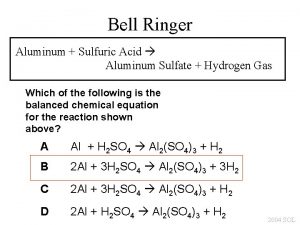
Ceramics Aluminum vs Alumina Demo Sketch the setup
















- Slides: 38

Ceramics

Aluminum vs. Alumina Demo Sketch the setup of the demo Mass of aluminum_____ Mass of Alumina_____ Describe WHAT you observed

Aluminum vs. Alumina Demo WHY it Happened Aluminum= Al=metal Aluminum Oxide, Alumina, Al 2 O 3 = ceramic • ceramics have higher melting points than pure metals • alumina has a melting point of 2054° C • aluminum melts at 660° C • ceramics don’t conduct electricity

What are Ceramics • Ceramics are non-metallic materials that are typically produced using clays and other minerals from the earth • The word ceramic, derives its name from the Greek word, keramos, meaning “pottery” – This word was derived from an older root, meaning “to burn".

Traditional Categories of Ceramics whiteware dinnerware, floor and wall tile, pottery, decorative ceramics Glass flat glass (windows), container glass (bottles), pressed and blown glass (dinnerware), glass fibers (home insulation) Abrasives Natural (garnet, diamond, etc. ) and synthetic (silicon carbide, diamond, fused alumina, etc. ) abrasives are used for grinding Structural, Clay Products Brick, sewer pipe, roofing tile, clay floor and wall tile (i. e. , quarry tile), flue linings Cement Concrete roads, bridges, buildings, dams, residential sidewalks, bricks/block Refractories Brick and monolithic products used in iron and steel, nonferrous metals, glass, cements, ceramics, energy conversion, petroleum, and chemicals industries, kiln furniture used in various industries

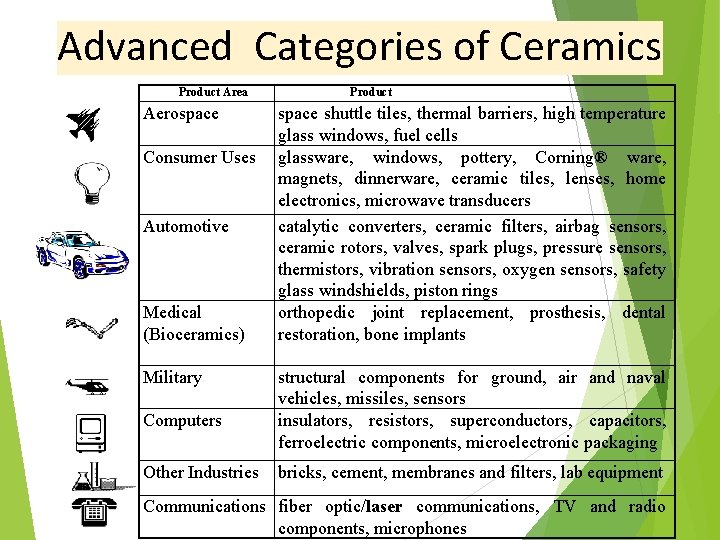
Advanced Categories of Ceramics Product Area Aerospace Consumer Uses Automotive Medical (Bioceramics) Military Computers Other Industries Product space shuttle tiles, thermal barriers, high temperature glass windows, fuel cells glassware, windows, pottery, Corning® ware, magnets, dinnerware, ceramic tiles, lenses, home electronics, microwave transducers catalytic converters, ceramic filters, airbag sensors, ceramic rotors, valves, spark plugs, pressure sensors, thermistors, vibration sensors, oxygen sensors, safety glass windshields, piston rings orthopedic joint replacement, prosthesis, dental restoration, bone implants structural components for ground, air and naval vehicles, missiles, sensors insulators, resistors, superconductors, capacitors, ferroelectric components, microelectronic packaging bricks, cement, membranes and filters, lab equipment Communications fiber optic/laser communications, TV and radio components, microphones

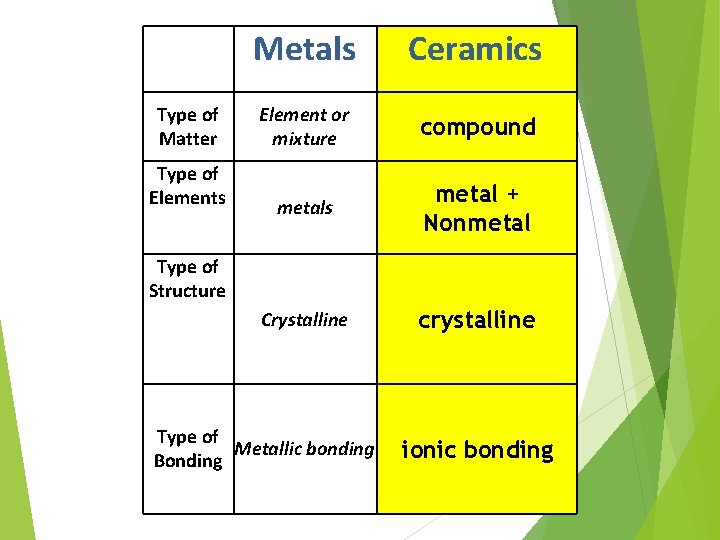
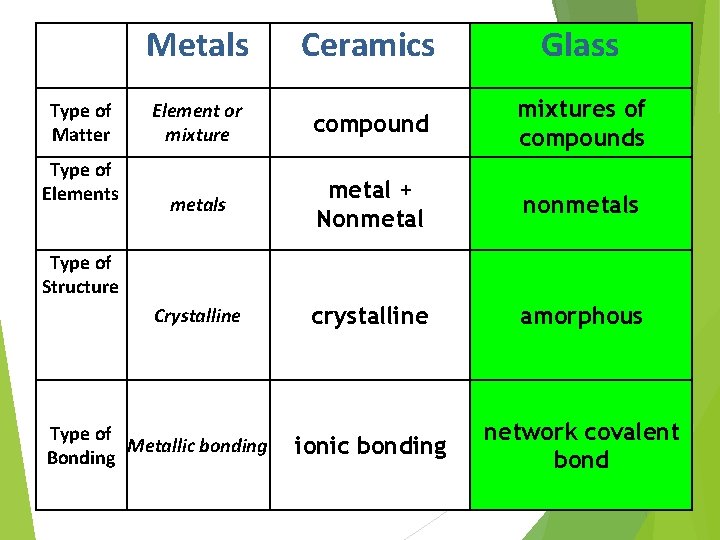
Type of Matter Type of Elements Metals Ceramics Element or mixture compound metals metal + Nonmetal Crystalline crystalline Type of Structure Type of Metallic bonding Bonding ionic bonding

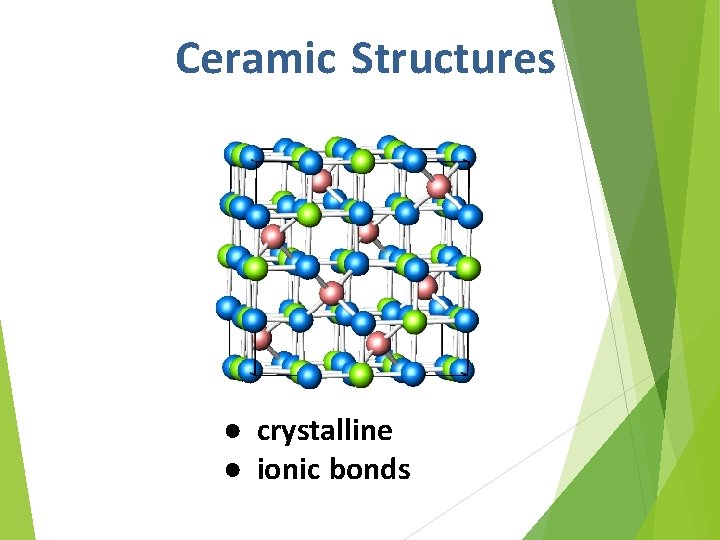
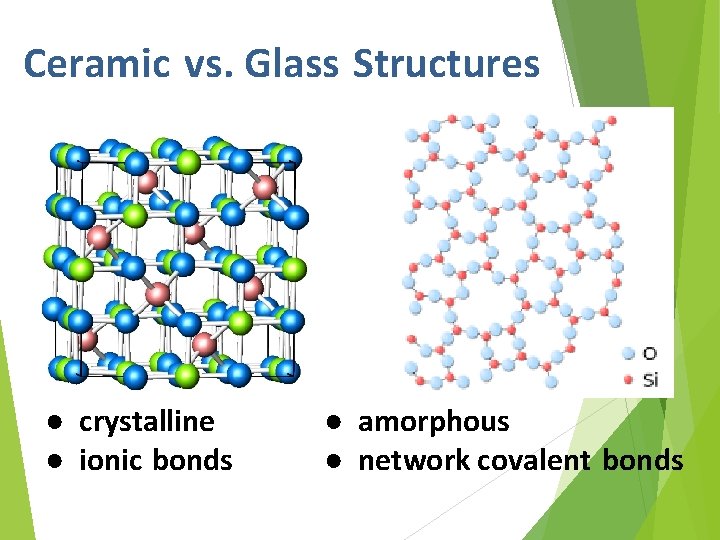
Ceramic Structures ● crystalline ● ionic bonds

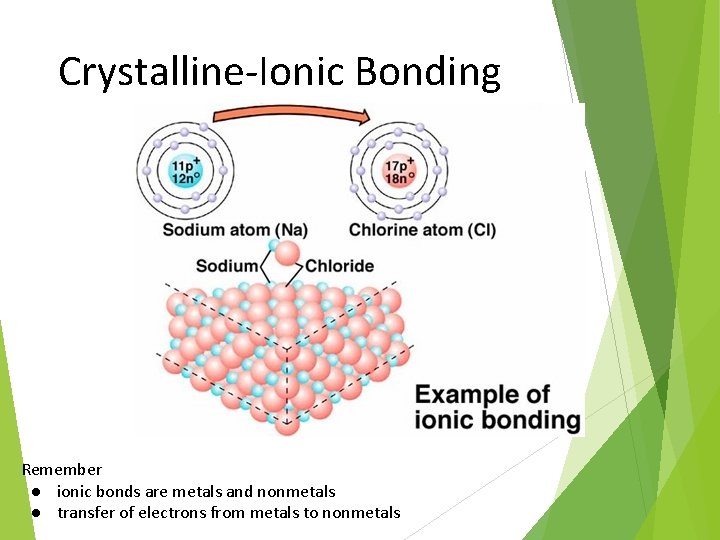
Crystalline-Ionic Bonding Remember ● ionic bonds are metals and nonmetals ● transfer of electrons from metals to nonmetals

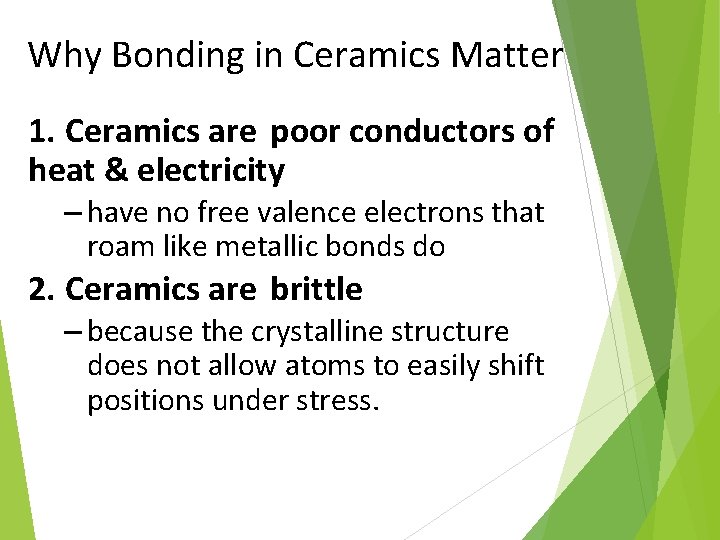
Why Bonding in Ceramics Matter 1. Ceramics are poor conductors of heat & electricity – have no free valence electrons that roam like metallic bonds do 2. Ceramics are brittle – because the crystalline structure does not allow atoms to easily shift positions under stress.

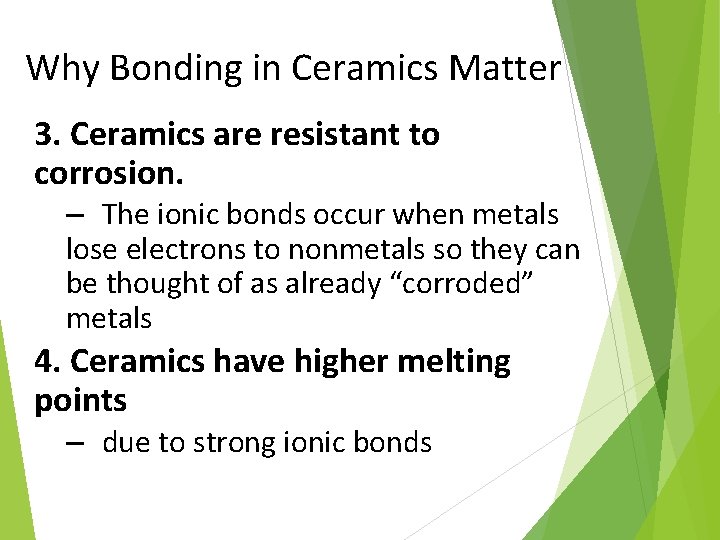
Why Bonding in Ceramics Matter 3. Ceramics are resistant to corrosion. – The ionic bonds occur when metals lose electrons to nonmetals so they can be thought of as already “corroded” metals 4. Ceramics have higher melting points – due to strong ionic bonds

Type of Matter Type of Elements Metals Ceramics Glass Element or mixture compound mixtures of compounds metal + Nonmetal nonmetals Crystalline crystalline amorphous ionic bonding network covalent bond Type of Structure Type of Metallic bonding Bonding

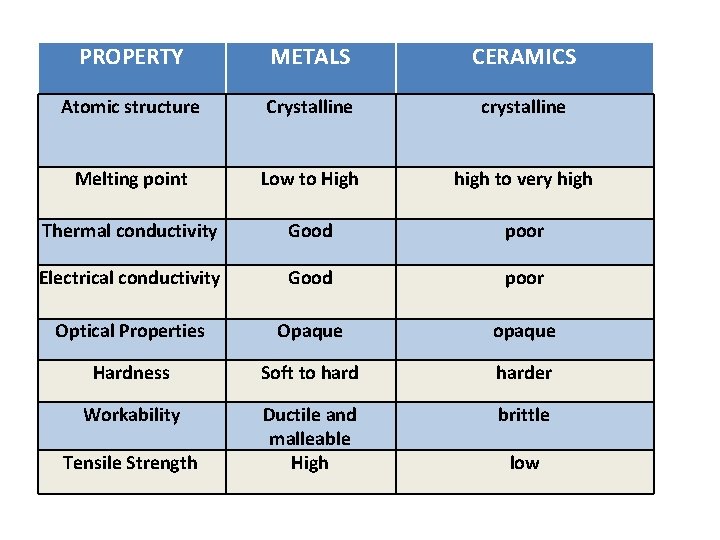
PROPERTY METALS CERAMICS Atomic structure Crystalline crystalline Melting point Low to High high to very high Thermal conductivity Good poor Electrical conductivity Good poor Optical Properties Opaque opaque Hardness Soft to harder Workability Ductile and malleable High brittle Tensile Strength low

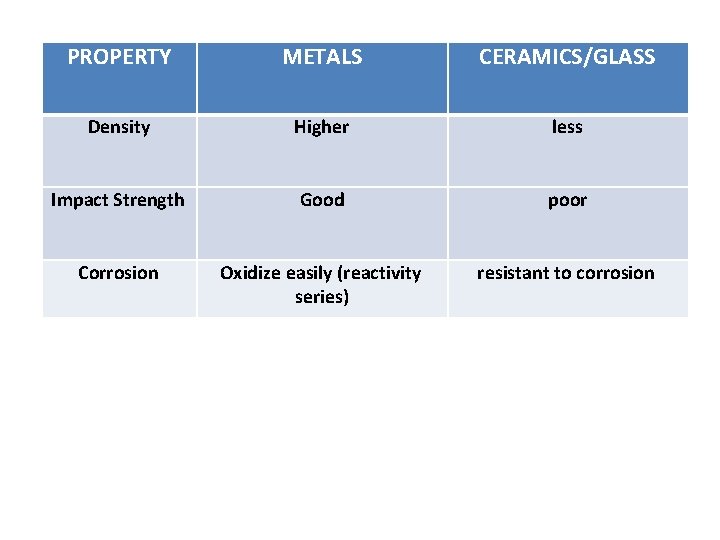
PROPERTY METALS CERAMICS/GLASS Density Higher less Impact Strength Good poor Corrosion Oxidize easily (reactivity series) resistant to corrosion

Ceramic vs. Glass Structures ● crystalline ● ionic bonds ● amorphous ● network covalent bonds

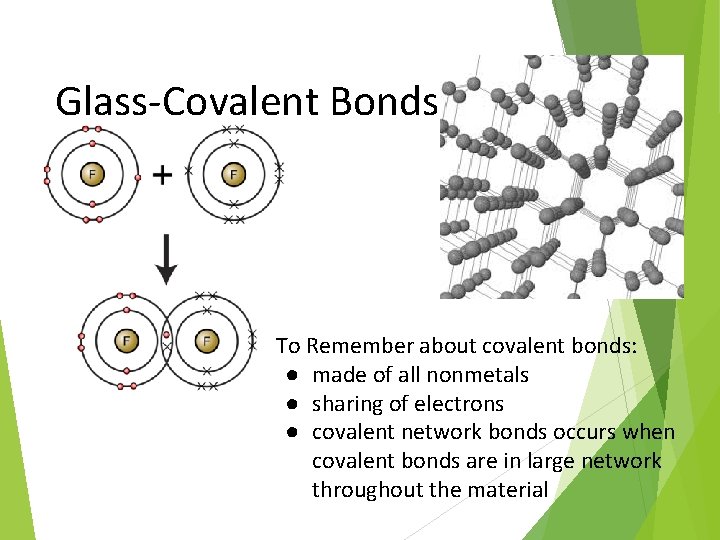
Glass-Covalent Bonds To Remember about covalent bonds: ● made of all nonmetals ● sharing of electrons ● covalent network bonds occurs when covalent bonds are in large network throughout the material

General Glass Info • Doesn’t have a definite melting point-just gradually softens – b/c glass is amorphous • very viscous • Rather poor conductor of electricity and heat • resistant to chemical attack – useful for food containers and laboratory apparatus

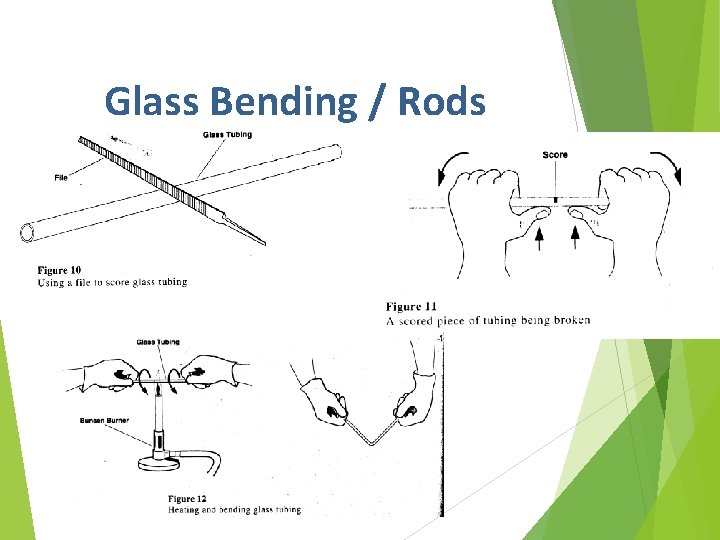
Glass Bending / Rods

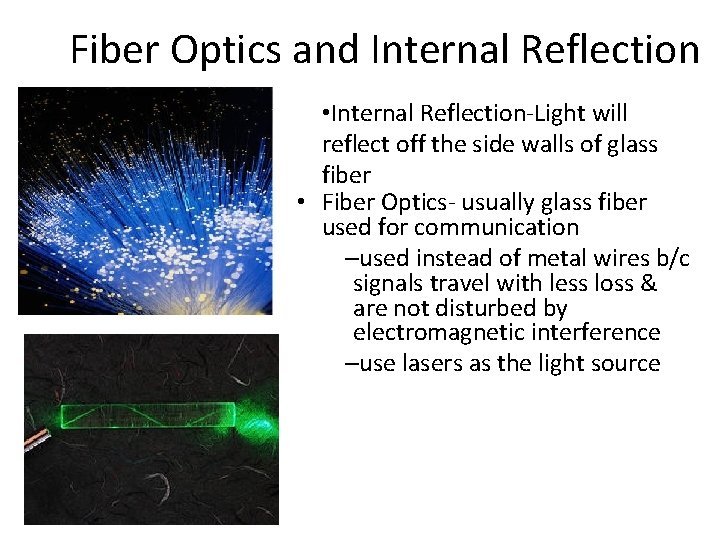
Fiber Optics and Internal Reflection • Internal Reflection-Light will reflect off the side walls of glass fiber • Fiber Optics- usually glass fiber used for communication –used instead of metal wires b/c signals travel with less loss & are not disturbed by electromagnetic interference –use lasers as the light source

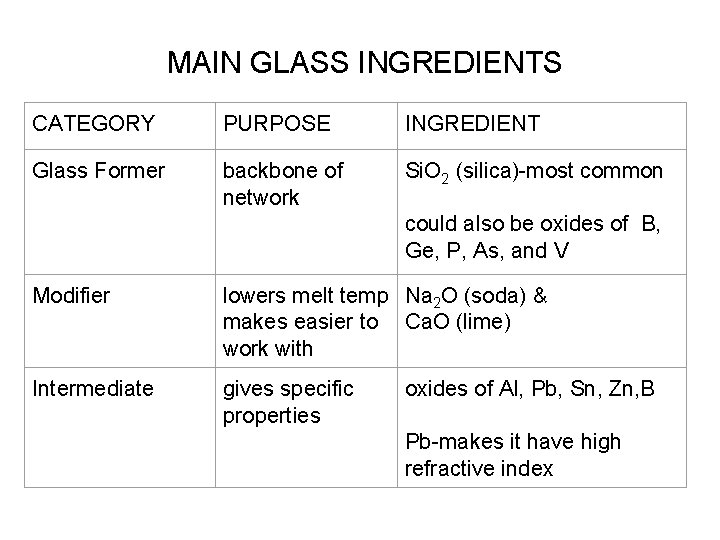
MAIN GLASS INGREDIENTS CATEGORY PURPOSE INGREDIENT Glass Former backbone of network Si. O 2 (silica)-most common could also be oxides of B, Ge, P, As, and V Modifier lowers melt temp Na 2 O (soda) & makes easier to Ca. O (lime) work with Intermediate gives specific properties oxides of Al, Pb, Sn, Zn, B Pb-makes it have high refractive index

Main Ingredients for Glass 1. Silica(silicon dioxide, Si. O 2) – – the chief constituent of sand quartz-silica in crystalline form=mineral fused silica=silica in amorphous form=glass However, fused silica is not used for commercial glass products for 2 reasons: • • it has a super high melting point (1650 C or 3000 F) when molten it has a very high viscosity and is difficult to form.


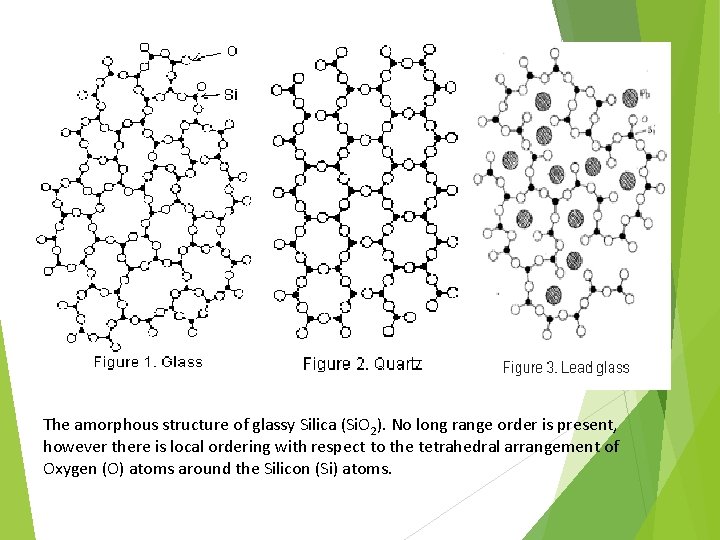
The amorphous structure of glassy Silica (Si. O 2). No long range order is present, however there is local ordering with respect to the tetrahedral arrangement of Oxygen (O) atoms around the Silicon (Si) atoms.

Other Ingredients in Glass • Fining agents – small amounts of other compounds added to help get rid of gas bubbles formed during melting process. • Many other ingredients -give specific colors or other properties. – For example, iron oxides can be added to give glass a green color and help absorb heat

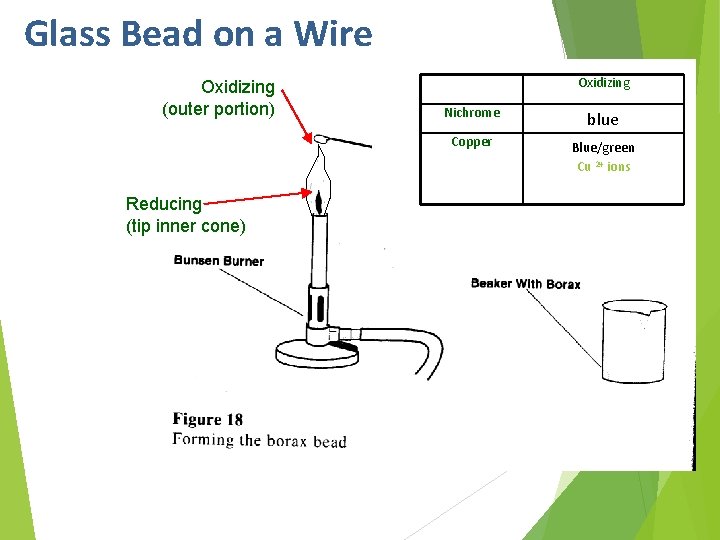
Glass Bead on a Wire Oxidizing (outer portion) Reducing (tip inner cone) Oxidizing Nichrome blue Copper Blue/green Cu 2+ ions



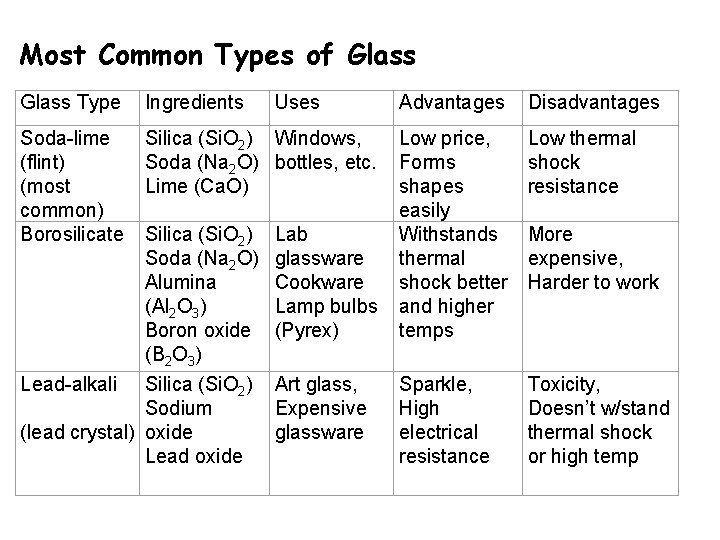
Most Common Types of Glass Type Ingredients Soda-lime (flint) (most common) Borosilicate Silica (Si. O 2) Windows, Soda (Na 2 O) bottles, etc. Lime (Ca. O) Lead-alkali Silica (Si. O 2) Soda (Na 2 O) Alumina (Al 2 O 3) Boron oxide (B 2 O 3) Silica (Si. O 2) Sodium (lead crystal) oxide Lead oxide Uses Lab glassware Cookware Lamp bulbs (Pyrex) Art glass, Expensive glassware Advantages Disadvantages Low price, Forms shapes easily Withstands thermal shock better and higher temps Low thermal shock resistance Sparkle, High electrical resistance Toxicity, Doesn’t w/stand thermal shock or high temp More expensive, Harder to work

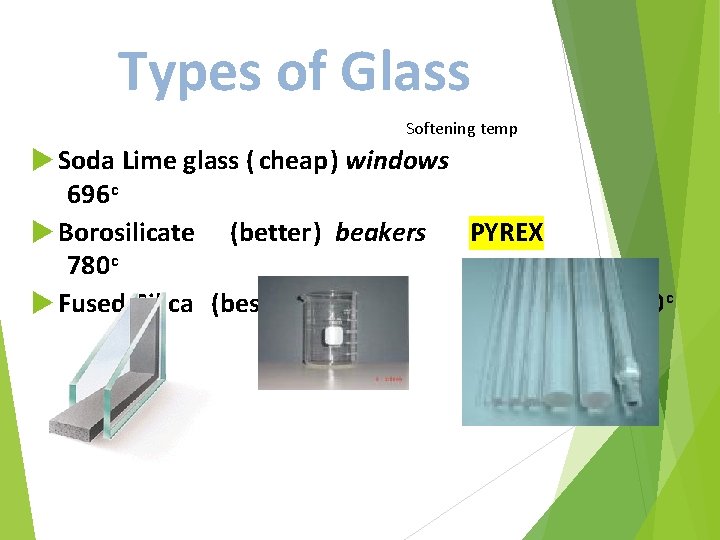
Types of Glass Softening temp Soda Lime glass ( cheap) windows 696 c Borosilicate (better) beakers PYREX 780 c Fused Silica (best) 1580 c

Thermal Shock Demo

Thermal Shock • Glass tends to shatter or crack when exposed to extreme temps too quickly • The outside will cool/contract or heat/expand quicker than the inside A lot of stress is put on the glass and it fails

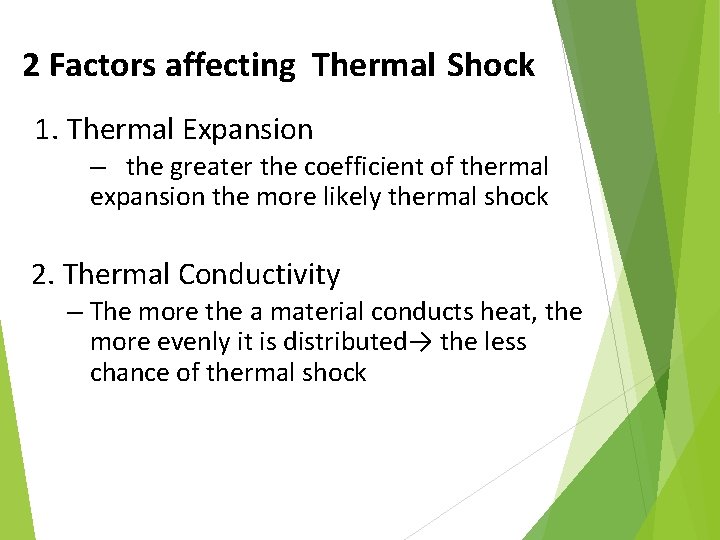
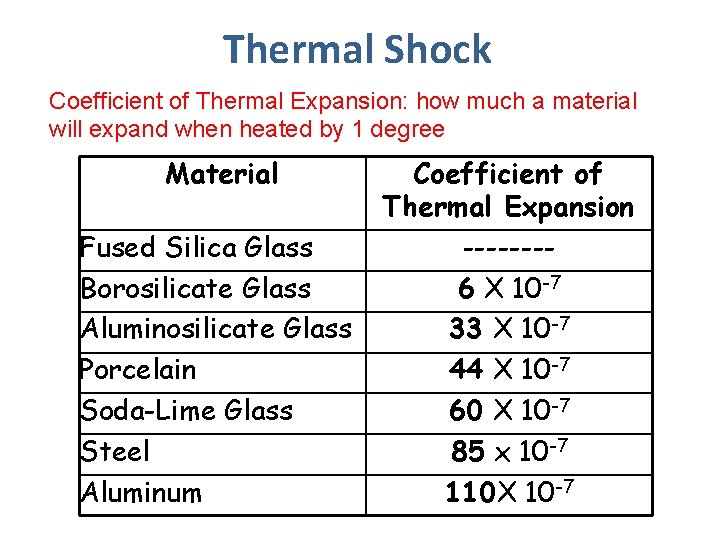
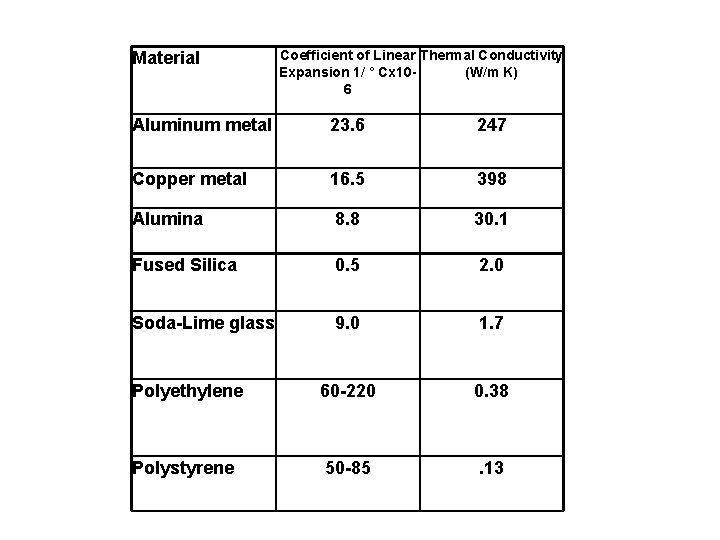
2 Factors affecting Thermal Shock 1. Thermal Expansion – the greater the coefficient of thermal expansion the more likely thermal shock 2. Thermal Conductivity – The more the a material conducts heat, the more evenly it is distributed→ the less chance of thermal shock

Thermal Shock Coefficient of Thermal Expansion: how much a material will expand when heated by 1 degree Material Fused Silica Glass Borosilicate Glass Aluminosilicate Glass Porcelain Soda-Lime Glass Steel Aluminum Coefficient of Thermal Expansion -------6 X 10 -7 33 X 10 -7 44 X 10 -7 60 X 10 -7 85 x 10 -7 110 X 10 -7

Material Coefficient of Linear Thermal Conductivity Expansion 1/ ° Cx 10(W/m K) 6 Aluminum metal 23. 6 247 Copper metal 16. 5 398 Alumina 8. 8 30. 1 Fused Silica 0. 5 2. 0 Soda-Lime glass 9. 0 1. 7 Polyethylene 60 -220 0. 38 Polystyrene 50 -85 . 13

Based on this information predict which glass is less likely to thermal shock Watch Video: https: //www. youtube. com/watch ? v=2 kx. Ttn. PGHSo (2 ½ minutes)

Processing—Heat Treating 1. Annealing – stresses build up in glass objects as they are cooled b/c glass can cool unevenly - weakens glass and may cause spontaneous fracture. – glass is reheated in ovens called lehrs, and then allowed to cool slowly to relieve the stresses Annealing Video (3 minutes): https: //www. youtube. com/watch? v=q. Bls. NNPRse Q&list=PLAEC 682 A 8 C 50 D 1 FF 8&index=8

Processing: Heat Treating 2. Tempering (2 minutes) – glass is heated to just below the softening temperature and is quenched to intentionally stress the entire surface uniformly. - outside is under compression - cooled/contracting - inside of glass is under tension- hot/ expanding – Advantages - much tougher than regular glass - when breaks it breaks into tiny pieces for safety – Disadvantages - Tempered glass cannot be cut or drilled. - weak on its edges Video: https: //www. youtube. com/watch? v=0 Qe. Lz_i. UF 6 c

Cool Glass Videos! • Rupert’s drop video (7 minutes) https: //www. youtube. com/watch? v=xef 4 gok. RBs • Glass Blowing Compilation (14 minutes) https: //www. youtube. com/watch? v=1 rt. QQ Rikrw. Y
 H2so4+al
H2so4+al Noranda alumina
Noranda alumina Porous alumina
Porous alumina Sketch meta demo lab
Sketch meta demo lab What is cross projection sketch
What is cross projection sketch Rough sketch vs final sketch crime scene
Rough sketch vs final sketch crime scene Willingham fire
Willingham fire Greenware def
Greenware def Ceramics science definition
Ceramics science definition Processing of ceramics
Processing of ceramics Bisqueware ceramics definition
Bisqueware ceramics definition Ceramics def
Ceramics def Charlotte manser ceramics
Charlotte manser ceramics Things made up of ceramic
Things made up of ceramic Powder metallurgy and metal ceramics
Powder metallurgy and metal ceramics Traditional ceramics examples
Traditional ceramics examples Most common coordination numbers for ceramic materials
Most common coordination numbers for ceramic materials Kiln god ideas easy
Kiln god ideas easy Robert arneson ceramics
Robert arneson ceramics Silicate ceramics dentistry
Silicate ceramics dentistry Sprigging ceramics definition
Sprigging ceramics definition Recent advances in ceramics
Recent advances in ceramics Blasch precision ceramics
Blasch precision ceramics Coiling clay definition
Coiling clay definition Hand building ceramics definition
Hand building ceramics definition Coil pot techniques
Coil pot techniques Hand building definition
Hand building definition Pinching ceramics definition
Pinching ceramics definition Functional ceramic applications
Functional ceramic applications Pinch pot ceramics definition
Pinch pot ceramics definition Raku-nn
Raku-nn Ceramics 101
Ceramics 101 Structures and properties of ceramics
Structures and properties of ceramics Maxit 603
Maxit 603 Timeline of pottery
Timeline of pottery Particulate forming process in ceramics
Particulate forming process in ceramics Ceramic classification
Ceramic classification Abstract expressionist ceramics
Abstract expressionist ceramics Sgraffito pottery
Sgraffito pottery