. L of Hg in grams? Solve the problem using DIMENSIONAL ANALYSIS.

PROBLEM: Mercury (Hg) has a density of 13. 6 g/cm 3. What is the mass of 95 m. L of Hg? 1. Analyze: knowns V = 95 m. L D = 13. 6 g/cm 3 or 13. 6 g = 1 cm 3 = 1 m. L unknowns - mass 2. Setup, calculate, round 95 cm 3 x 13. 6 g = 1 cm 3 1292 g =1300 g 3. Evalute: Is our answer reasonable?

Learning Check Osmium has a density of 22. 5 g/cm 3. What is the volume of a 50. 00 g sample? 1) 2) 3) 0. 45 2. 22 11. 3 cm 3 50. 00 g x 1 cm 3 22. 5 g

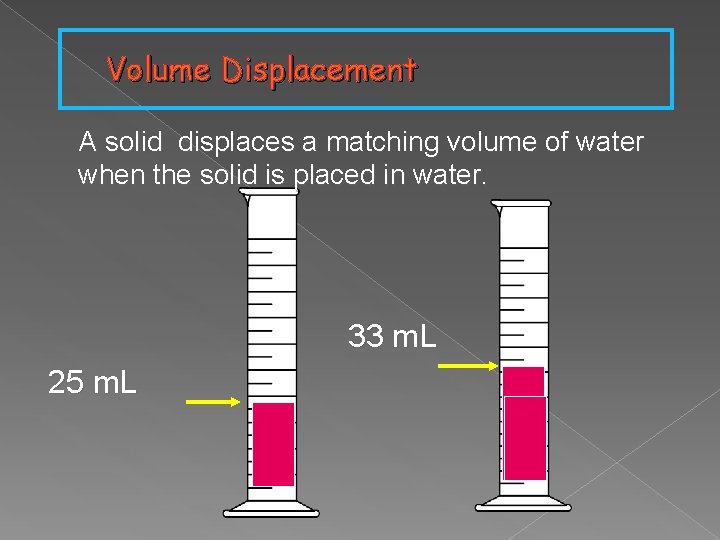
Volume Displacement A solid displaces a matching volume of water when the solid is placed in water. 33 m. L 25 m. L

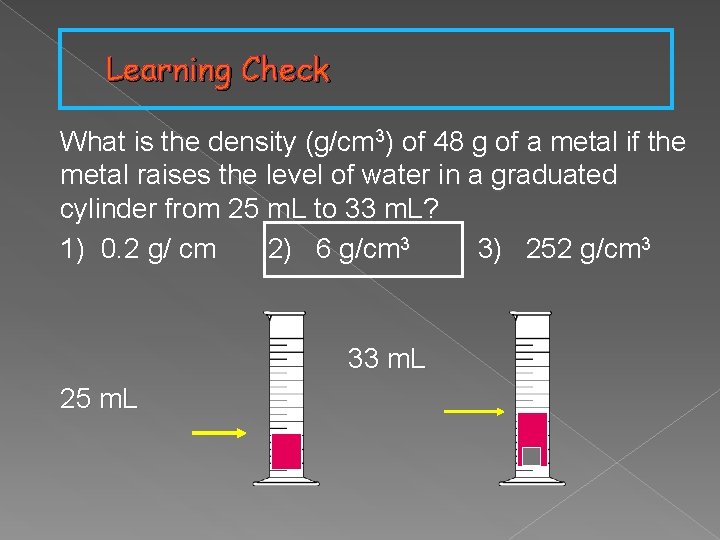
Learning Check What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? 1) 0. 2 g/ cm 2) 6 g/cm 3 3) 252 g/cm 3 33 m. L 25 m. L