CH 11 Gases 11 1 Gasses and Pressure
- Slides: 12

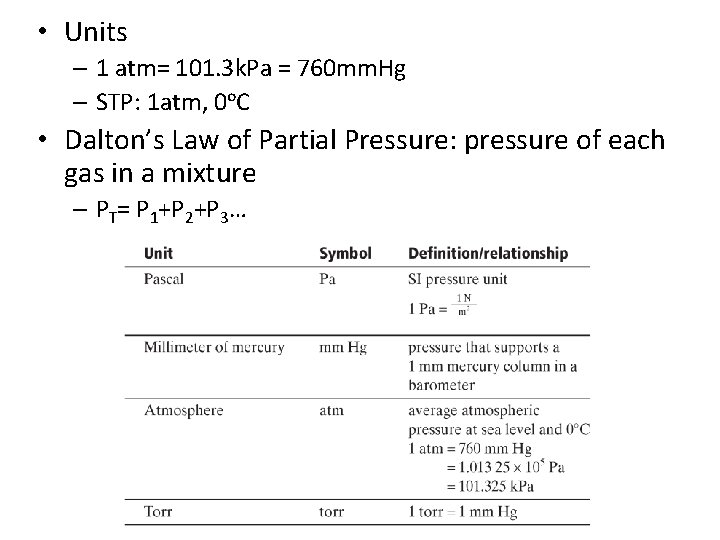
CH 11 Gases 11. 1 Gasses and Pressure • • Pressure = force/area Newton (N): increase speed of 1 kg mass by 1 m/s Earth’s gravity: 9. 8 m/s 2 1 atm= 101. 3 k. Pa = 760 mm. Hg

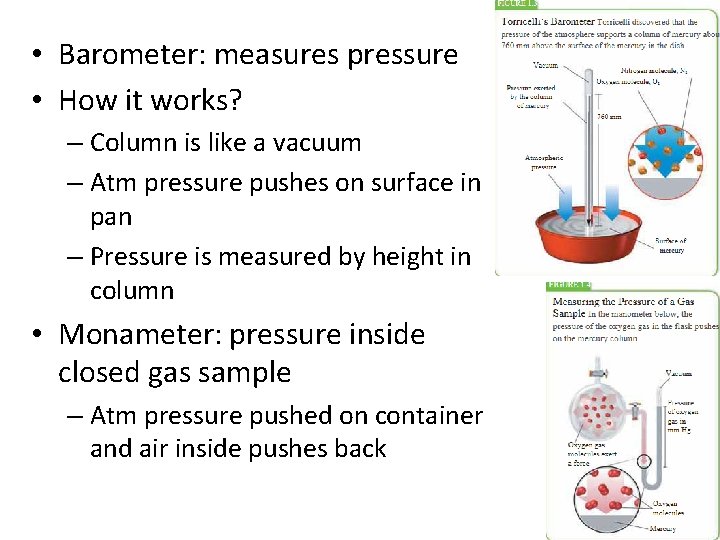
• Barometer: measures pressure • How it works? – Column is like a vacuum – Atm pressure pushes on surface in pan – Pressure is measured by height in column • Monameter: pressure inside closed gas sample – Atm pressure pushed on container and air inside pushes back

• Units – 1 atm= 101. 3 k. Pa = 760 mm. Hg – STP: 1 atm, 0ᵒC • Dalton’s Law of Partial Pressure: pressure of each gas in a mixture – PT= P 1+P 2+P 3…

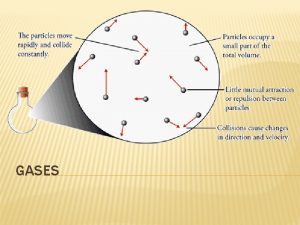
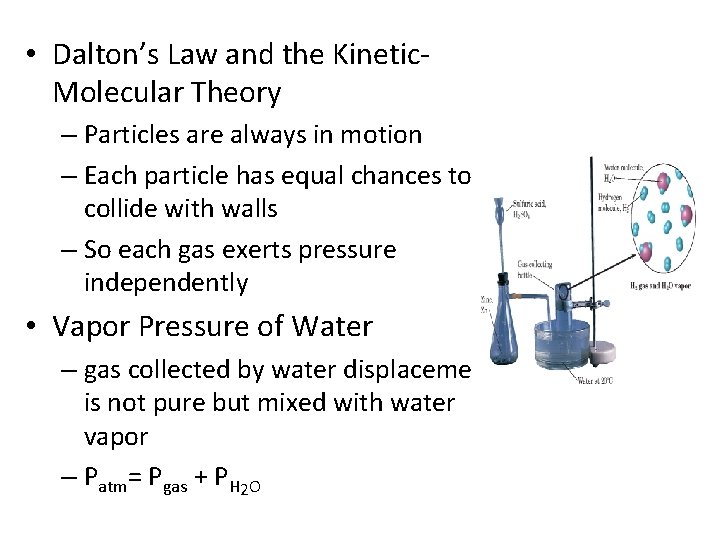
• Dalton’s Law and the Kinetic. Molecular Theory – Particles are always in motion – Each particle has equal chances to collide with walls – So each gas exerts pressure independently • Vapor Pressure of Water – gas collected by water displacement is not pure but mixed with water vapor – Patm= Pgas + PH 2 O

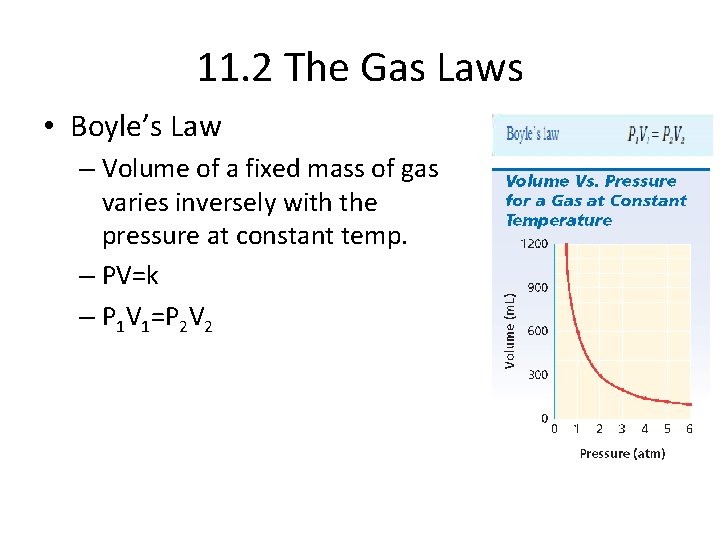
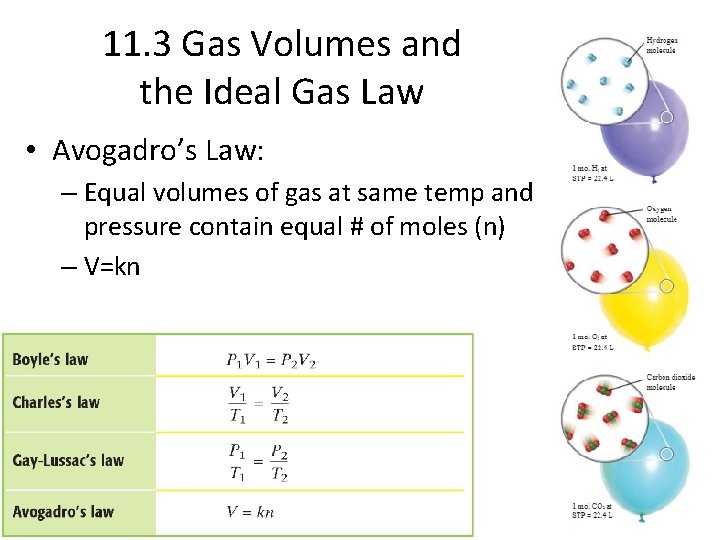
11. 2 The Gas Laws • Boyle’s Law – Volume of a fixed mass of gas varies inversely with the pressure at constant temp. – PV=k – P 1 V 1=P 2 V 2

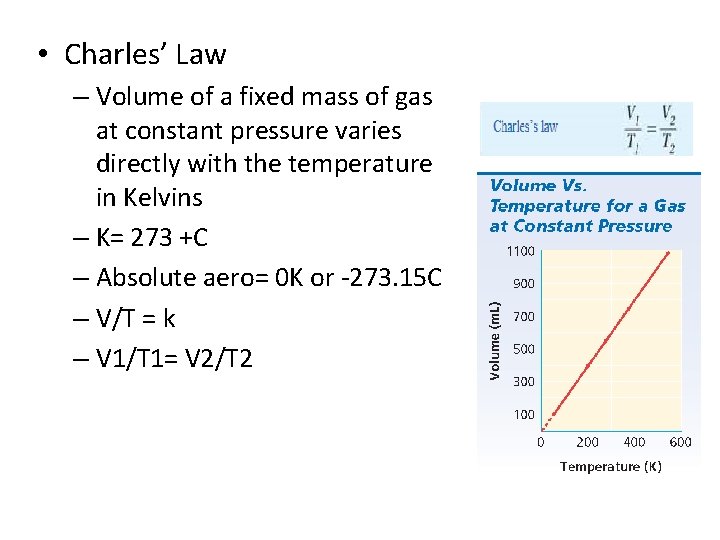
• Charles’ Law – Volume of a fixed mass of gas at constant pressure varies directly with the temperature in Kelvins – K= 273 +C – Absolute aero= 0 K or -273. 15 C – V/T = k – V 1/T 1= V 2/T 2

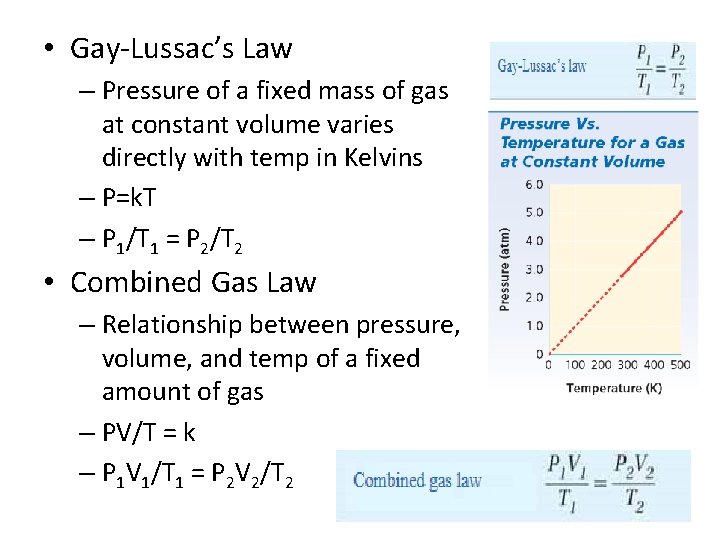
• Gay-Lussac’s Law – Pressure of a fixed mass of gas at constant volume varies directly with temp in Kelvins – P=k. T – P 1/T 1 = P 2/T 2 • Combined Gas Law – Relationship between pressure, volume, and temp of a fixed amount of gas – PV/T = k – P 1 V 1/T 1 = P 2 V 2/T 2

11. 3 Gas Volumes and the Ideal Gas Law • Avogadro’s Law: – Equal volumes of gas at same temp and pressure contain equal # of moles (n) – V=kn

• STP: Standard Temperature and Pressure – Vol occupied by 1 mol is 22. 4 L • Coefficients – Use amounts, moles, or volume ratios

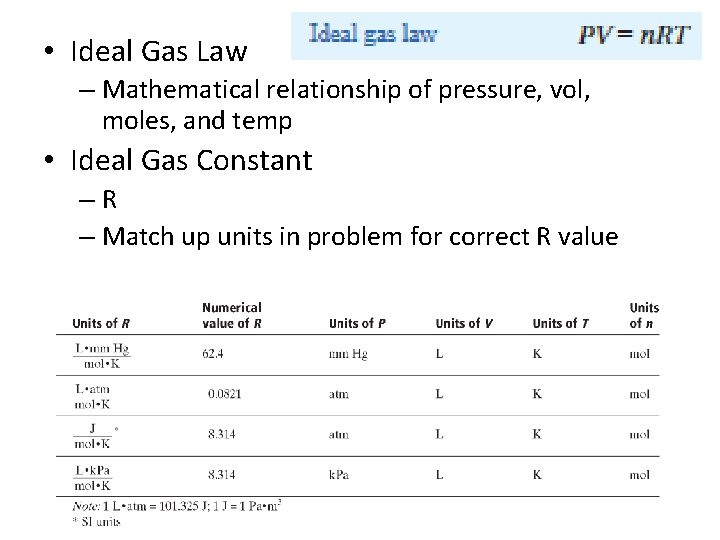
• Ideal Gas Law – Mathematical relationship of pressure, vol, moles, and temp • Ideal Gas Constant –R – Match up units in problem for correct R value

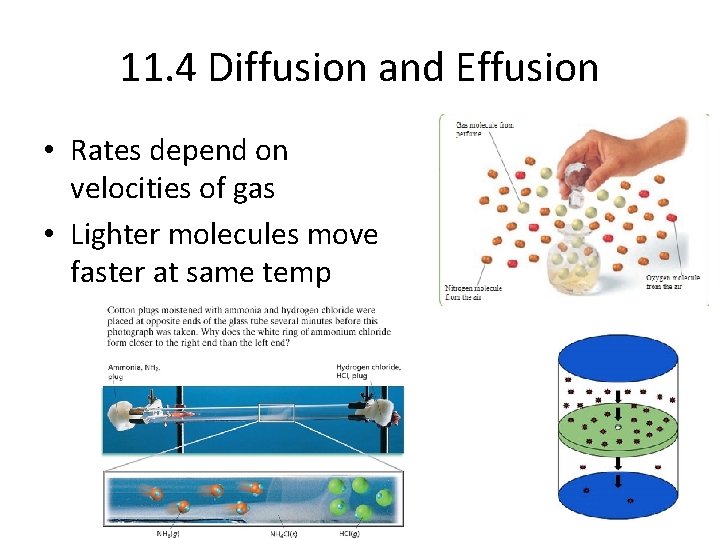
11. 4 Diffusion and Effusion • Rates depend on velocities of gas • Lighter molecules move faster at same temp

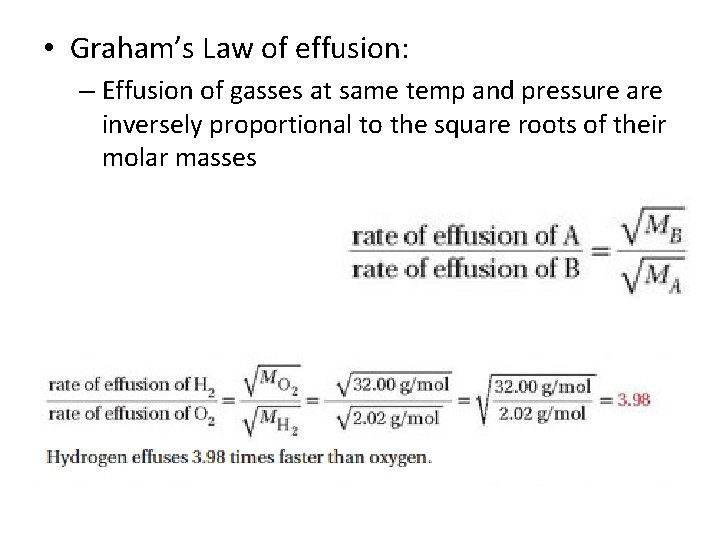
• Graham’s Law of effusion: – Effusion of gasses at same temp and pressure are inversely proportional to the square roots of their molar masses
 Gasses or gases
Gasses or gases A gas occupies 473 cm3 at 36°c. find its volume at 94°c
A gas occupies 473 cm3 at 36°c. find its volume at 94°c How many elements are gasses at room temperature
How many elements are gasses at room temperature Electron configuration for noble gases
Electron configuration for noble gases Most weather reports for the general public use
Most weather reports for the general public use Gasses
Gasses Blue gasses
Blue gasses Properties of gases
Properties of gases Magma volatile gasses definition
Magma volatile gasses definition Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them Bernoulli rule of integration
Bernoulli rule of integration What is oncotic pressure of blood
What is oncotic pressure of blood