Chapter 2 Inorganic Solids in Soil continued http




- Slides: 20

Chapter 2 Inorganic Solids in Soil continued

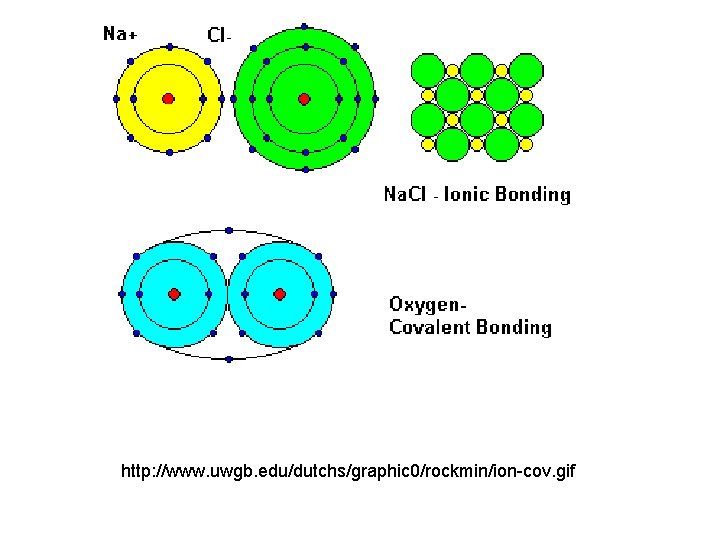
http: //www. uwgb. edu/dutchs/graphic 0/rockmin/ion-cov. gif

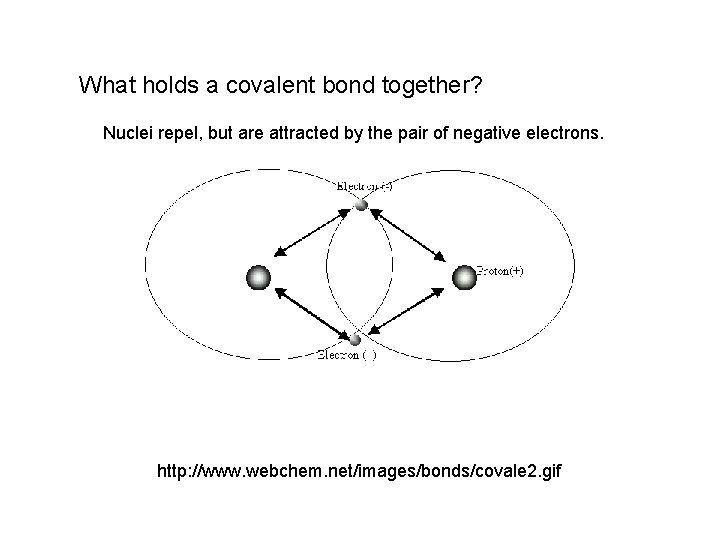
What holds a covalent bond together? Nuclei repel, but are attracted by the pair of negative electrons. http: //www. webchem. net/images/bonds/covale 2. gif

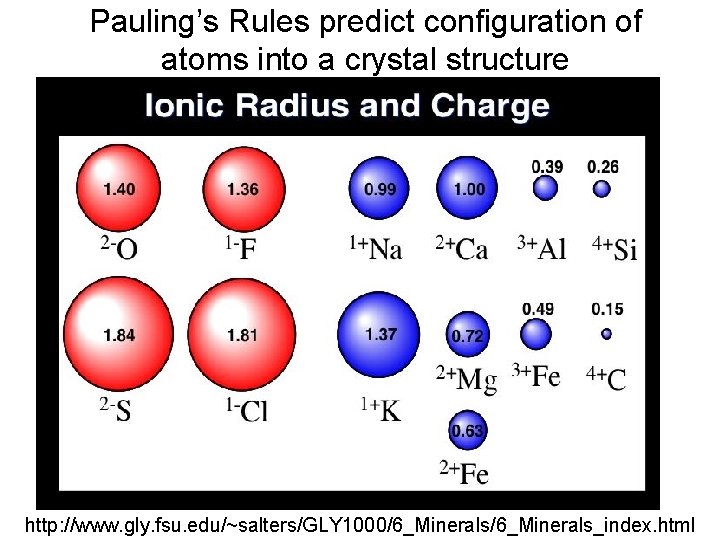
Pauling’s Rules predict configuration of atoms into a crystal structure http: //www. gly. fsu. edu/~salters/GLY 1000/6_Minerals_index. html

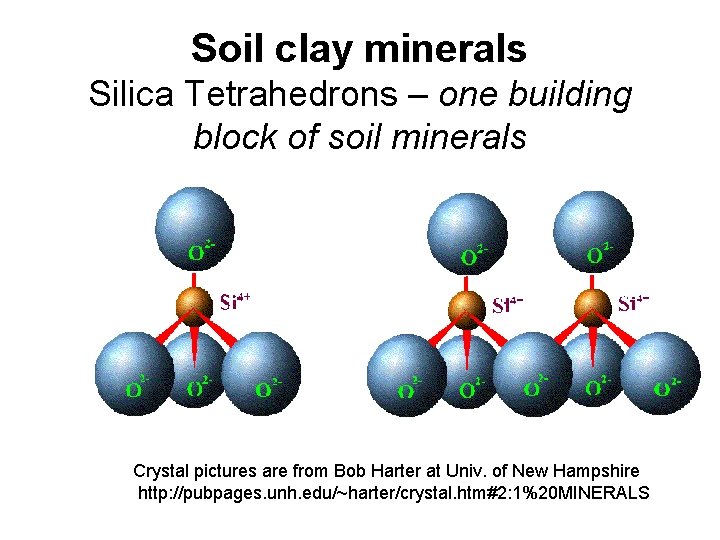
Soil clay minerals Silica Tetrahedrons – one building block of soil minerals Crystal pictures are from Bob Harter at Univ. of New Hampshire http: //pubpages. unh. edu/~harter/crystal. htm#2: 1%20 MINERALS

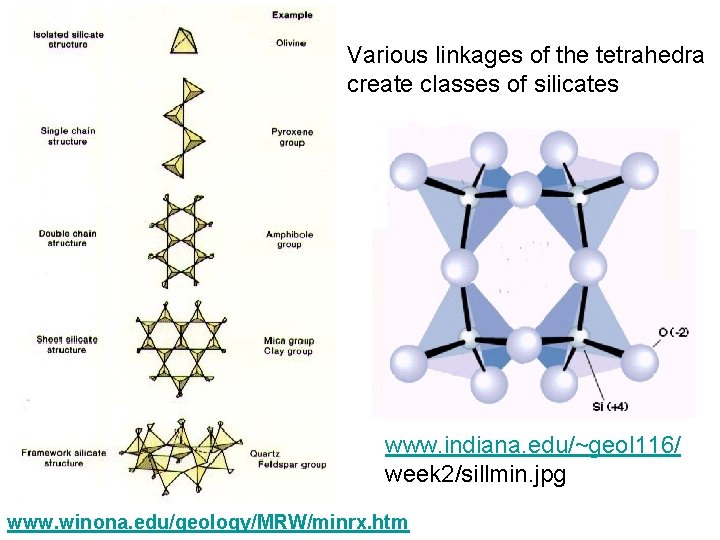
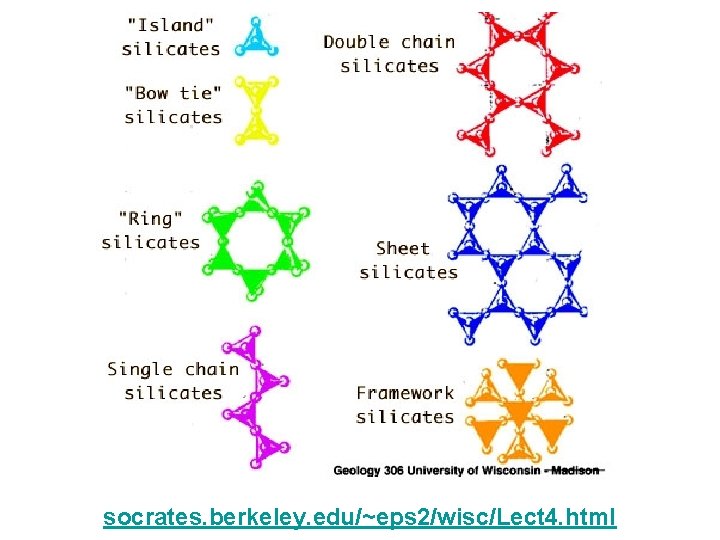
Various linkages of the tetrahedra create classes of silicates www. indiana. edu/~geol 116/ week 2/sillmin. jpg www. winona. edu/geology/MRW/minrx. htm

socrates. berkeley. edu/~eps 2/wisc/Lect 4. html

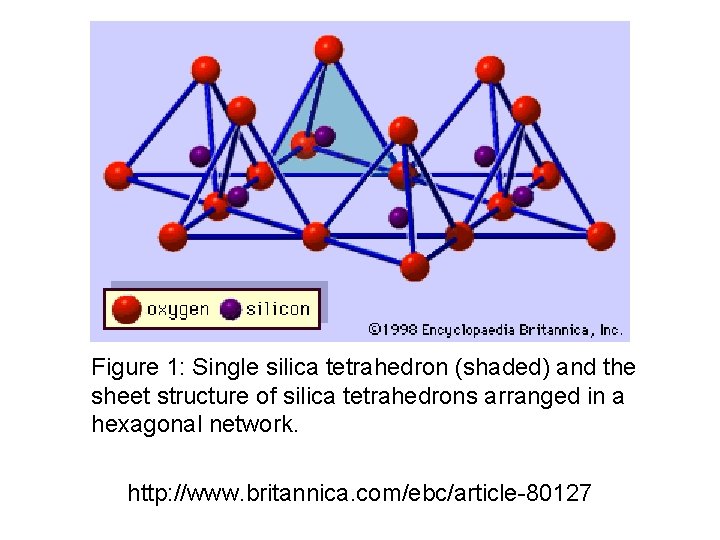
Figure 1: Single silica tetrahedron (shaded) and the sheet structure of silica tetrahedrons arranged in a hexagonal network. http: //www. britannica. com/ebc/article-80127

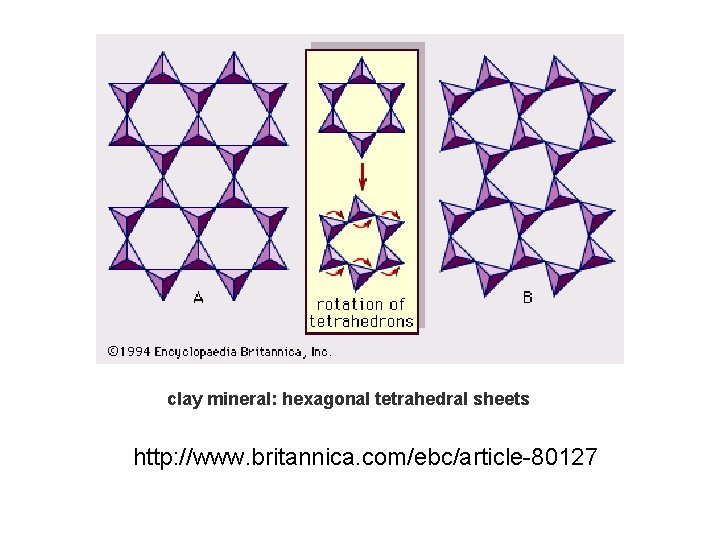
clay mineral: hexagonal tetrahedral sheets http: //www. britannica. com/ebc/article-80127

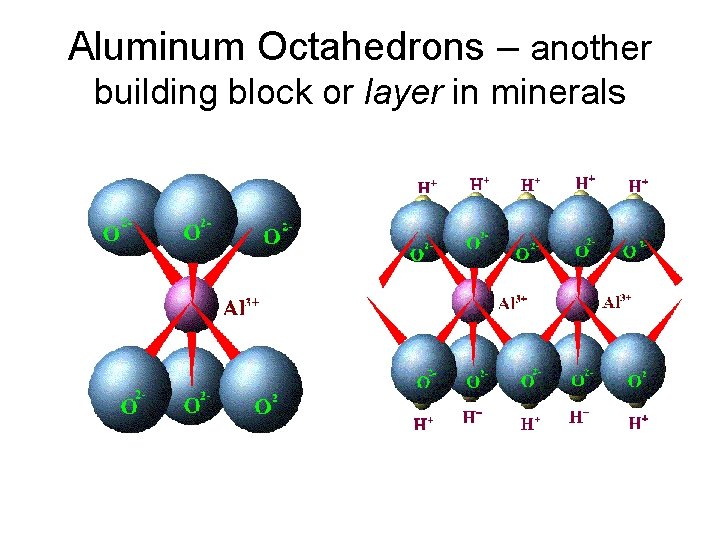
Aluminum Octahedrons – another building block or layer in minerals

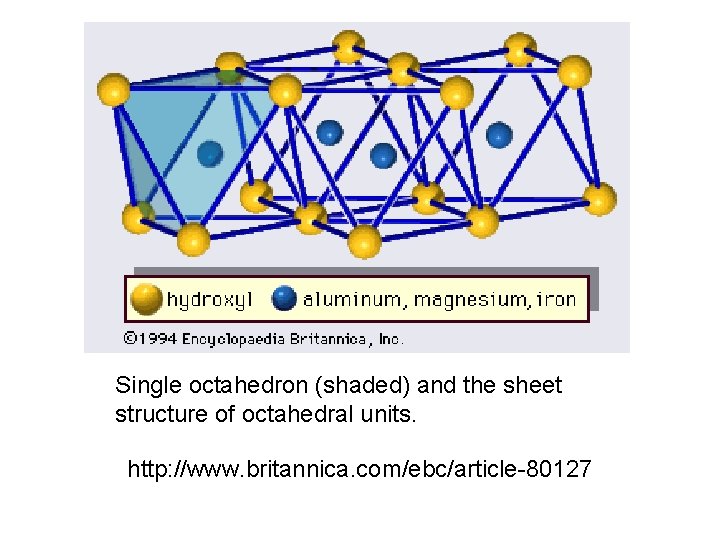
Single octahedron (shaded) and the sheet structure of octahedral units. http: //www. britannica. com/ebc/article-80127

Primary Soil Minerals • Not chemically altered or significantly weathered • Inherited from original crystallization or deposition • Found mostly in the sand silt fractions • Ex: Quartz, Feldspars/Plagioclases, Amphiboles, Pyroxenes, etc. (Sparks, Table 2. 2 p. 46) • Source of Na, Mg, K, Ca, Mn, and Fe ions as they weather/decompose. • Also source of trace elements and heavy metals in soils.

http: //www. mineralogie. uni-wuerzburg. de/gallery/Seiten/quartz 2. htm Photograph taken by Klaus-Peter Kelber

Secondary Soil Minerals • Low-temperature weathering product of primary minerals – Structural alteration of primary minerals – Precipitation out of solution – Inherited from sedimentary rocks • Predominant in clay fraction (<2µm) • Very reactive chemically and physically • Source of readily available nutrient ions (Ca, Mg, K, NH 4, S, Fe…) • Ex: Phyllosilicates (kaolins, smectites, illites); oxides and hydroxides, carbonates, sulfates, etc.

Phyllosilicates or “layer” silicates • Most abundant in the clay-sized fraction (hence referred to as “clay minerals” • Very high surface area + unsatisfied charges = very reactive (both physically and chemically) • Composed of sheets of Si. O 4 tetrahedra + Al or Mg octahedra • Strong ionic/covalent internal bonding and weaker H or van der Waals bonding between the layers

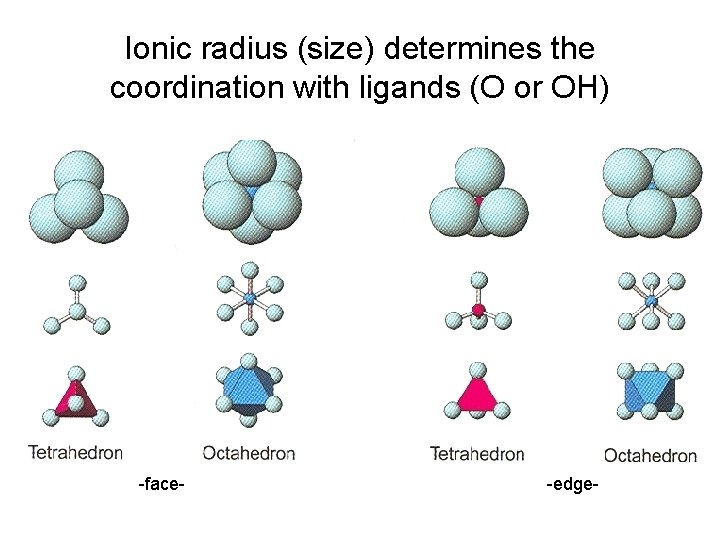
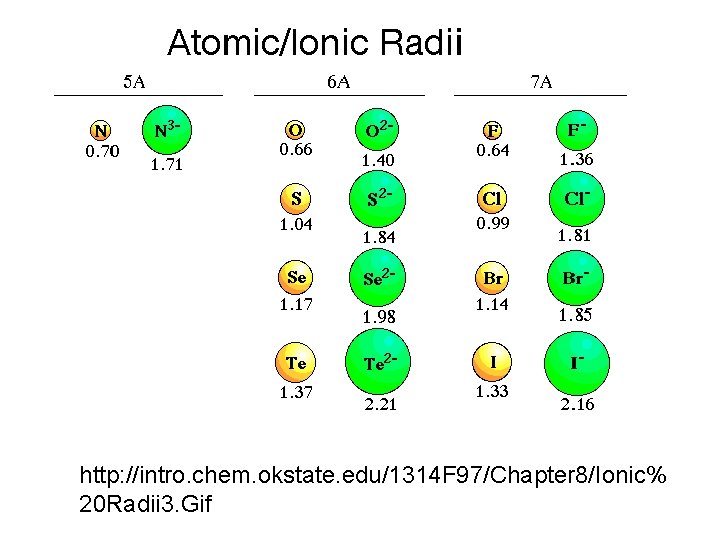
Ionic radius (size) determines the coordination with ligands (O or OH) -face- -edge-

http: //intro. chem. okstate. edu/1314 F 97/Chapter 8/Ionic% 20 Radii 3. Gif

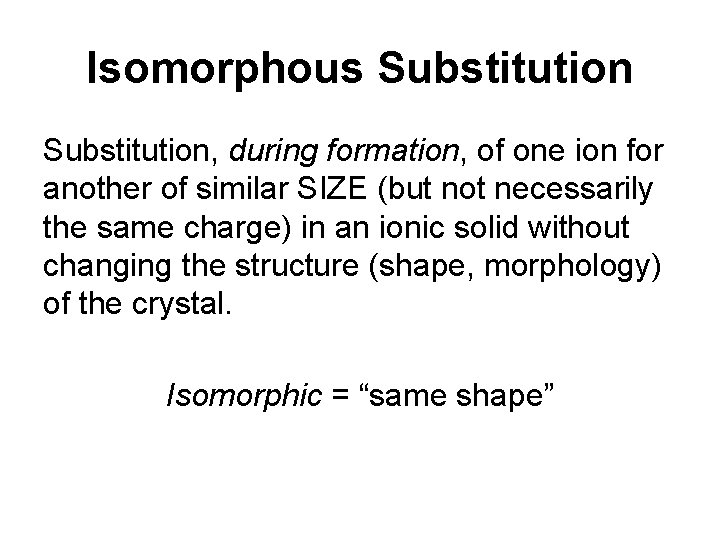
Isomorphous Substitution, during formation, of one ion for another of similar SIZE (but not necessarily the same charge) in an ionic solid without changing the structure (shape, morphology) of the crystal. Isomorphic = “same shape”

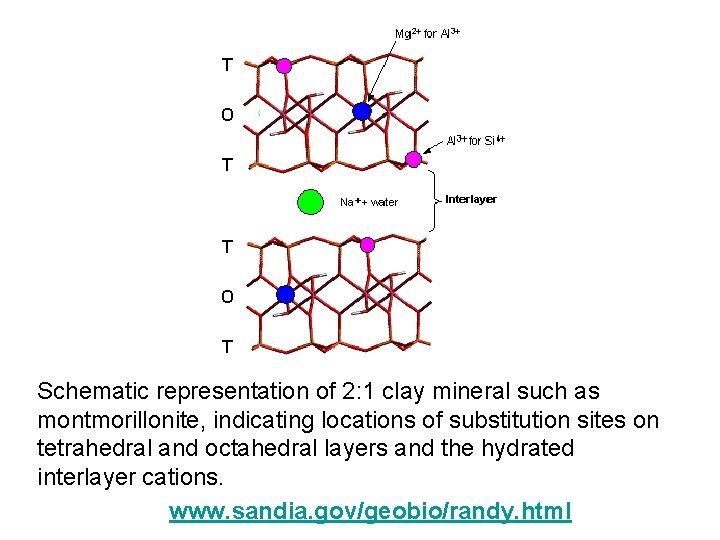
Schematic representation of 2: 1 clay mineral such as montmorillonite, indicating locations of substitution sites on tetrahedral and octahedral layers and the hydrated interlayer cations. www. sandia. gov/geobio/randy. html

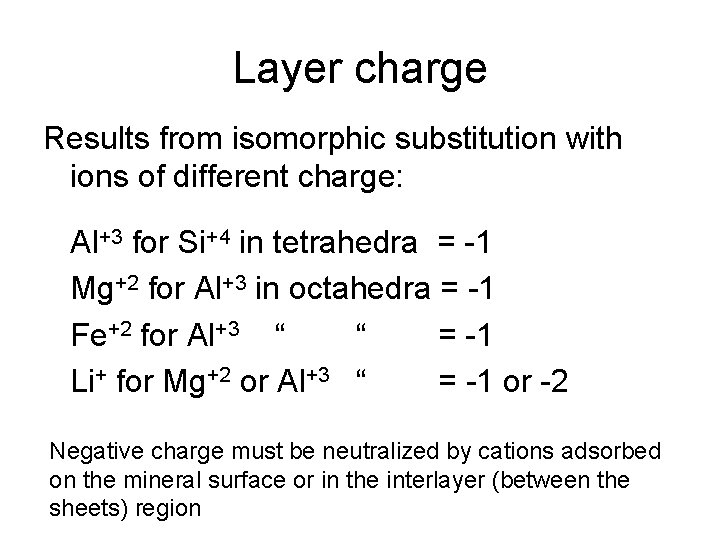
Layer charge Results from isomorphic substitution with ions of different charge: Al+3 for Si+4 in tetrahedra = -1 Mg+2 for Al+3 in octahedra = -1 Fe+2 for Al+3 “ “ = -1 Li+ for Mg+2 or Al+3 “ = -1 or -2 Negative charge must be neutralized by cations adsorbed on the mineral surface or in the interlayer (between the sheets) region