Chapter 3 FLUID STATIC PRESSURE PRESSURE l l










- Slides: 46

Chapter 3 FLUID STATIC - PRESSURE

PRESSURE l l l Pressure is defined as a normal force exerted by a fluid per unit area. Units of pressure are N/m 2, which is called a pascal (Pa). Since the unit Pa is too small for pressures encountered in practice, kilopascal (1 k. Pa = 103 Pa) and megapascal (1 MPa = 106 Pa) are commonly used. 1 atm = 101, 325 Pa = 101. 325 k. Pa = 1. 01325 bar Other units include kgf/cm 2, lbf/in 2=psi. 1 kgf/cm 2 = 9. 807 N/cm 2 = 14. 223 psi Sphygmometer A few pictures of Pressure applications Compressor Clinical mercury Manometer Spray Gun

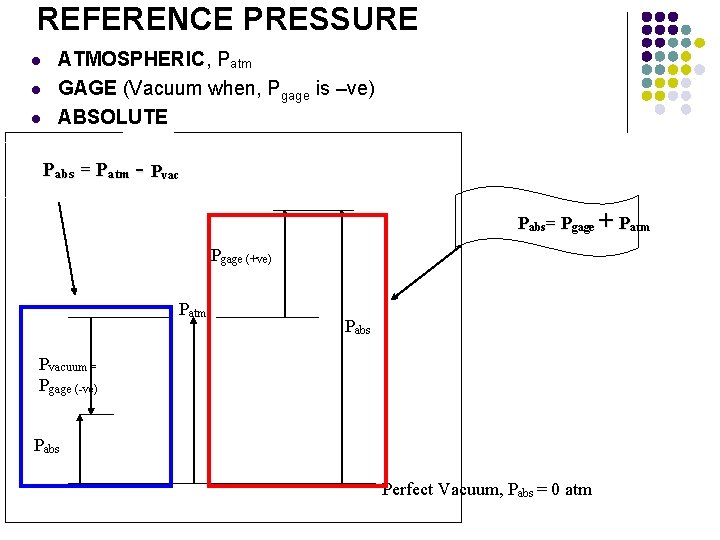
REFERENCE PRESSURE l l l ATMOSPHERIC, Patm GAGE (Vacuum when, Pgage is –ve) ABSOLUTE Pabs = Patm - Pvac Pabs= Pgage + Patm Pgage (+ve) Patm Pabs Pvacuum = Pgage (-ve) Pabs Perfect Vacuum, Pabs = 0 atm

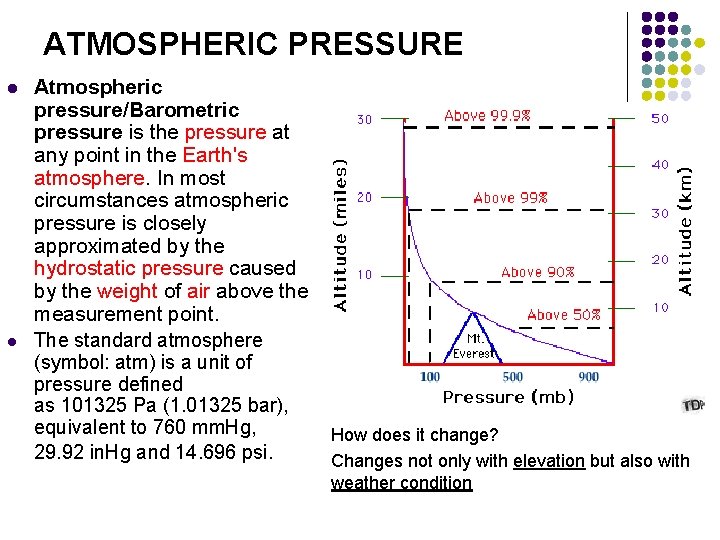
ATMOSPHERIC PRESSURE l l Atmospheric pressure/Barometric pressure is the pressure at any point in the Earth's atmosphere. In most circumstances atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point. The standard atmosphere (symbol: atm) is a unit of pressure defined as 101325 Pa (1. 01325 bar), equivalent to 760 mm. Hg, How does it change? 29. 92 in. Hg and 14. 696 psi. Changes not only with elevation but also with weather condition

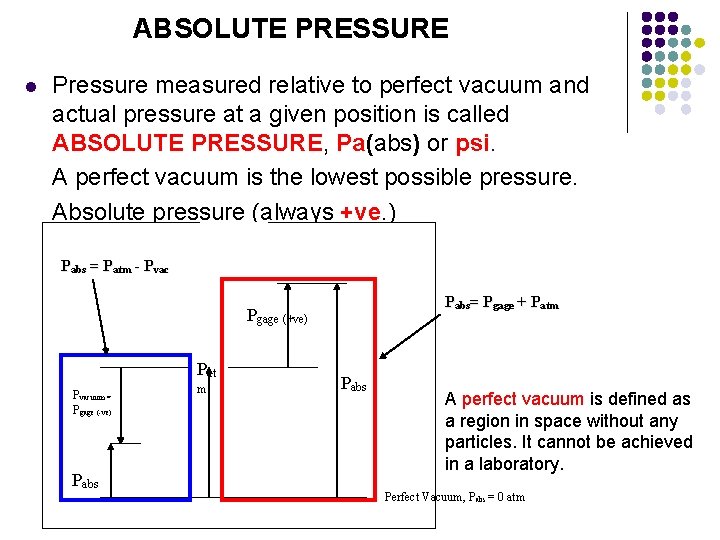
ABSOLUTE PRESSURE l Pressure measured relative to perfect vacuum and actual pressure at a given position is called ABSOLUTE PRESSURE, Pa(abs) or psi. A perfect vacuum is the lowest possible pressure. Absolute pressure (always +ve. ) Pabs = Patm - Pvac Pabs= Pgage + Patm Pgage (+ve) Pat Pvacuum = Pgage (-ve) Pabs m Pabs A perfect vacuum is defined as a region in space without any particles. It cannot be achieved in a laboratory. Perfect Vacuum, Pabs = 0 atm

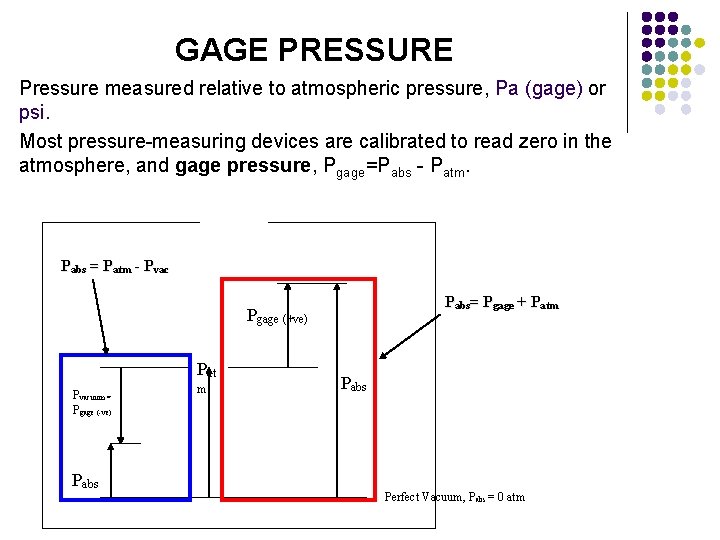
GAGE PRESSURE Pressure measured relative to atmospheric pressure, Pa (gage) or psi. Most pressure-measuring devices are calibrated to read zero in the atmosphere, and gage pressure, Pgage=Pabs - Patm. Pabs = Patm - Pvac Pabs= Pgage + Patm Pgage (+ve) Pat Pvacuum = Pgage (-ve) m Pabs Perfect Vacuum, Pabs = 0 atm

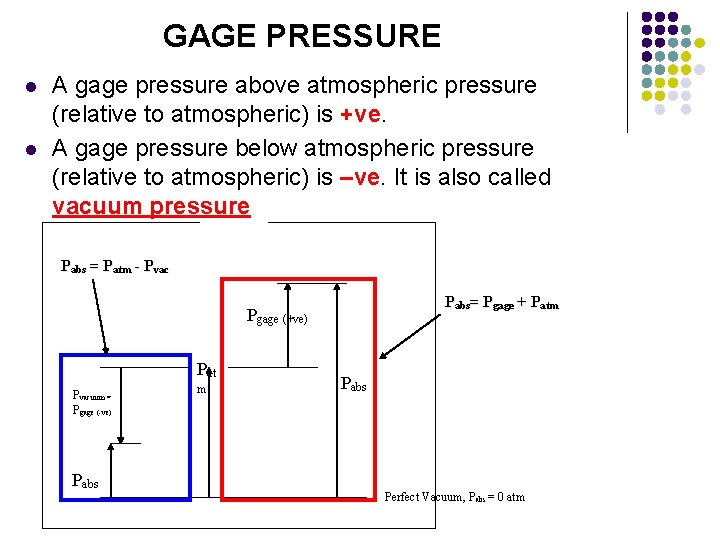
GAGE PRESSURE l l A gage pressure above atmospheric pressure (relative to atmospheric) is +ve. A gage pressure below atmospheric pressure (relative to atmospheric) is –ve. It is also called vacuum pressure Pabs = Patm - Pvac Pabs= Pgage + Patm Pgage (+ve) Pat Pvacuum = Pgage (-ve) m Pabs Perfect Vacuum, Pabs = 0 atm

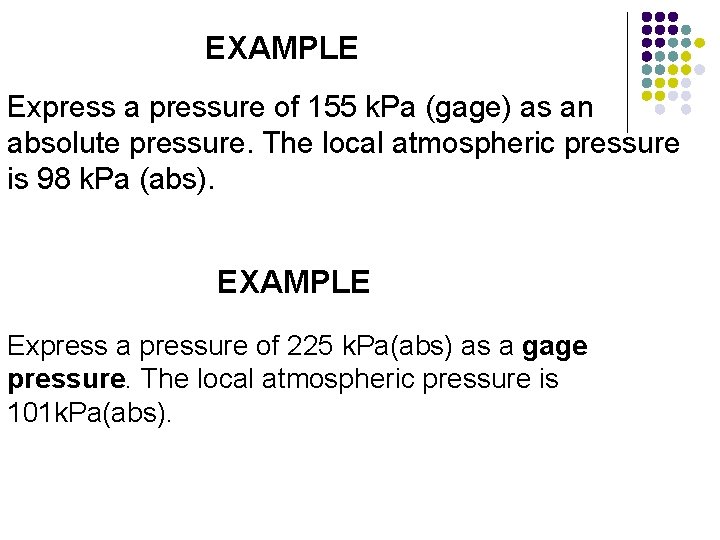
EXAMPLE Express a pressure of 155 k. Pa (gage) as an absolute pressure. The local atmospheric pressure is 98 k. Pa (abs). EXAMPLE Express a pressure of 225 k. Pa(abs) as a gage pressure. The local atmospheric pressure is 101 k. Pa(abs).

EXAMPLE Express a pressure of 75. 2 k. Pa (abs) as a gage pressure. The local atmospheric pressure is 103. 4 k. Pa. EXAMPLE Express a pressure of – 42. 7 k. Pa as an absolute pressure. Assume, Patm=101 k. Pa:

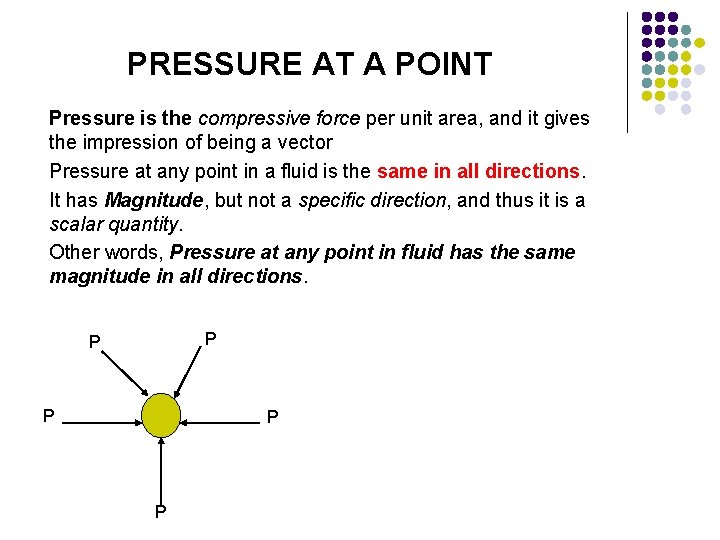
PRESSURE AT A POINT Pressure is the compressive force per unit area, and it gives the impression of being a vector Pressure at any point in a fluid is the same in all directions. It has Magnitude, but not a specific direction, and thus it is a scalar quantity. Other words, Pressure at any point in fluid has the same magnitude in all directions. P P P

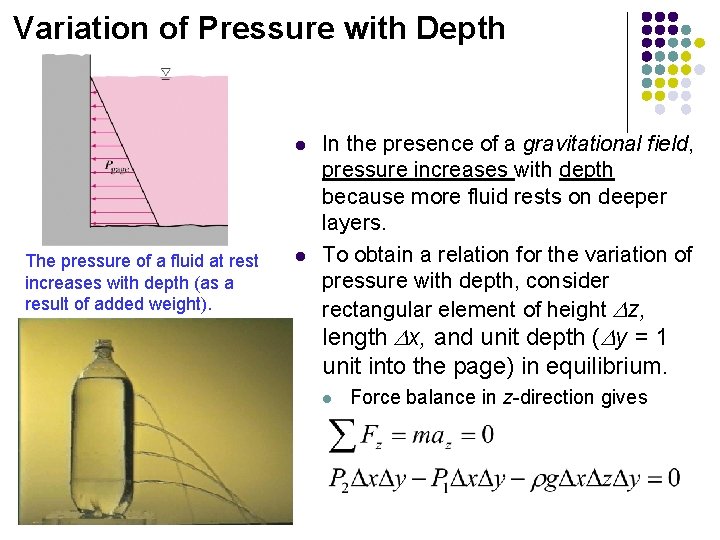
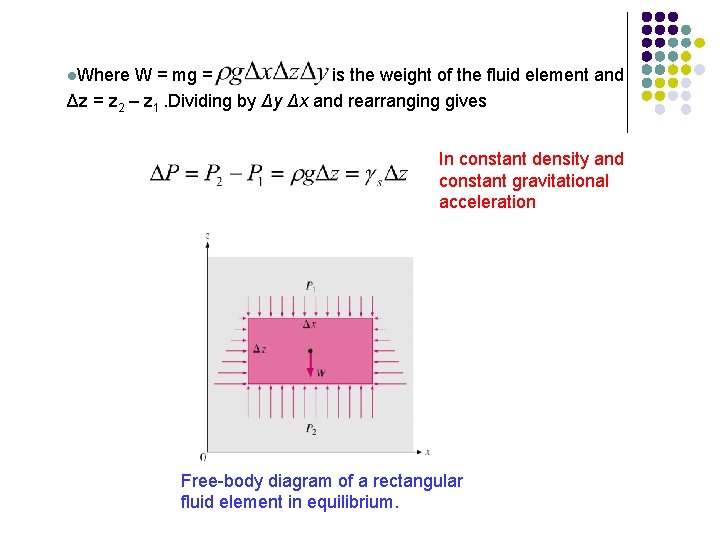
Variation of Pressure with Depth l The pressure of a fluid at rest increases with depth (as a result of added weight). l In the presence of a gravitational field, pressure increases with depth because more fluid rests on deeper layers. To obtain a relation for the variation of pressure with depth, consider rectangular element of height Dz, length Dx, and unit depth (Dy = 1 unit into the page) in equilibrium. l Force balance in z-direction gives

l. Where W = mg = is the weight of the fluid element and Δz = z 2 – z 1. Dividing by Δy Δx and rearranging gives In constant density and constant gravitational acceleration Free-body diagram of a rectangular fluid element in equilibrium.

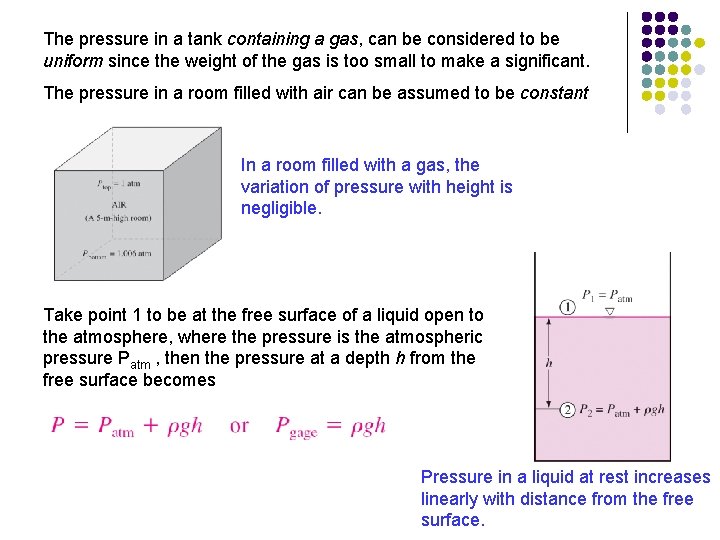
The pressure in a tank containing a gas, can be considered to be uniform since the weight of the gas is too small to make a significant. The pressure in a room filled with air can be assumed to be constant In a room filled with a gas, the variation of pressure with height is negligible. Take point 1 to be at the free surface of a liquid open to the atmosphere, where the pressure is the atmospheric pressure Patm , then the pressure at a depth h from the free surface becomes Pressure in a liquid at rest increases linearly with distance from the free surface.

When the variation of density with elevation is known, the pressure difference between point 1 and 2 can be determined by integration

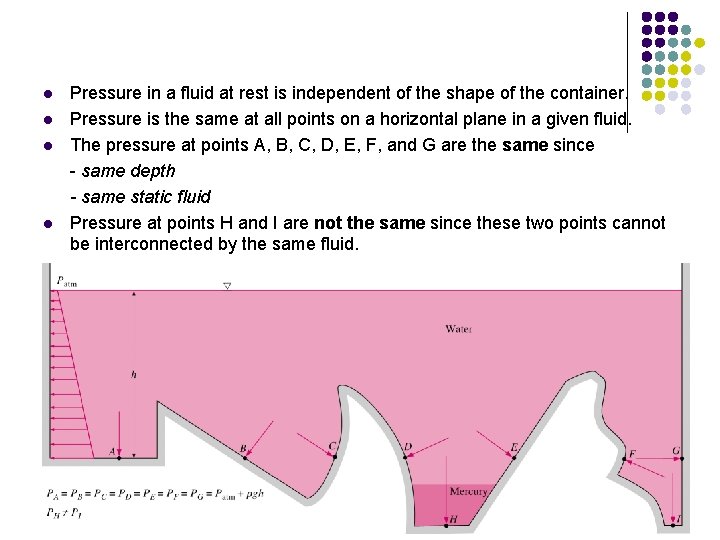
l l Pressure in a fluid at rest is independent of the shape of the container. Pressure is the same at all points on a horizontal plane in a given fluid. The pressure at points A, B, C, D, E, F, and G are the same since - same depth - same static fluid Pressure at points H and I are not the same since these two points cannot be interconnected by the same fluid.

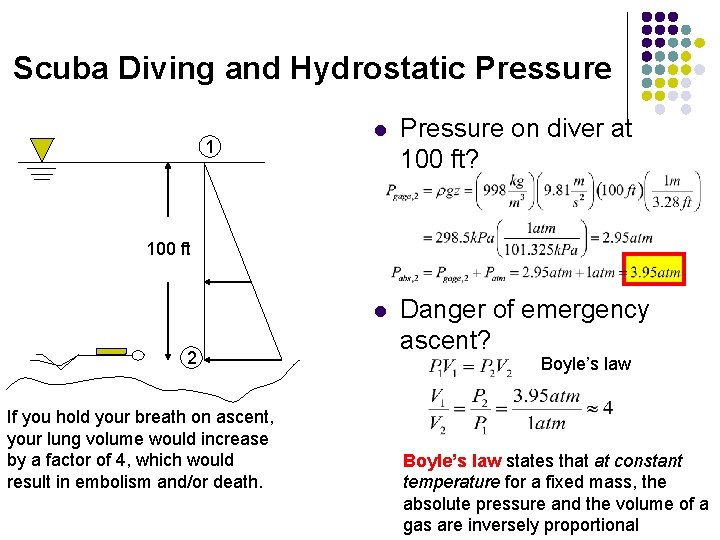
Scuba Diving and Hydrostatic Pressure

Scuba Diving and Hydrostatic Pressure 1 l Pressure on diver at 100 ft? l Danger of emergency ascent? 100 ft 2 If you hold your breath on ascent, your lung volume would increase by a factor of 4, which would result in embolism and/or death. Boyle’s law states that at constant temperature for a fixed mass, the absolute pressure and the volume of a gas are inversely proportional

EXAMPLE Calculate the change in water pressure from the surface (exposed to atmosphere) to a depth of 5 m. ANSWER If the surface of the water is exposed to the atmosphere, the pressure there is 0 Pa(gage). Descending in the water (decreasing elevation) produces an increase in pressure. Therefore, at 5 m the pressure is 49. 05 k. Pa(gage). P = ρgh = (1000 kg/m 3)(9. 81 m/s 2)(5 m) Pa.

*GAS PRESSURE VARIATION WITH ELEVATION Air density at sea level, 15 degree Celcius is 1. 225 kg/m 3. Pressure difference at 5 m height difference; ρgh = (1. 225 kg/m 3) * 9. 807 ms-2 * 5 m * (1 N/1 kgms-2) * ( 1 k. Pa/ 1000 N/m 2) = 0. 06 k. Pa 1 atm = 101. 325 k. Pa For gas, the variation of pressure with height is negligible, because of their low density. Also, weight is too small. However is accuracy is desired, it becomes significant. Gravitational Effect g = 9. 807 m/s 2 at sea level, at elevation 14, 000 m above sea level, g = 9. 764 m/s 2, which is 0. 4% change. Therefore, g variation is so small and g can be considered constant.

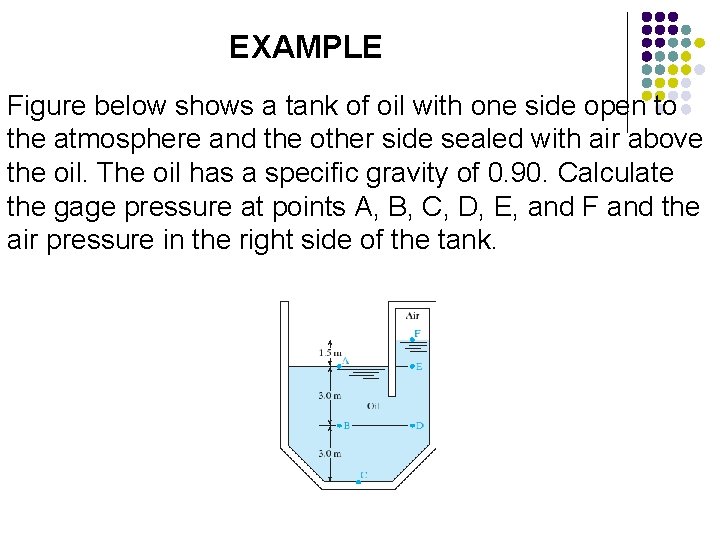
EXAMPLE Figure below shows a tank of oil with one side open to the atmosphere and the other side sealed with air above the oil. The oil has a specific gravity of 0. 90. Calculate the gage pressure at points A, B, C, D, E, and F and the air pressure in the right side of the tank.

EXAMPLE 6 Point A At this point, the oil is exposed to the atmosphere, and therefore Point B The change in elevation between point A and point B is 3. 0 m, with B lower than A. The specific weight of the oil: Then we have

EXAMPLE 6 Now, the pressure at B is Point C The change in elevation from point A to point C is 6. 0 m, with C lower than A. Then, the pressure at point C is Point D Because point D is at the same level as point B, the pressure is the same. That is, we have

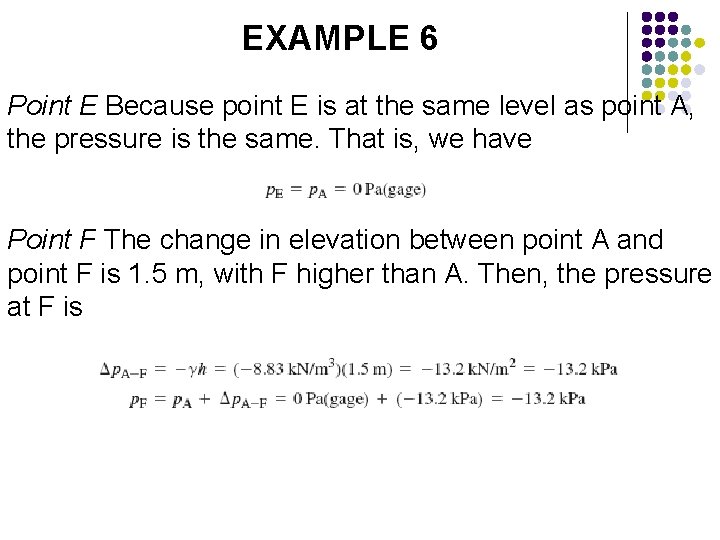
EXAMPLE 6 Point E Because point E is at the same level as point A, the pressure is the same. That is, we have Point F The change in elevation between point A and point F is 1. 5 m, with F higher than A. Then, the pressure at F is

EXAMPLE 6 Air Pressure in the right side of the tank is exposed to the surface of the oil, where p. F = -13. 2 k. Pa the air pressure is also -13. 2 k. Pa or 13. 2 k. Pa below atmospheric pressure.

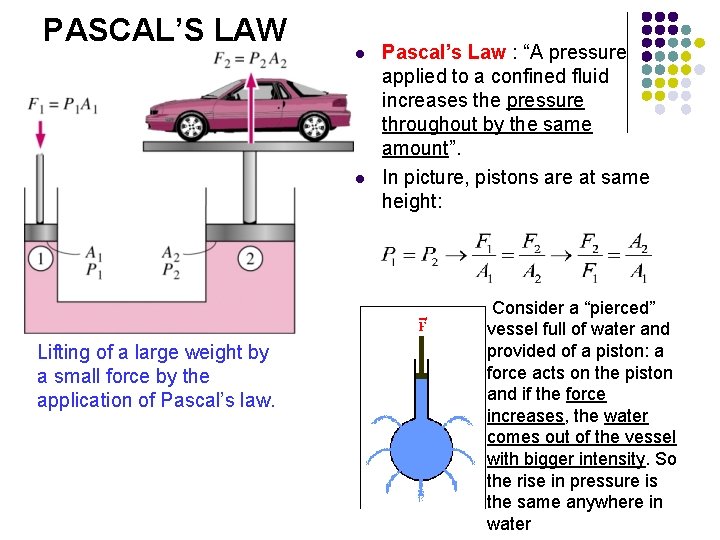
PASCAL’S LAW l l Lifting of a large weight by a small force by the application of Pascal’s law. Pascal’s Law : “A pressure applied to a confined fluid increases the pressure throughout by the same amount”. In picture, pistons are at same height: Consider a “pierced” vessel full of water and provided of a piston: a force acts on the piston and if the force increases, the water comes out of the vessel with bigger intensity. So the rise in pressure is the same anywhere in water

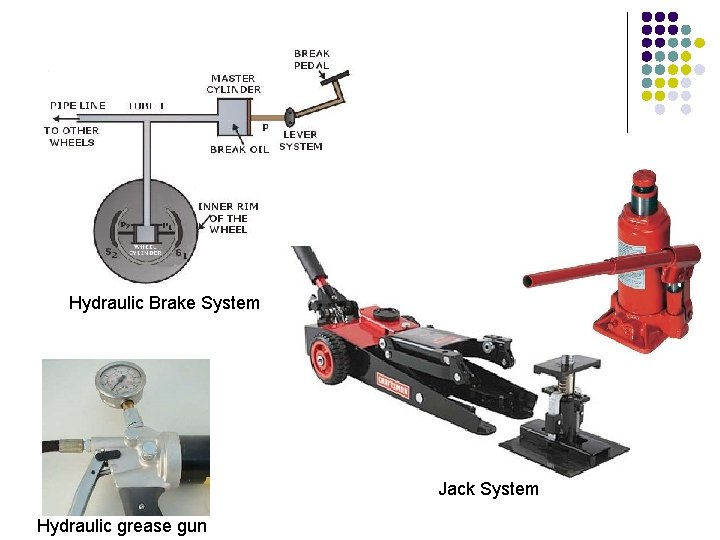
Hydraulic Brake System Jack System Hydraulic grease gun

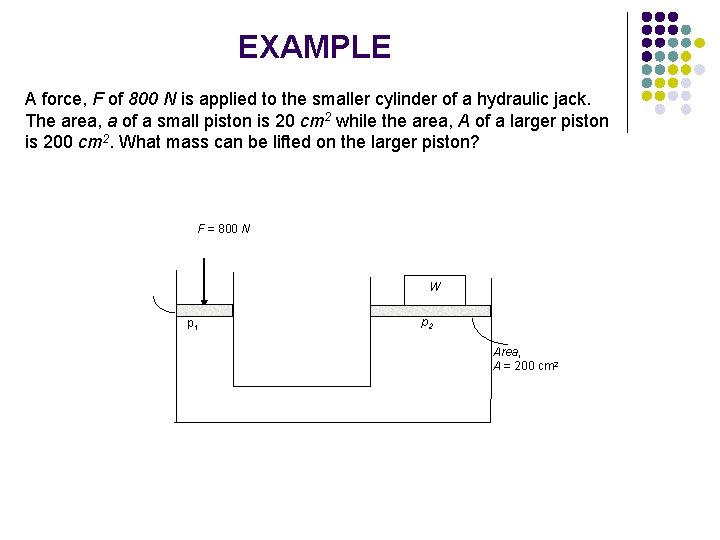
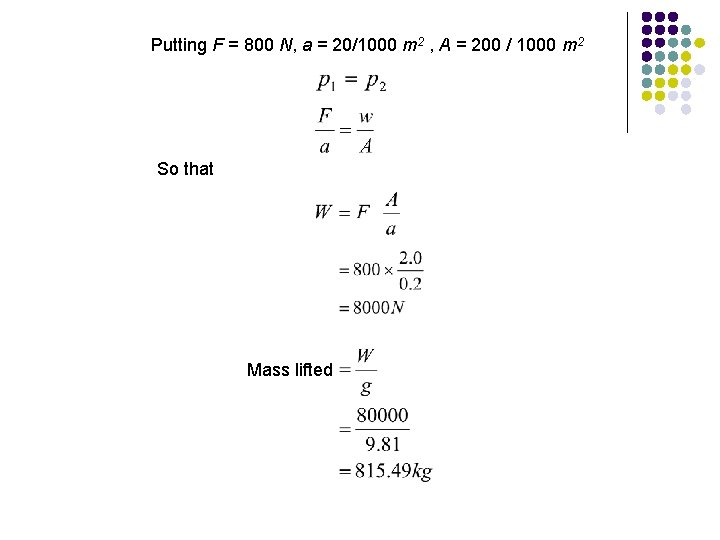
EXAMPLE A force, F of 800 N is applied to the smaller cylinder of a hydraulic jack. The area, a of a small piston is 20 cm 2 while the area, A of a larger piston is 200 cm 2. What mass can be lifted on the larger piston? F = 800 N W p 1 p 2 Area, A = 200 cm 2

Putting F = 800 N, a = 20/1000 m 2 , A = 200 / 1000 m 2 So that Mass lifted

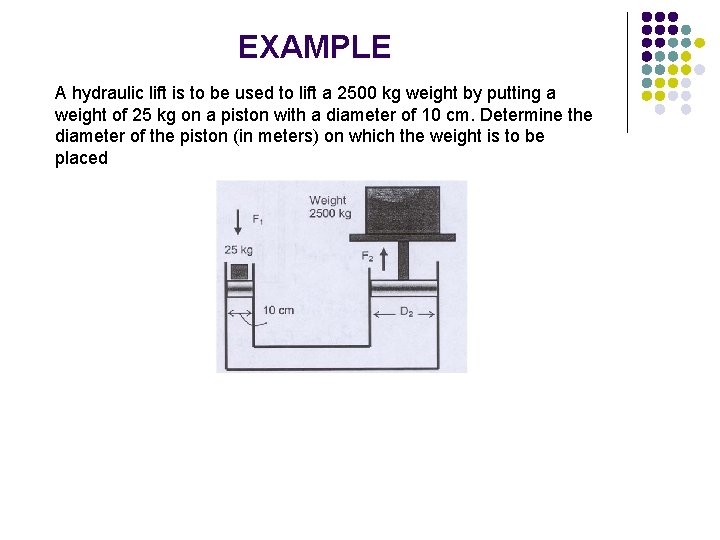
EXAMPLE A hydraulic lift is to be used to lift a 2500 kg weight by putting a weight of 25 kg on a piston with a diameter of 10 cm. Determine the diameter of the piston (in meters) on which the weight is to be placed

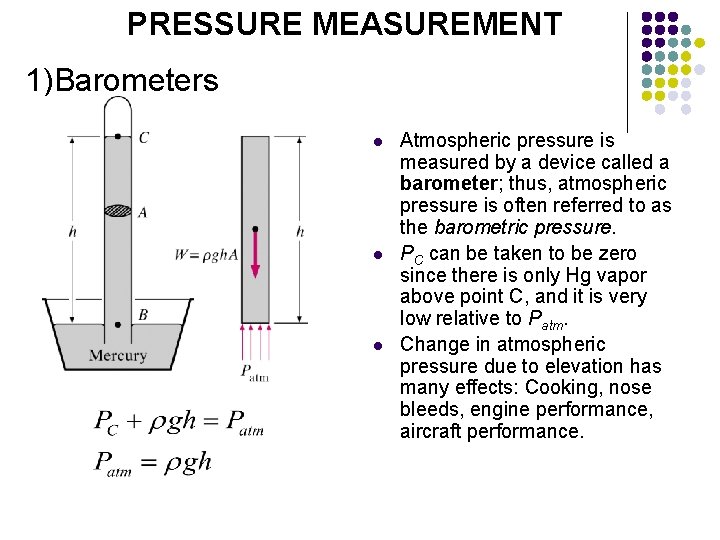
PRESSURE MEASUREMENT 1)Barometers l l l Atmospheric pressure is measured by a device called a barometer; thus, atmospheric pressure is often referred to as the barometric pressure. PC can be taken to be zero since there is only Hg vapor above point C, and it is very low relative to Patm. Change in atmospheric pressure due to elevation has many effects: Cooking, nose bleeds, engine performance, aircraft performance.

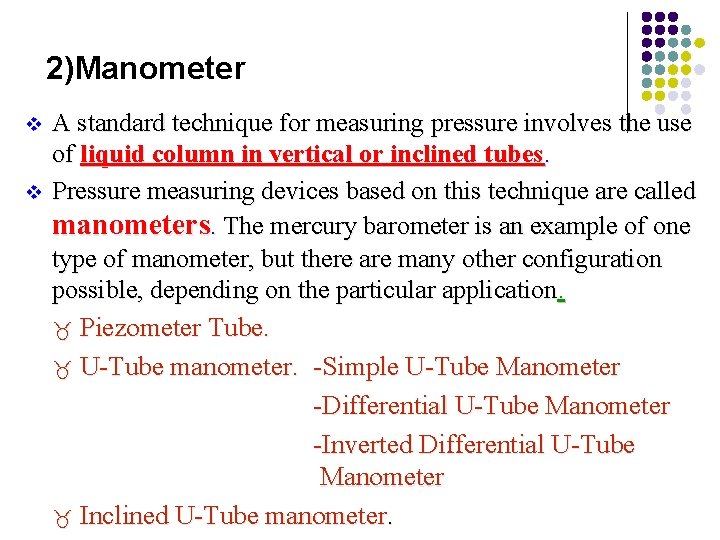
2)Manometer v v A standard technique for measuring pressure involves the use of liquid column in vertical or inclined tubes. Pressure measuring devices based on this technique are called manometers. The mercury barometer is an example of one type of manometer, but there are many other configuration possible, depending on the particular application. _ Piezometer Tube. _ U-Tube manometer. -Simple U-Tube Manometer -Differential U-Tube Manometer -Inverted Differential U-Tube Manometer _ Inclined U-Tube manometer.

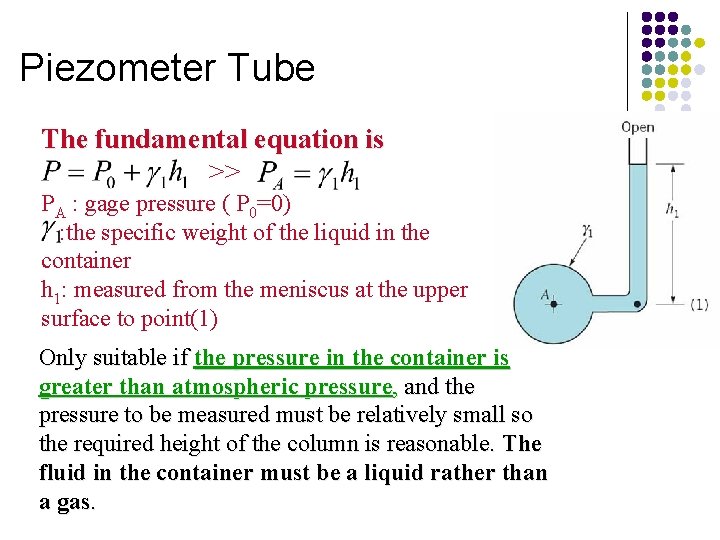
Piezometer Tube The fundamental equation is >> PA : gage pressure ( P 0=0) : the specific weight of the liquid in the container h 1: measured from the meniscus at the upper surface to point(1) Only suitable if the pressure in the container is greater than atmospheric pressure, and the pressure to be measured must be relatively small so the required height of the column is reasonable. The fluid in the container must be a liquid rather than a gas.

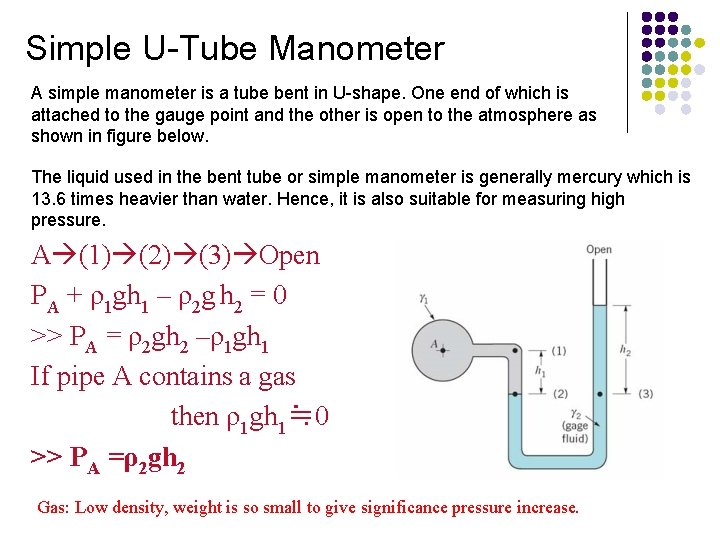
Simple U-Tube Manometer A simple manometer is a tube bent in U-shape. One end of which is attached to the gauge point and the other is open to the atmosphere as shown in figure below. The liquid used in the bent tube or simple manometer is generally mercury which is 13. 6 times heavier than water. Hence, it is also suitable for measuring high pressure. A (1) (2) (3) Open PA + ρ1 gh 1 – ρ2 g h 2 = 0 >> PA = ρ2 gh 2 –ρ1 gh 1 If pipe A contains a gas then ρ1 gh 1≒ 0 >> PA =ρ2 gh 2 Gas: Low density, weight is so small to give significance pressure increase.

Digital Manometer A volunteer for the RAF blowing into a 'U-tube manometer' to check his lung power.

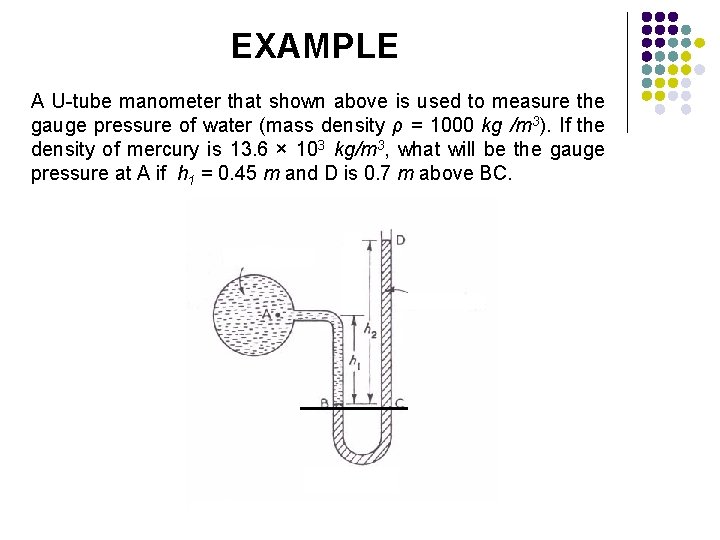
EXAMPLE A U-tube manometer that shown above is used to measure the gauge pressure of water (mass density ρ = 1000 kg /m 3). If the density of mercury is 13. 6 × 103 kg/m 3, what will be the gauge pressure at A if h 1 = 0. 45 m and D is 0. 7 m above BC.

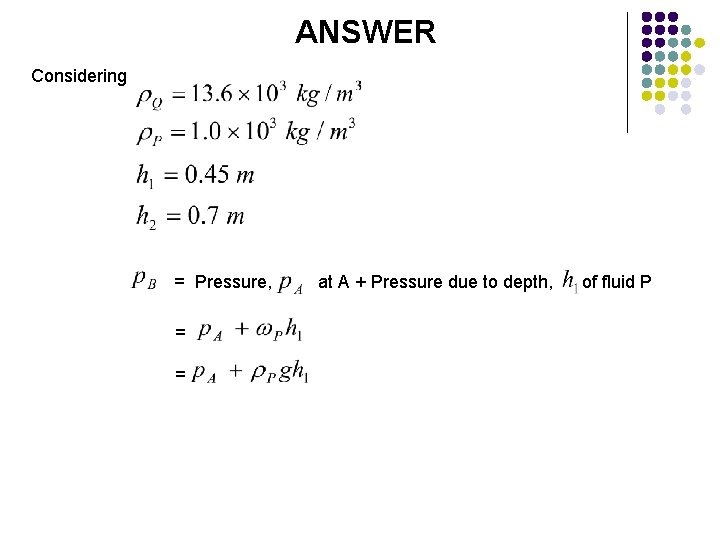
ANSWER Considering = Pressure, = at A + Pressure due to depth, of fluid P

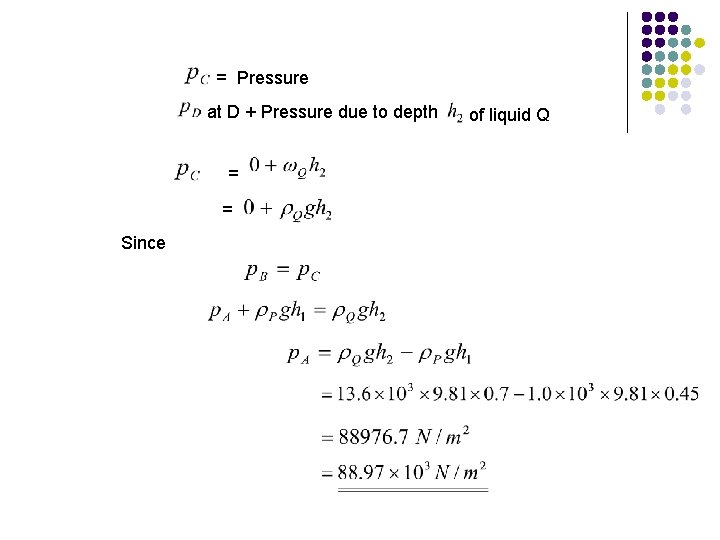
= Pressure at D + Pressure due to depth = Since of liquid Q

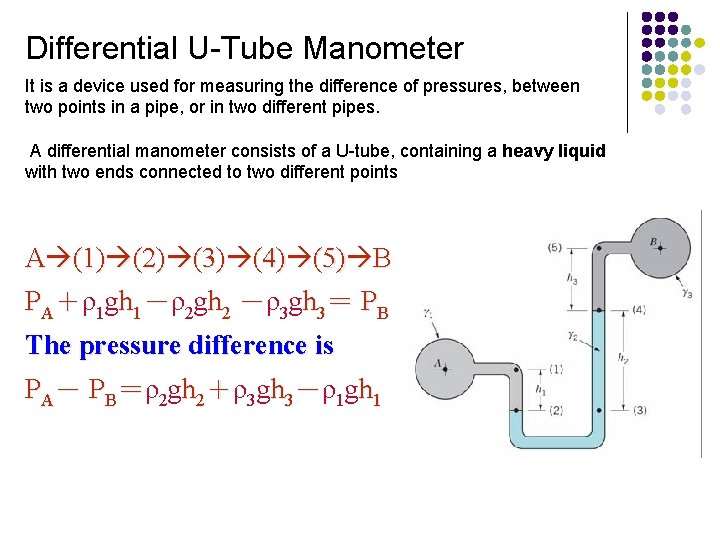
Differential U-Tube Manometer It is a device used for measuring the difference of pressures, between two points in a pipe, or in two different pipes. A differential manometer consists of a U-tube, containing a heavy liquid with two ends connected to two different points A (1) (2) (3) (4) (5) B PA+ρ1 gh 1-ρ2 gh 2 -ρ3 gh 3= PB The pressure difference is PA- PB=ρ2 gh 2+ρ3 gh 3-ρ1 gh 1

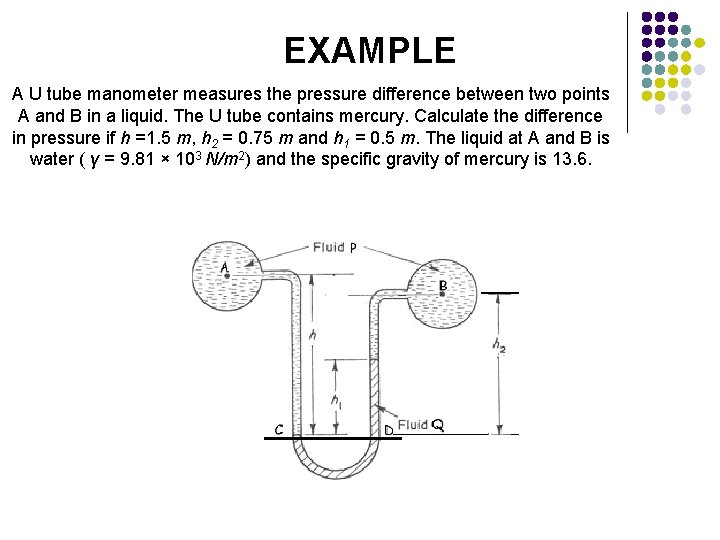
EXAMPLE A U tube manometer measures the pressure difference between two points A and B in a liquid. The U tube contains mercury. Calculate the difference in pressure if h =1. 5 m, h 2 = 0. 75 m and h 1 = 0. 5 m. The liquid at A and B is water ( γ = 9. 81 × 103 N/m 2) and the specific gravity of mercury is 13. 6.

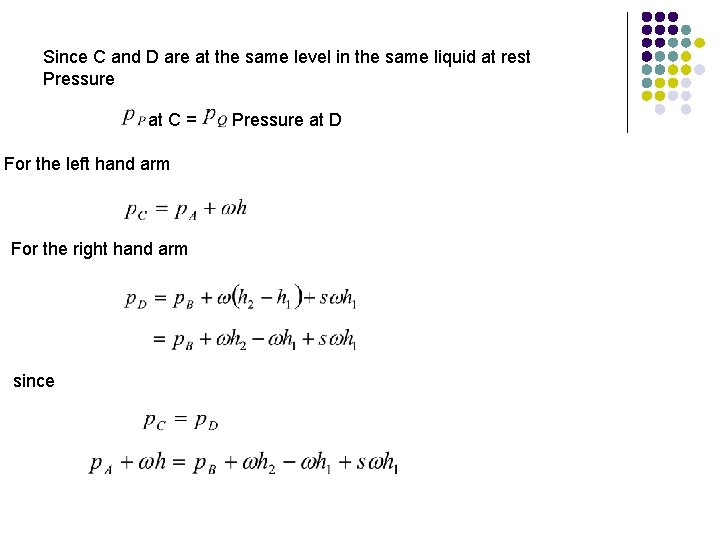
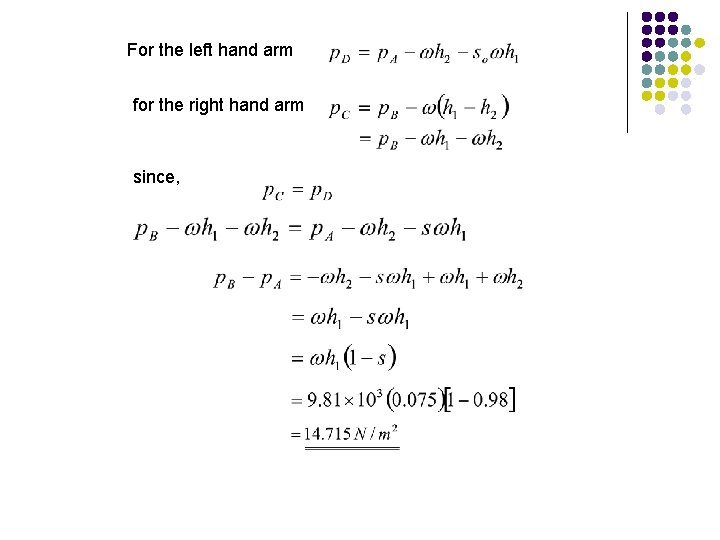
Since C and D are at the same level in the same liquid at rest Pressure at C = Pressure at D For the left hand arm For the right hand arm since

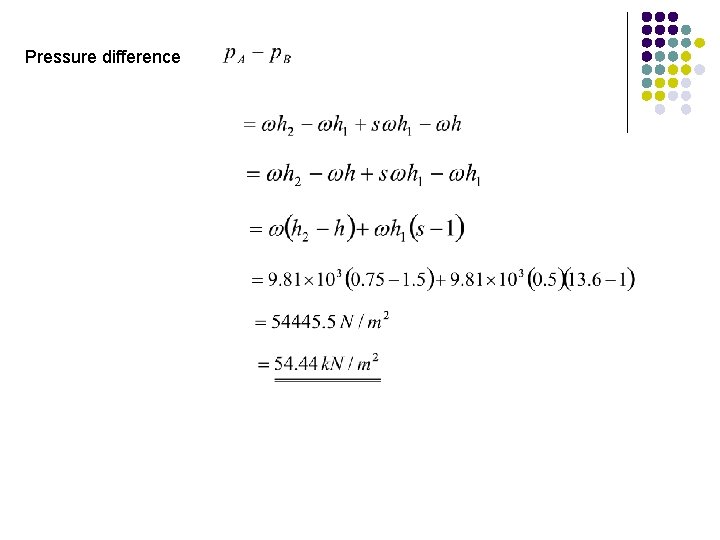
Pressure difference

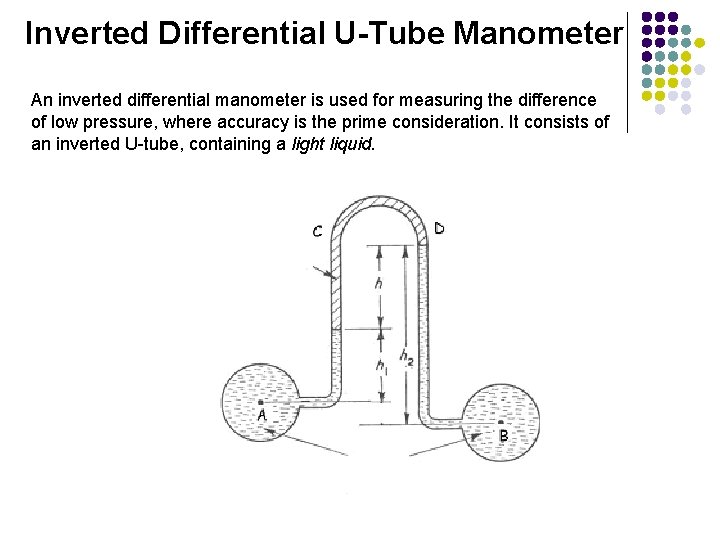
Inverted Differential U-Tube Manometer An inverted differential manometer is used for measuring the difference of low pressure, where accuracy is the prime consideration. It consists of an inverted U-tube, containing a light liquid.

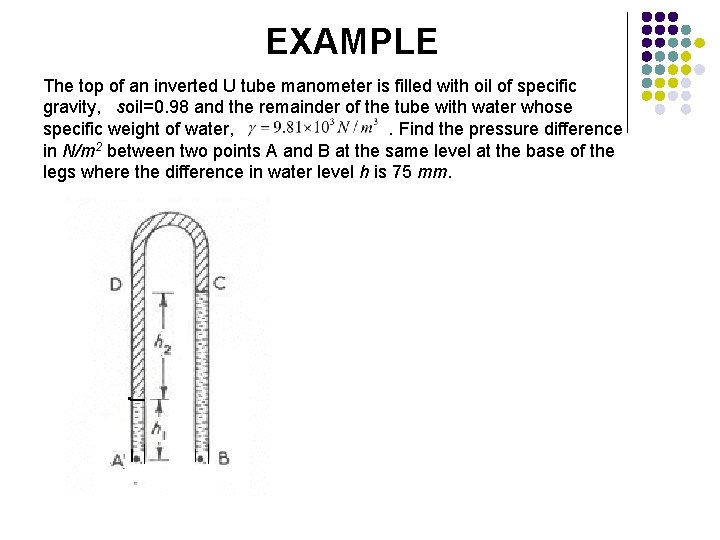
EXAMPLE The top of an inverted U tube manometer is filled with oil of specific gravity, soil=0. 98 and the remainder of the tube with water whose specific weight of water, . Find the pressure difference in N/m 2 between two points A and B at the same level at the base of the legs where the difference in water level h is 75 mm.

For the left hand arm for the right hand arm since,

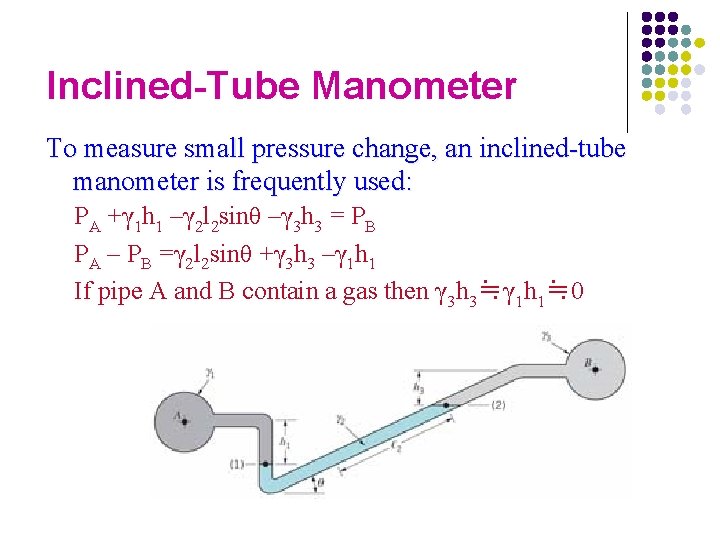
Inclined-Tube Manometer To measure small pressure change, an inclined-tube manometer is frequently used: PA +γ 1 h 1 –γ 2 l 2 sinθ –γ 3 h 3 = PB PA – PB =γ 2 l 2 sinθ +γ 3 h 3 –γ 1 h 1 If pipe A and B contain a gas then γ 3 h 3≒γ 1 h 1≒ 0

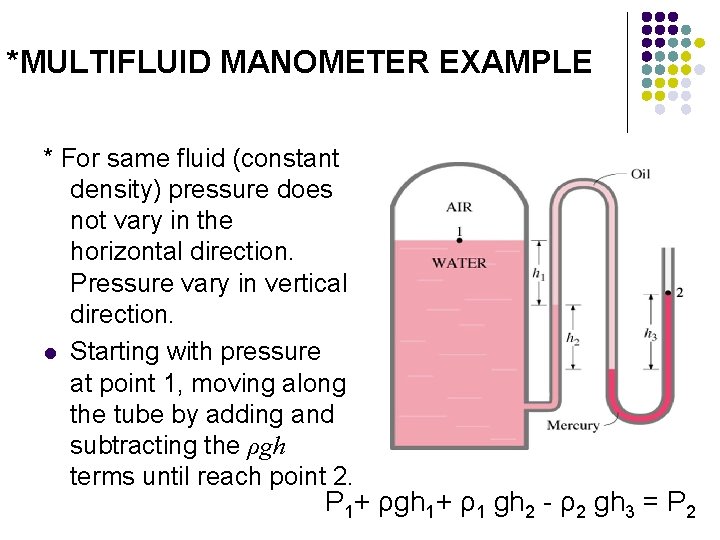
*MULTIFLUID MANOMETER EXAMPLE * For same fluid (constant density) pressure does not vary in the horizontal direction. Pressure vary in vertical direction. l Starting with pressure at point 1, moving along the tube by adding and subtracting the ρgh terms until reach point 2. P 1+ ρgh 1+ ρ1 gh 2 - ρ2 gh 3 = P 2