Q 19 Second Law of Thermodynamics 1 An
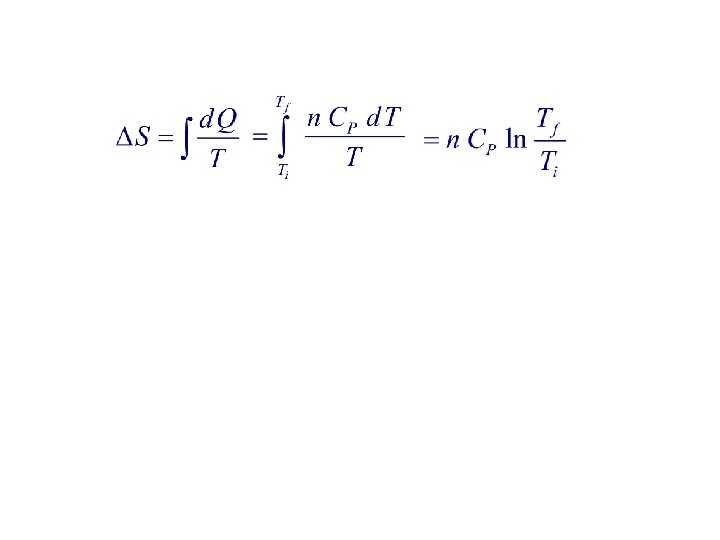



- Slides: 17

Q 19. Second Law of Thermodynamics

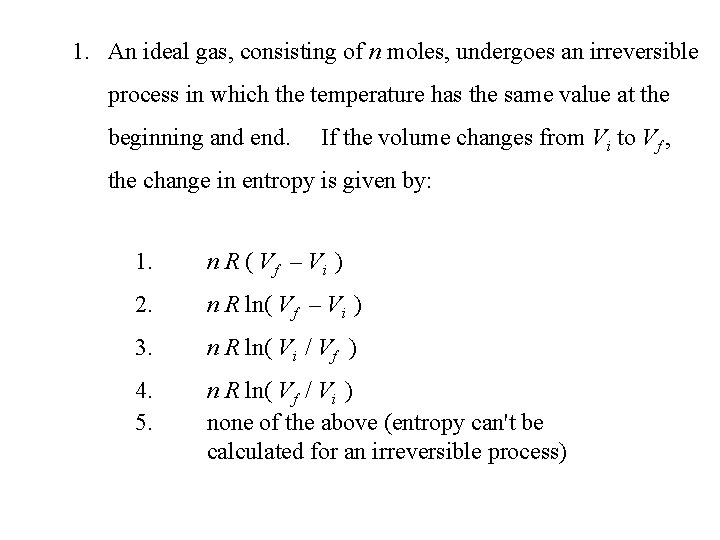
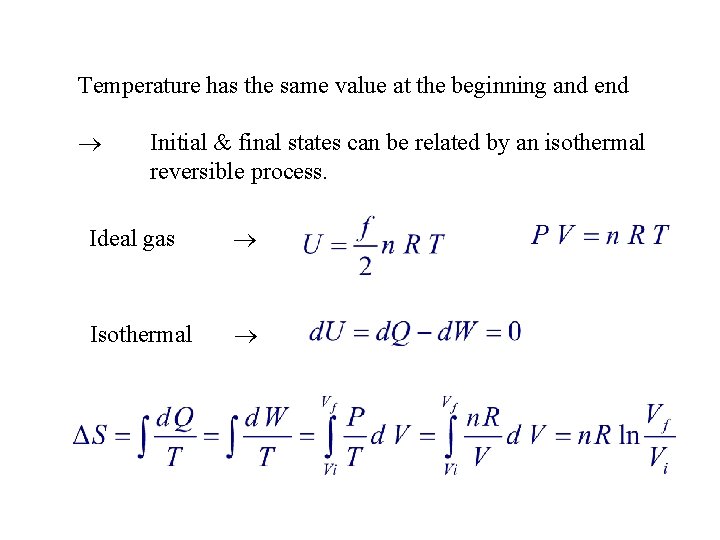
1. An ideal gas, consisting of n moles, undergoes an irreversible process in which the temperature has the same value at the beginning and end. If the volume changes from Vi to Vf , the change in entropy is given by: 1. n R ( Vf – Vi ) 2. n R ln( Vf – Vi ) 3. n R ln( Vi / Vf ) 4. 5. n R ln( Vf / Vi ) none of the above (entropy can't be calculated for an irreversible process)

Temperature has the same value at the beginning and end Initial & final states can be related by an isothermal reversible process. Ideal gas Isothermal

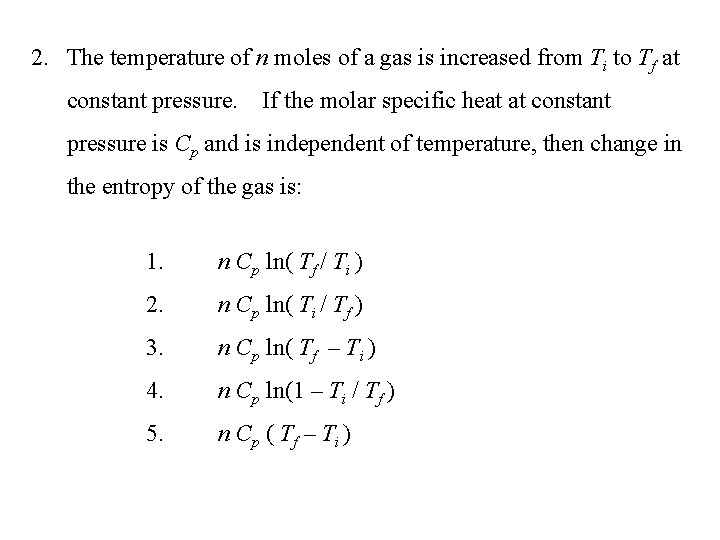
2. The temperature of n moles of a gas is increased from Ti to Tf at constant pressure. If the molar specific heat at constant pressure is Cp and is independent of temperature, then change in the entropy of the gas is: 1. n Cp ln( Tf / Ti ) 2. n Cp ln( Ti / Tf ) 3. n Cp ln( Tf – Ti ) 4. n Cp ln(1 – Ti / Tf ) 5. n C p ( Tf – Ti )
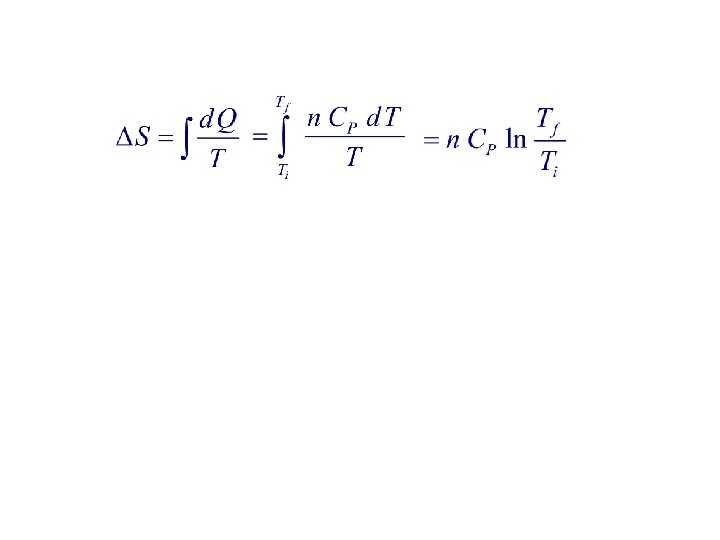

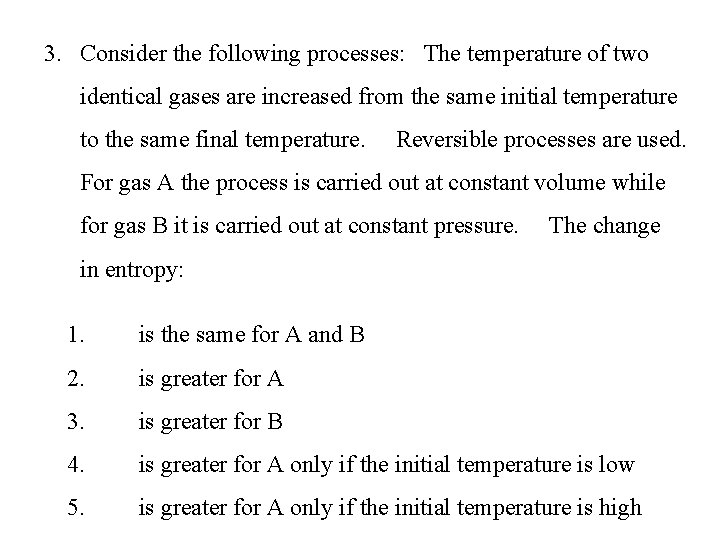
3. Consider the following processes: The temperature of two identical gases are increased from the same initial temperature to the same final temperature. Reversible processes are used. For gas A the process is carried out at constant volume while for gas B it is carried out at constant pressure. The change in entropy: 1. is the same for A and B 2. is greater for A 3. is greater for B 4. is greater for A only if the initial temperature is low 5. is greater for A only if the initial temperature is high


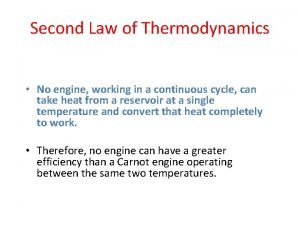
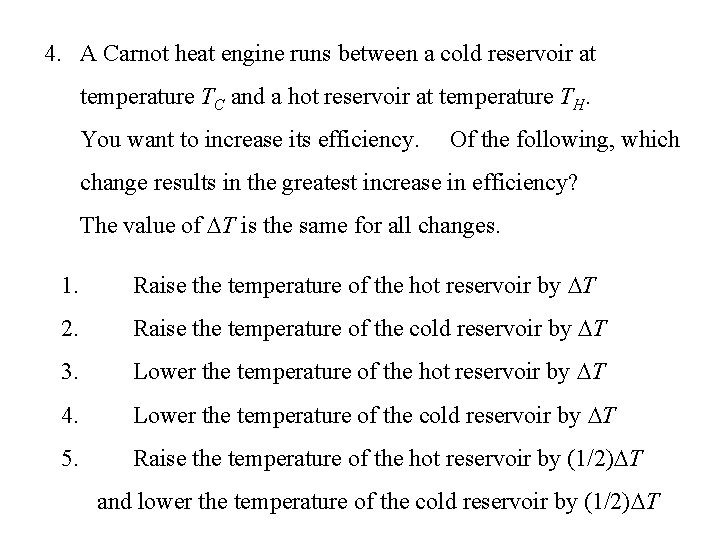
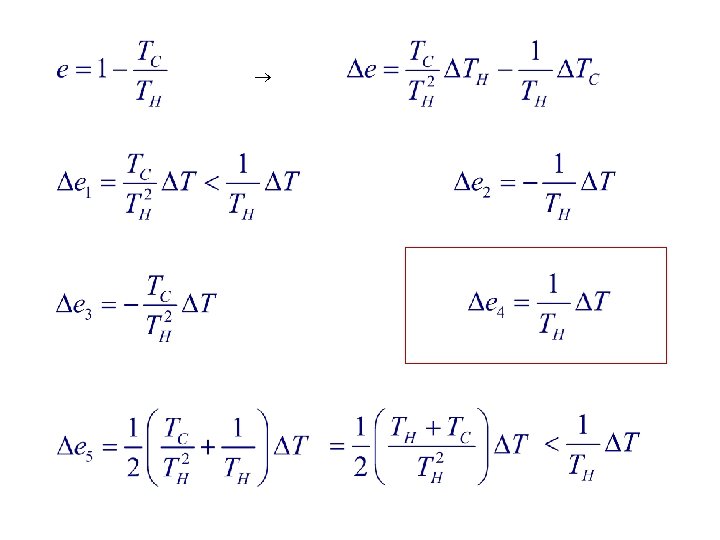
4. A Carnot heat engine runs between a cold reservoir at temperature TC and a hot reservoir at temperature TH. You want to increase its efficiency. Of the following, which change results in the greatest increase in efficiency? The value of T is the same for all changes. 1. Raise the temperature of the hot reservoir by T 2. Raise the temperature of the cold reservoir by T 3. Lower the temperature of the hot reservoir by T 4. Lower the temperature of the cold reservoir by T 5. Raise the temperature of the hot reservoir by (1/2) T and lower the temperature of the cold reservoir by (1/2) T


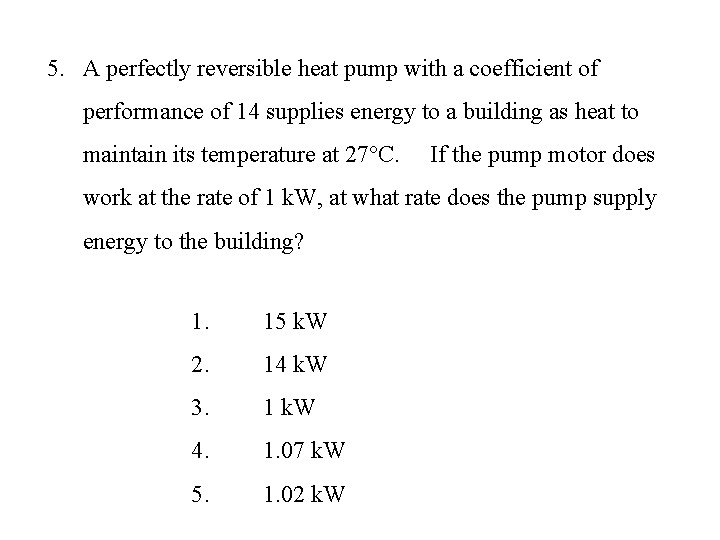
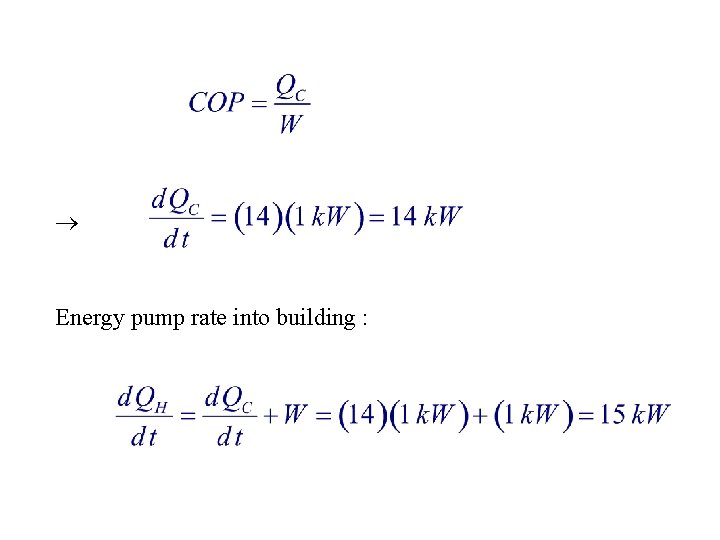
5. A perfectly reversible heat pump with a coefficient of performance of 14 supplies energy to a building as heat to maintain its temperature at 27°C. If the pump motor does work at the rate of 1 k. W, at what rate does the pump supply energy to the building? 1. 15 k. W 2. 14 k. W 3. 1 k. W 4. 1. 07 k. W 5. 1. 02 k. W

Energy pump rate into building :

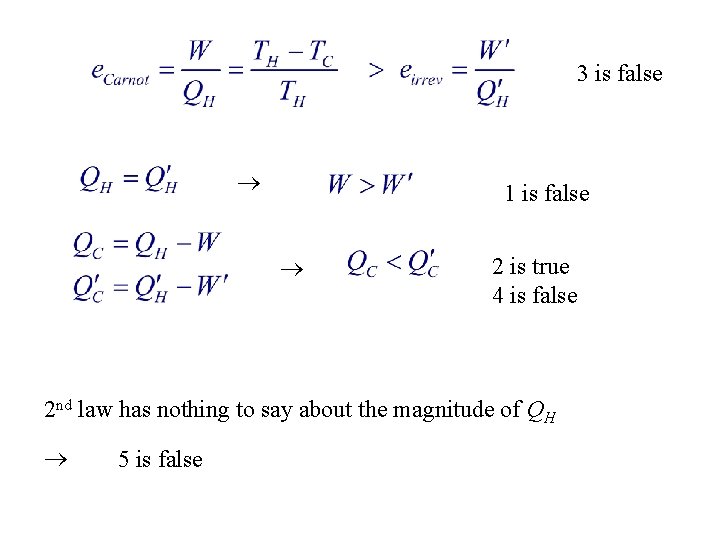
6. A Carnot heat engine and an irreversible heat engine both operate between the same high temperature and low temperature reservoirs. They absorb the same heat from the high temperature reservoir as heat. The irreversible engine: 1. does more work 2. rejects more energy to the low temperature reservoir as heat 3. has the greater efficiency 4. 5. rejects less energy to the low temperature reservoir as heat cannot absorb the same energy from the high temperature reservoir as heat without violating the second law of thermodynamics

3 is false 1 is false 2 is true 4 is false 2 nd law has nothing to say about the magnitude of QH 5 is false

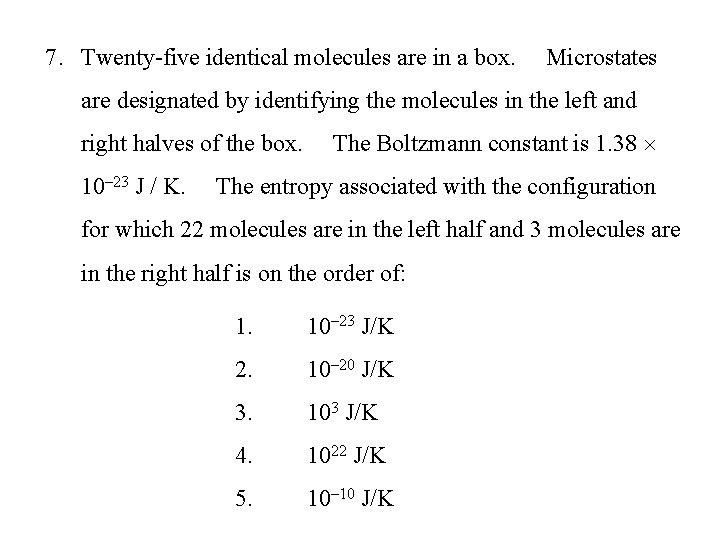
7. Twenty-five identical molecules are in a box. Microstates are designated by identifying the molecules in the left and right halves of the box. 10– 23 J / K. The Boltzmann constant is 1. 38 The entropy associated with the configuration for which 22 molecules are in the left half and 3 molecules are in the right half is on the order of: 1. 10– 23 J/K 2. 10– 20 J/K 3. 103 J/K 4. 1022 J/K 5. 10– 10 J/K


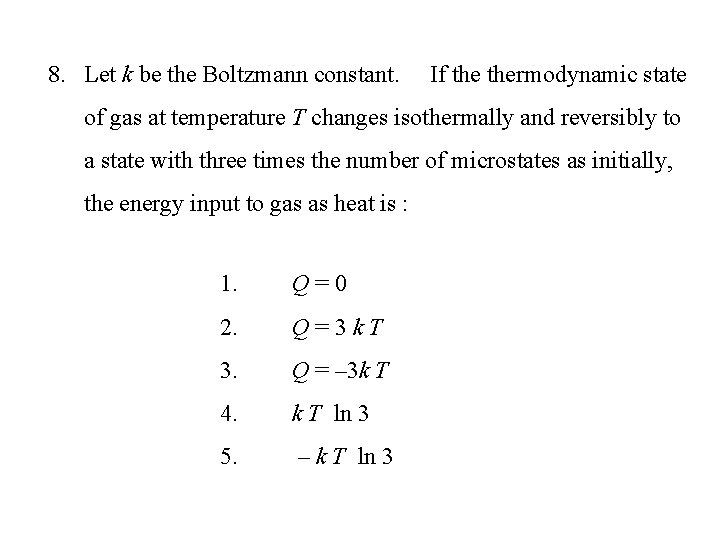
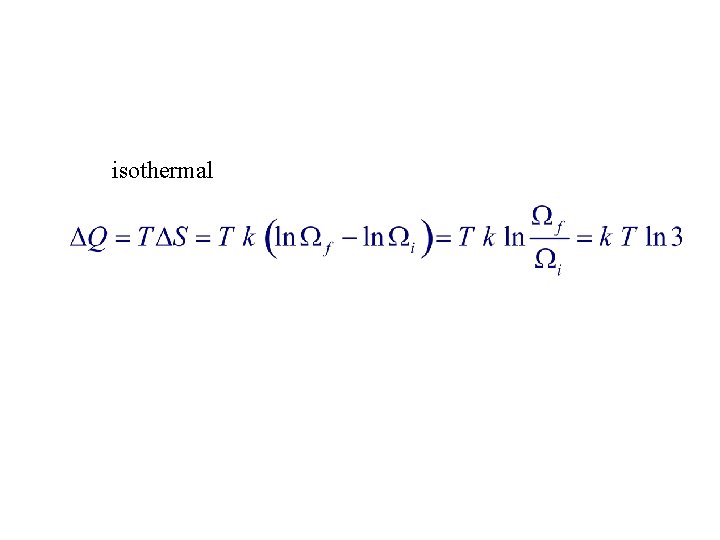
8. Let k be the Boltzmann constant. If thermodynamic state of gas at temperature T changes isothermally and reversibly to a state with three times the number of microstates as initially, the energy input to gas as heat is : 1. Q=0 2. Q=3 k. T 3. Q = – 3 k T 4. k T ln 3 5. – k T ln 3

isothermal
 Entropy change formula
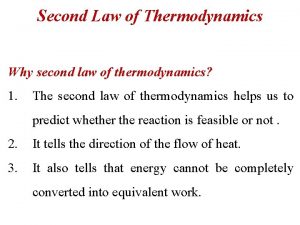
Entropy change formula State second law of thermodynamics
State second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2 nd law of thermodynamics
2 nd law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law 186 282 miles per second into meters per second
186 282 miles per second into meters per second Energy balance equation thermodynamics open system
Energy balance equation thermodynamics open system Zeroth law of thermodynamics
Zeroth law of thermodynamics Newtons third law of thermodynamics
Newtons third law of thermodynamics Third law of thermodynamics
Third law of thermodynamics Isobaric process formula
Isobaric process formula First law of thermodynamics for cyclic process
First law of thermodynamics for cyclic process Joule's first law of thermodynamics
Joule's first law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics