Chapter 17 The First Law of Thermodynamics Thermodynamic

- Slides: 19

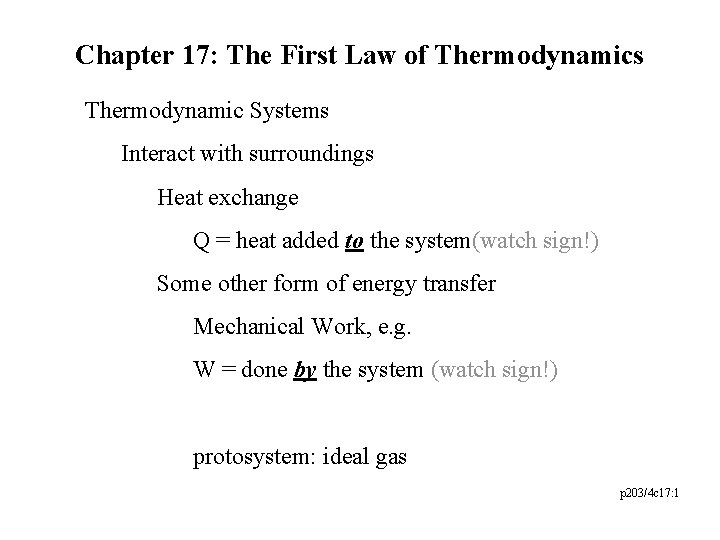
Chapter 17: The First Law of Thermodynamics Thermodynamic Systems Interact with surroundings Heat exchange Q = heat added to the system(watch sign!) Some other form of energy transfer Mechanical Work, e. g. W = done by the system (watch sign!) protosystem: ideal gas p 203/4 c 17: 1

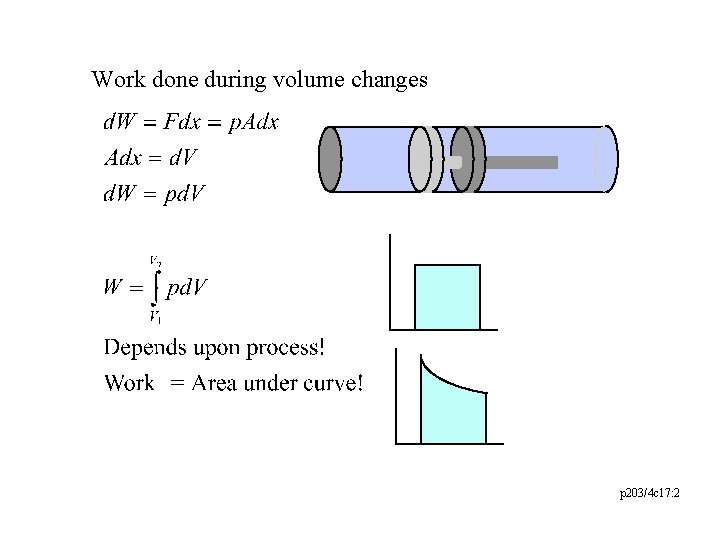
Work done during volume changes p 203/4 c 17: 2

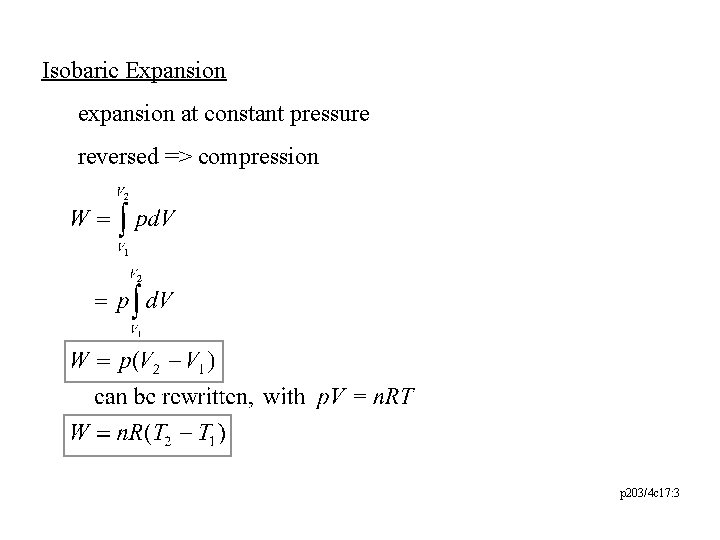
Isobaric Expansion expansion at constant pressure reversed => compression p 203/4 c 17: 3

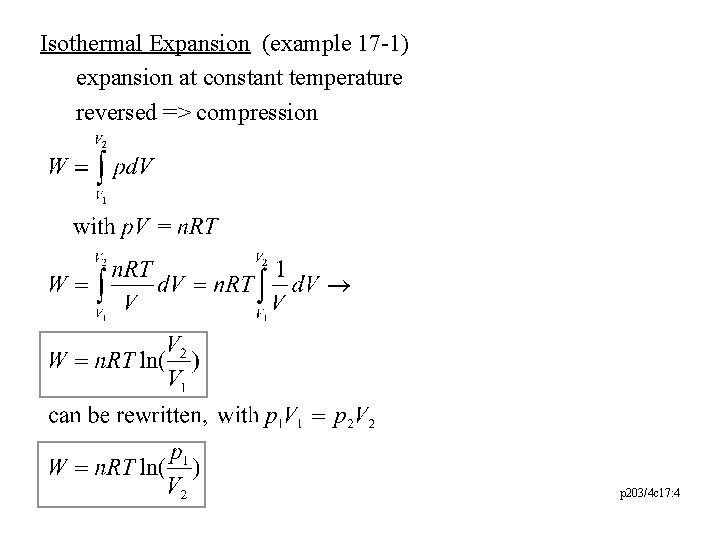
Isothermal Expansion (example 17 -1) expansion at constant temperature reversed => compression p 203/4 c 17: 4

Example: 1 m 3 of an ideal gas starting at 1 atm of pressure expands to twice its original volume by one of two processes: isobaric expansion or isothermal expansion. How much work is done in each case? p 203/4 c 17: 5

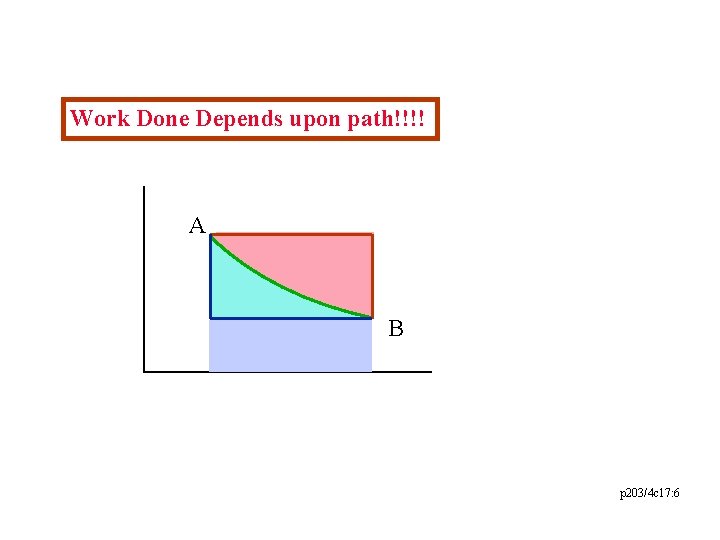
Work Done Depends upon path!!!! A B p 203/4 c 17: 6

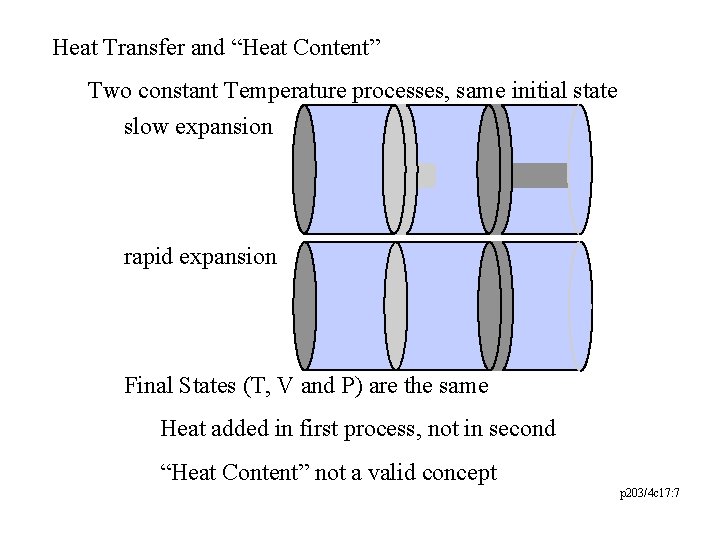
Heat Transfer and “Heat Content” Two constant Temperature processes, same initial state slow expansion rapid expansion Final States (T, V and P) are the same Heat added in first process, not in second “Heat Content” not a valid concept p 203/4 c 17: 7

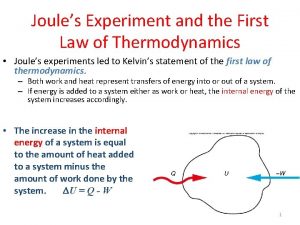
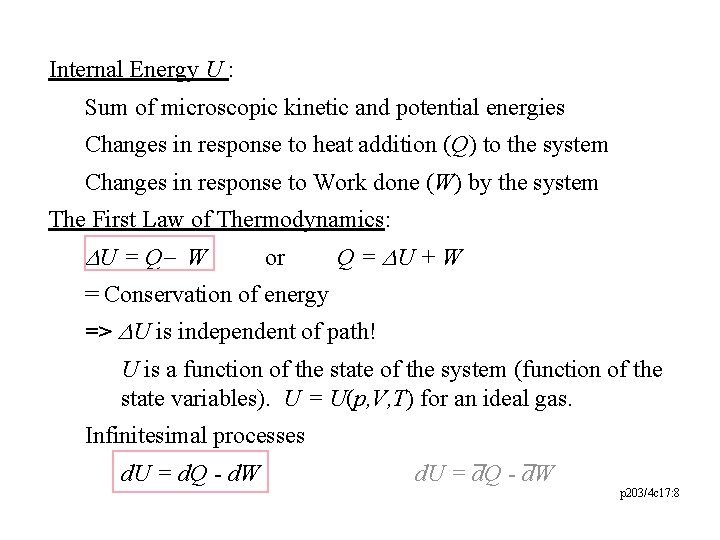
Internal Energy U : Sum of microscopic kinetic and potential energies Changes in response to heat addition (Q) to the system Changes in response to Work done (W) by the system The First Law of Thermodynamics: DU = Q - W or Q = DU + W = Conservation of energy => DU is independent of path! U is a function of the state of the system (function of the state variables). U = U(p, V, T) for an ideal gas. Infinitesimal processes d. U = d. Q - d. W p 203/4 c 17: 8

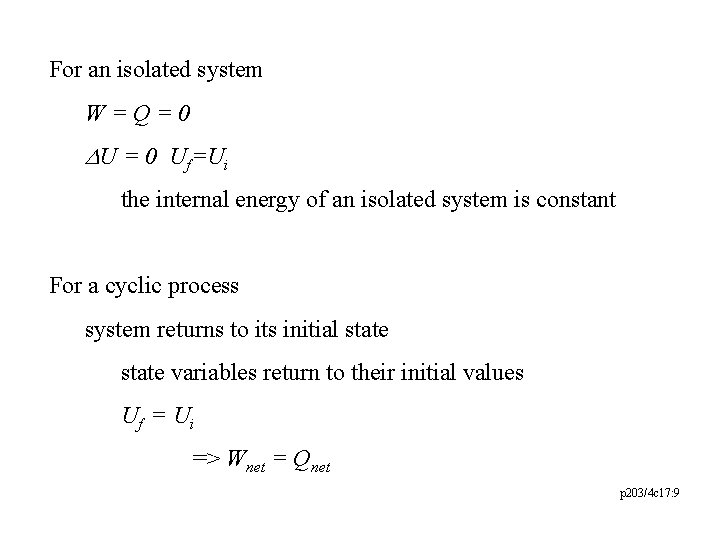
For an isolated system W=Q=0 DU = 0 Uf=Ui the internal energy of an isolated system is constant For a cyclic process system returns to its initial state variables return to their initial values Uf = U i => Wnet = Qnet p 203/4 c 17: 9

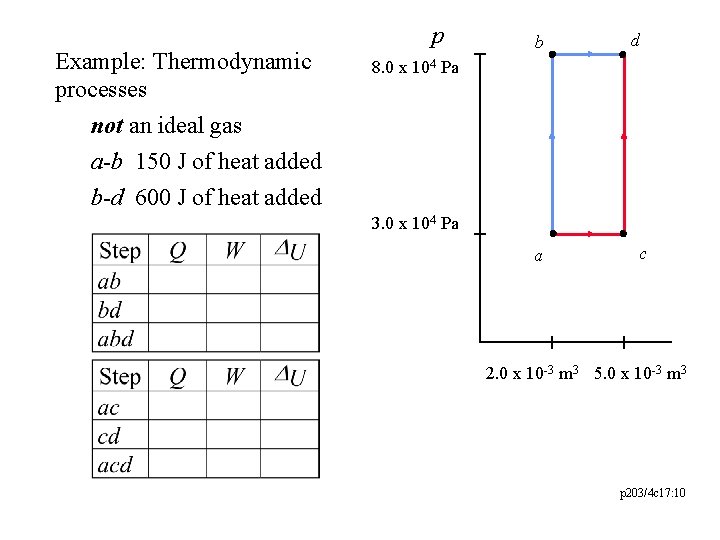
Example: Thermodynamic processes not an ideal gas a-b 150 J of heat added p b d 8. 0 x 104 Pa b-d 600 J of heat added 3. 0 x 104 Pa a c 2. 0 x 10 -3 m 3 5. 0 x 10 -3 m 3 p 203/4 c 17: 10

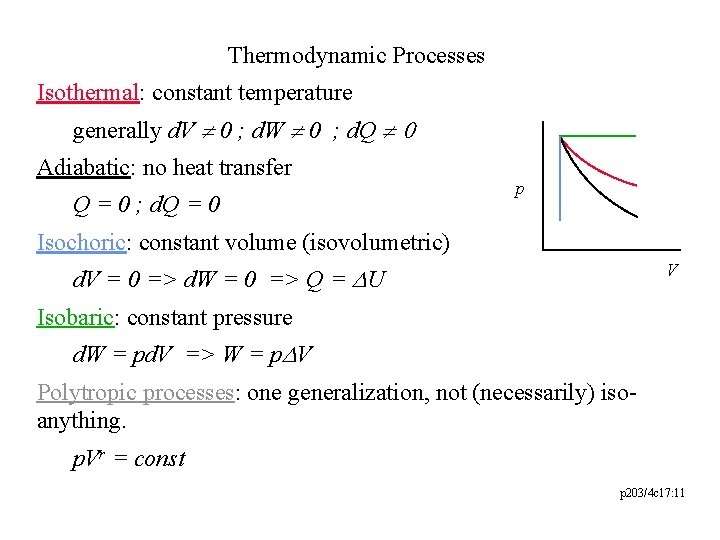
Thermodynamic Processes Isothermal: constant temperature generally d. V ¹ 0 ; d. W ¹ 0 ; d. Q ¹ 0 Adiabatic: no heat transfer Q = 0 ; d. Q = 0 p Isochoric: constant volume (isovolumetric) V d. V = 0 => d. W = 0 => Q = DU Isobaric: constant pressure d. W = pd. V => W = p. DV Polytropic processes: one generalization, not (necessarily) isoanything. p. Vr = const p 203/4 c 17: 11

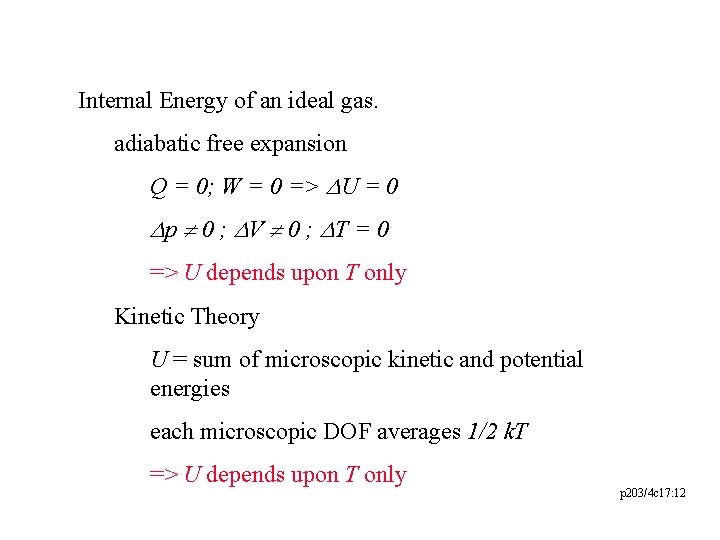
Internal Energy of an ideal gas. adiabatic free expansion Q = 0; W = 0 => DU = 0 Dp ¹ 0 ; DV ¹ 0 ; DT = 0 => U depends upon T only Kinetic Theory U = sum of microscopic kinetic and potential energies each microscopic DOF averages 1/2 k. T => U depends upon T only p 203/4 c 17: 12

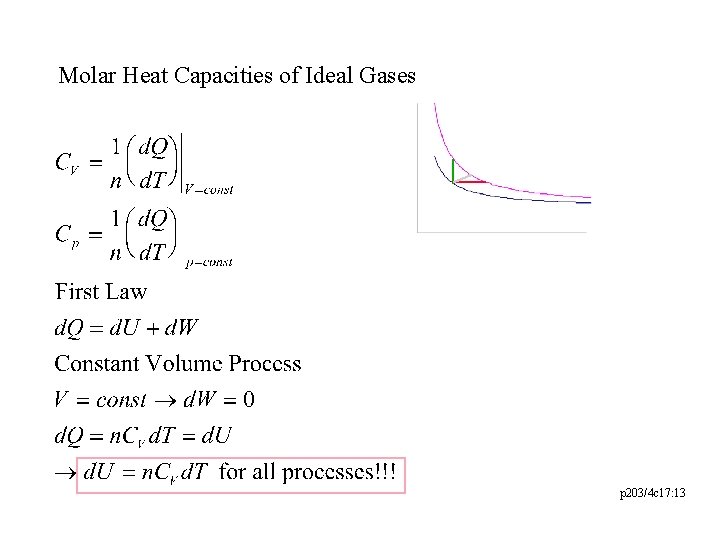
Molar Heat Capacities of Ideal Gases p 203/4 c 17: 13

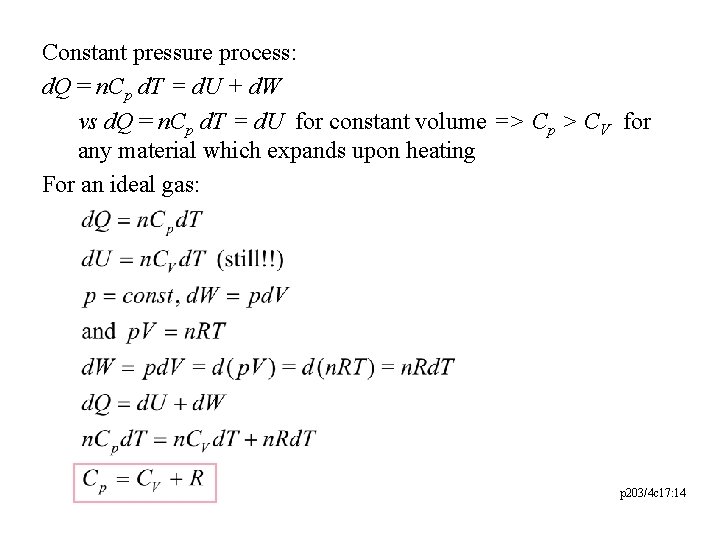
Constant pressure process: d. Q = n. Cp d. T = d. U + d. W vs d. Q = n. Cp d. T = d. U for constant volume => Cp > CV for any material which expands upon heating For an ideal gas: p 203/4 c 17: 14

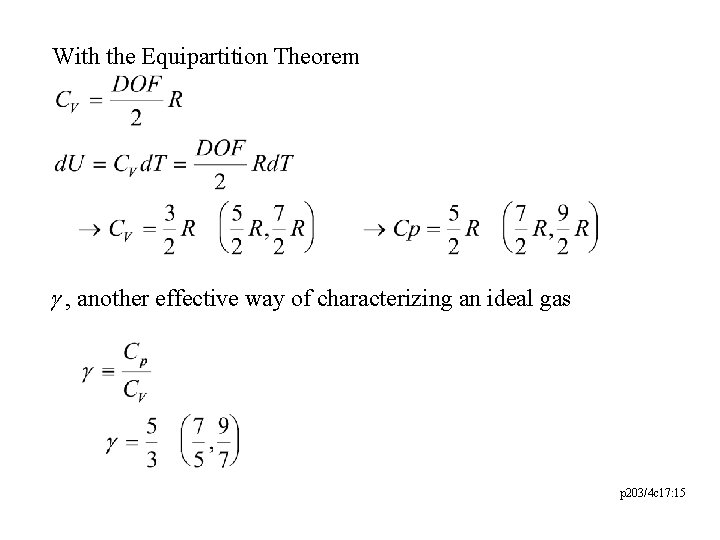
With the Equipartition Theorem g , another effective way of characterizing an ideal gas p 203/4 c 17: 15

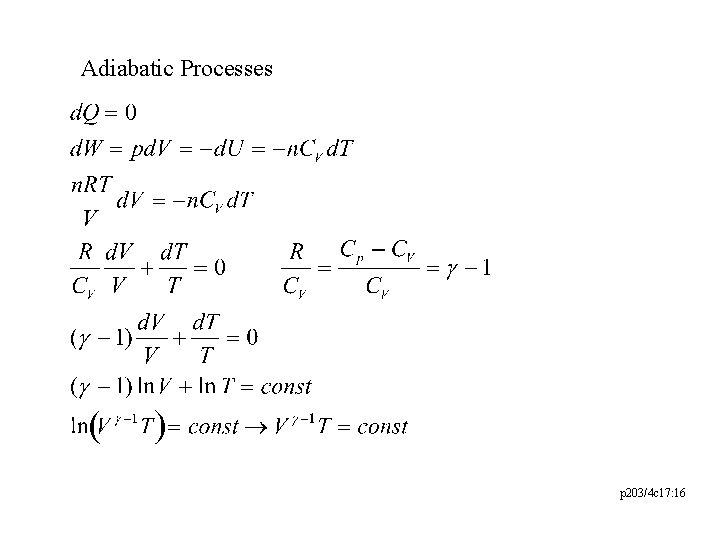
Adiabatic Processes p 203/4 c 17: 16

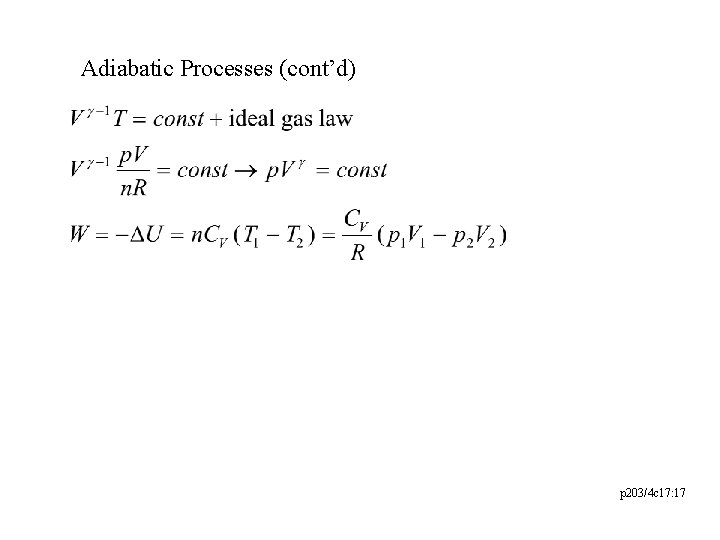
Adiabatic Processes (cont’d) p 203/4 c 17: 17

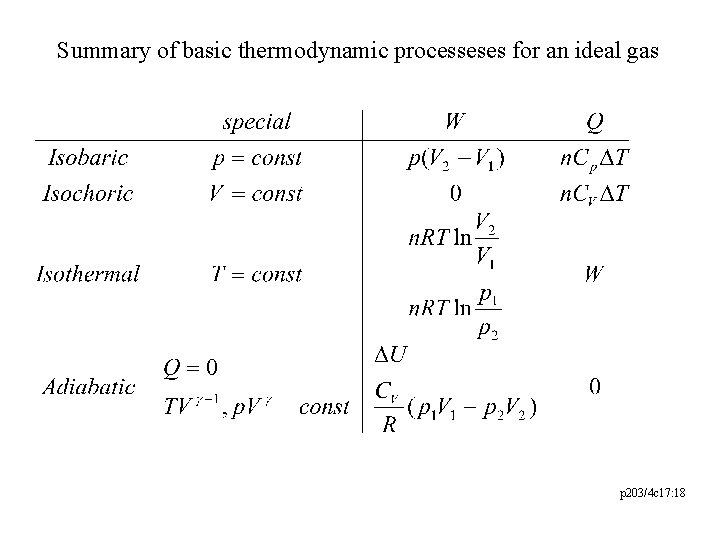
Summary of basic thermodynamic processeses for an ideal gas p 203/4 c 17: 18

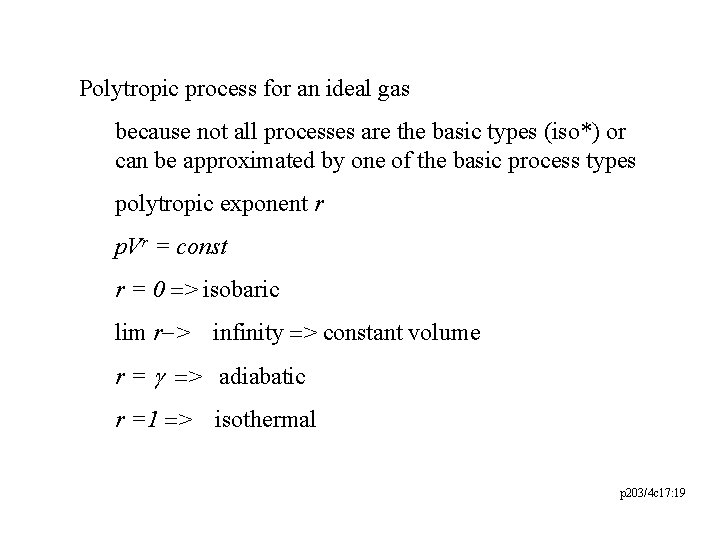
Polytropic process for an ideal gas because not all processes are the basic types (iso*) or can be approximated by one of the basic process types polytropic exponent r p. Vr = const r = 0 => isobaric lim r-> infinity => constant volume r = g => adiabatic r =1 => isothermal p 203/4 c 17: 19
 First law of thermodynamics in open system
First law of thermodynamics in open system Isobaric process formula
Isobaric process formula Brayton cycle
Brayton cycle First law of thermodynamics experiment
First law of thermodynamics experiment Thermodynamics first law
Thermodynamics first law What is steady flow process in thermodynamics
What is steady flow process in thermodynamics First law of thermodynamics sign convention
First law of thermodynamics sign convention Enthalpy vs internal energy
Enthalpy vs internal energy Free energy
Free energy Thermodynamics of ideal gases
Thermodynamics of ideal gases Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law and second law and third law
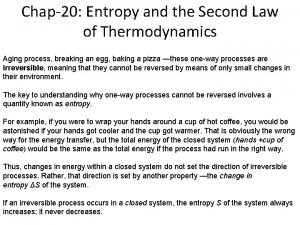
Newton's first law and second law and third law What is entropy in thermodynamics
What is entropy in thermodynamics Second law of thermodynamic
Second law of thermodynamic Second law of thermodynamic
Second law of thermodynamic Dr. erukhimova
Dr. erukhimova 0th law of thermodynamics definition
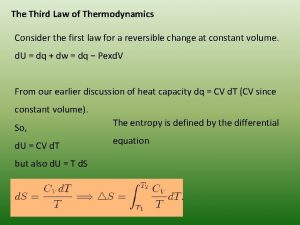
0th law of thermodynamics definition Newton's third law of thermodynamics
Newton's third law of thermodynamics Zeroth law of thermodynamics statement
Zeroth law of thermodynamics statement Kelvin statement
Kelvin statement