THE METRIC SYSTEM SCIENTIFIC NOTATION Metric Conversions Ladder













- Slides: 17

THE METRIC SYSTEM & SCIENTIFIC NOTATION

Metric Conversions Ladder Method T. Trimpe 2008 http: //sciencespot. net/

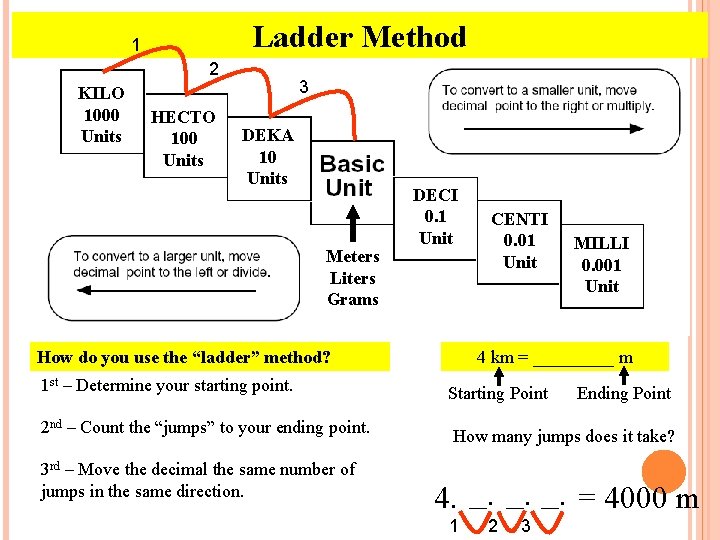
Ladder Method 1 2 KILO 1000 Units HECTO 100 Units 3 DEKA 10 Units Meters Liters Grams DECI 0. 1 Unit How do you use the “ladder” method? 1 st – Determine your starting point. 2 nd – Count the “jumps” to your ending point. 3 rd – Move the decimal the same number of jumps in the same direction. CENTI 0. 01 Unit MILLI 0. 001 Unit 4 km = _____ m Starting Point Ending Point How many jumps does it take? 4. __. __. = 4000 m 1 2 3

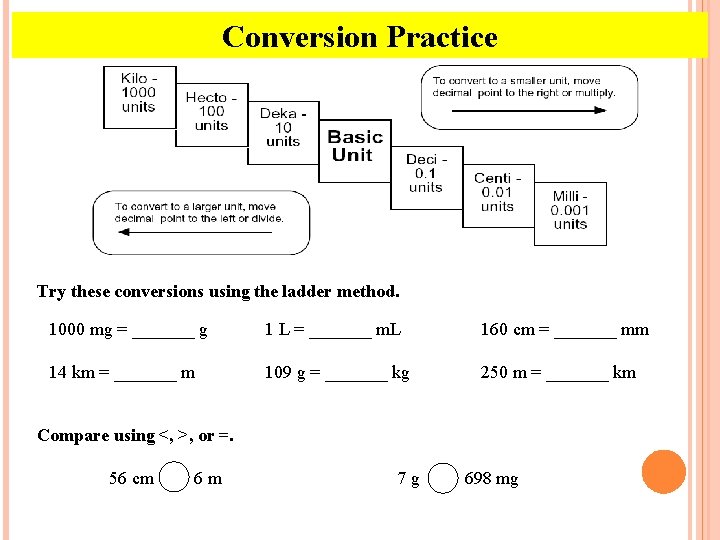
Conversion Practice Try these conversions using the ladder method. 1000 mg = _______ g 1 L = _______ m. L 160 cm = _______ mm 14 km = _______ m 109 g = _______ kg 250 m = _______ km Compare using <, >, or =. 56 cm 6 m 7 g 698 mg

SCIENTIFIC NOTATION Many of the numbers used in physics are extremely small or large.

SCIENTIFIC NOTATION Many of the numbers used in physics are extremely small or large. Therefore, it is important you understand scientific notation.

SCIENTIFIC NOTATION In scientific notation, the number is written as two parts. The first part is a number between 1 and 9.

SCIENTIFIC NOTATION The first part cannot be a number that starts or ends with a zero. Zeros can be in between but not at the beginning or end.

SCIENTIFIC NOTATION The second part is an exponent, written as a power of 10. The exponent tells you how many places to move the decimal point.

SCIENTIFIC NOTATION Example: 2. 6 x 103 ____ is the exponent and tells you that you need to move the decimal _____ places.

SCIENTIFIC NOTATION The sign of the exponent tells you which way to move the decimal point.

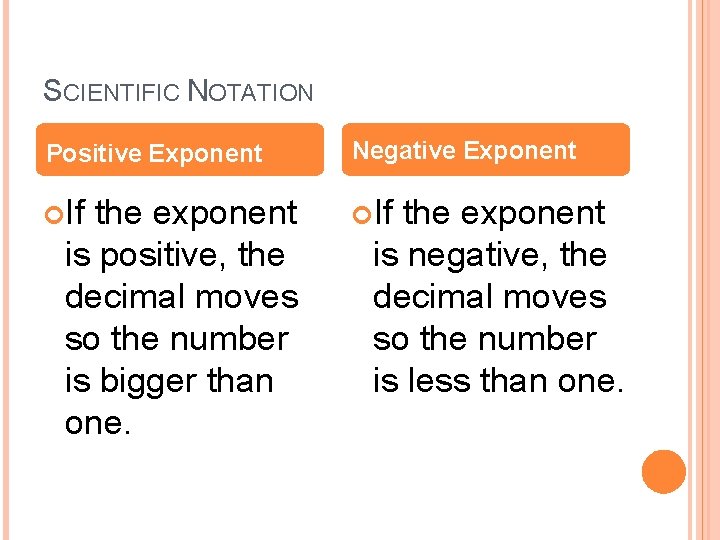
SCIENTIFIC NOTATION Positive Exponent Negative Exponent If the exponent is positive, the decimal moves so the number is bigger than one. the exponent is negative, the decimal moves so the number is less than one.

SCIENTIFIC NOTATION Examples: 3. 1 x 10 -3 6. 25 x 104

SCIENTIFIC NOTATION Putting numbers into scientific notation requires moving the decimal point so there is only one number in front of it.

If you don’t see a decimal point, it is at the end of the number. Remember, a zero can’t be the first number.

To put numbers into scientific notation, count the number of places you had to move the decimal point, so that only one number is in front of it. That number becomes your exponent.

The sign of the exponent depends on your original number. If the number was bigger than one, the sign is positive. If the number was less than one, the sign is negative.