CARE Effect of Lipid Lowering on Lipid Values




- Slides: 31

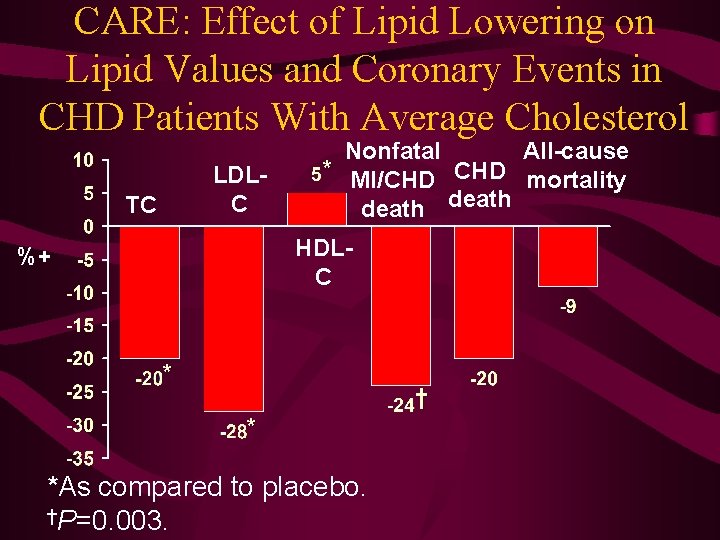
CARE: Effect of Lipid Lowering on Lipid Values and Coronary Events in CHD Patients With Average Cholesterol LDLC TC All-cause Nonfatal * MI/CHD mortality death HDLC %+ * † * *As compared to placebo. †P=0. 003.

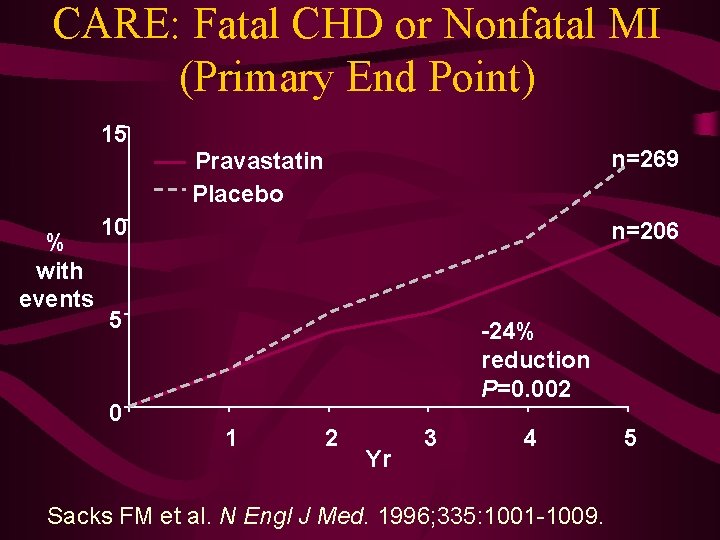
CARE: Fatal CHD or Nonfatal MI (Primary End Point) 15 n=269 Pravastatin Placebo % with events 10 n=206 5 0 -24% reduction P=0. 002 1 2 Yr 3 4 Sacks FM et al. N Engl J Med. 1996; 335: 1001 -1009. 5

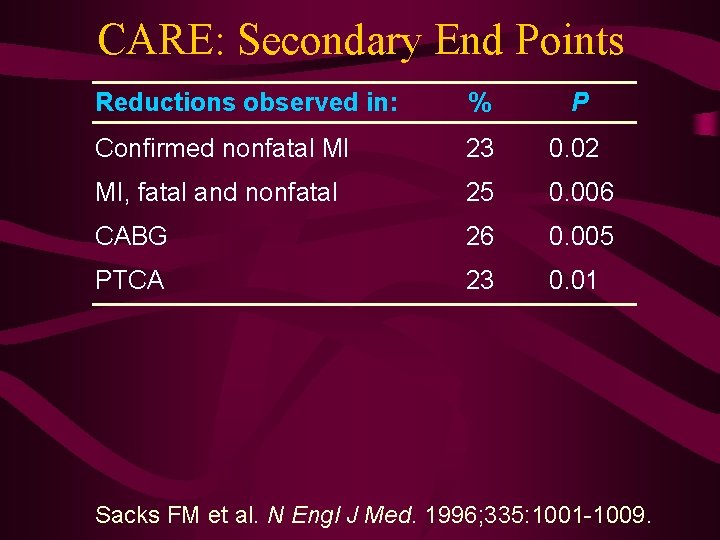
CARE: Secondary End Points Reductions observed in: % P Confirmed nonfatal MI 23 0. 02 MI, fatal and nonfatal 25 0. 006 CABG 26 0. 005 PTCA 23 0. 01 Sacks FM et al. N Engl J Med. 1996; 335: 1001 -1009.

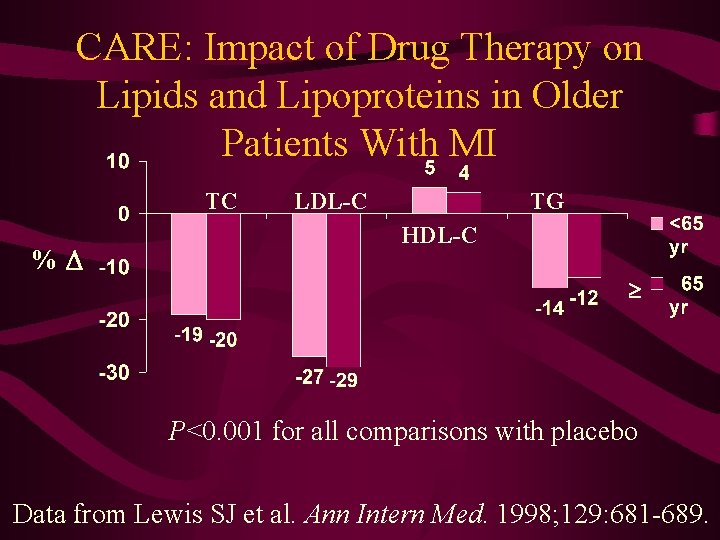
CARE: Impact of Drug Therapy on Lipids and Lipoproteins in Older Patients With MI TC % LDL-C TG HDL-C P<0. 001 for all comparisons with placebo Data from Lewis SJ et al. Ann Intern Med. 1998; 129: 681 -689.

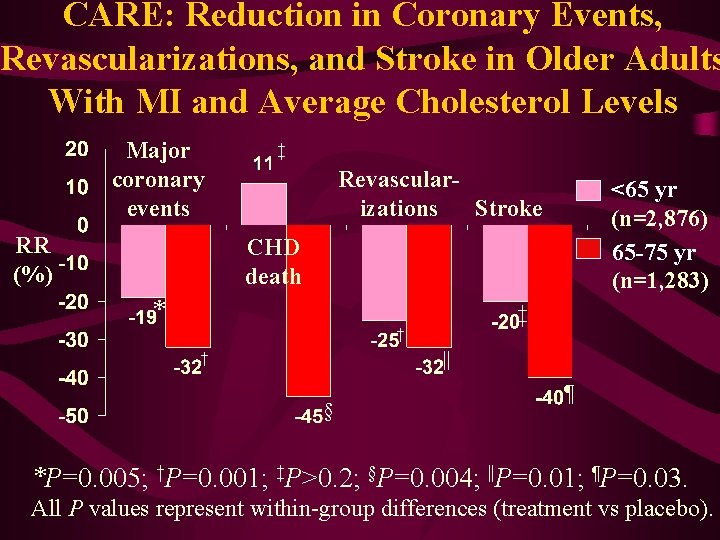
CARE: Reduction in Coronary Events, Revascularizations, and Stroke in Older Adults With MI and Average Cholesterol Levels Major coronary events RR (%) ‡ Revascularizations Stroke <65 yr (n=2, 876) 65 -75 yr (n=1, 283) CHD death * ‡ † || † § ¶ *P=0. 005; †P=0. 001; ‡P>0. 2; §P=0. 004; ||P=0. 01; ¶P=0. 03. All P values represent within-group differences (treatment vs placebo).

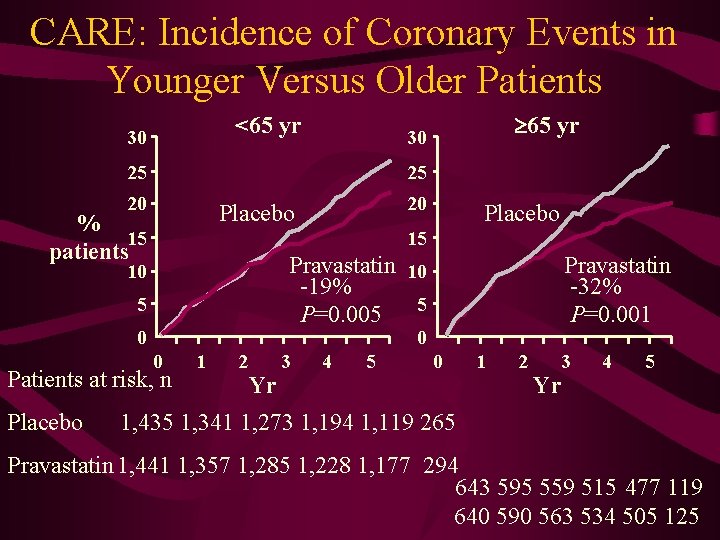
CARE: Incidence of Coronary Events in Younger Versus Older Patients <65 yr 30 25 25 20 20 Placebo % 15 patients Placebo 15 Pravastatin 10 -19% P=0. 005 5 10 5 0 Pravastatin -32% P=0. 001 0 0 Patients at risk, n Placebo 65 yr 30 1 2 Yr 3 4 5 0 1 2 3 Yr 4 5 1, 435 1, 341 1, 273 1, 194 1, 119 265 Pravastatin 1, 441 1, 357 1, 285 1, 228 1, 177 294 643 595 559 515 477 119 640 590 563 534 505 125

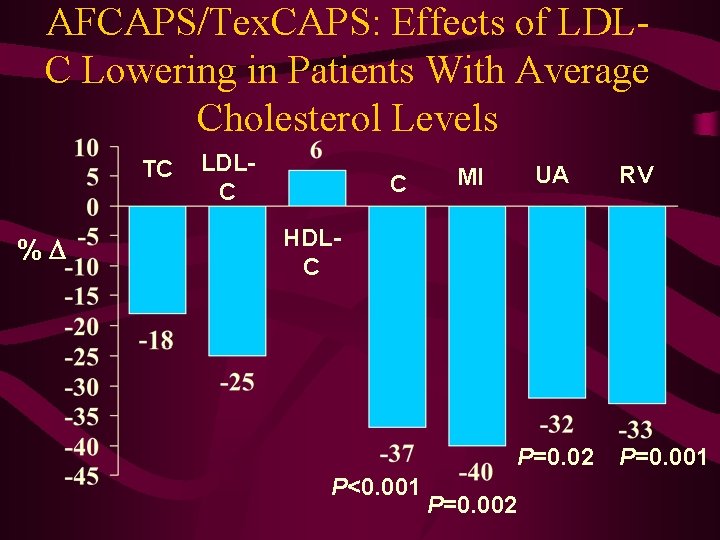
AFCAPS/Tex. CAPS: Effects of LDLC Lowering in Patients With Average Cholesterol Levels TC % LDLC C UA MI RV HDLC P=0. 02 P<0. 001 P=0. 002 P=0. 001

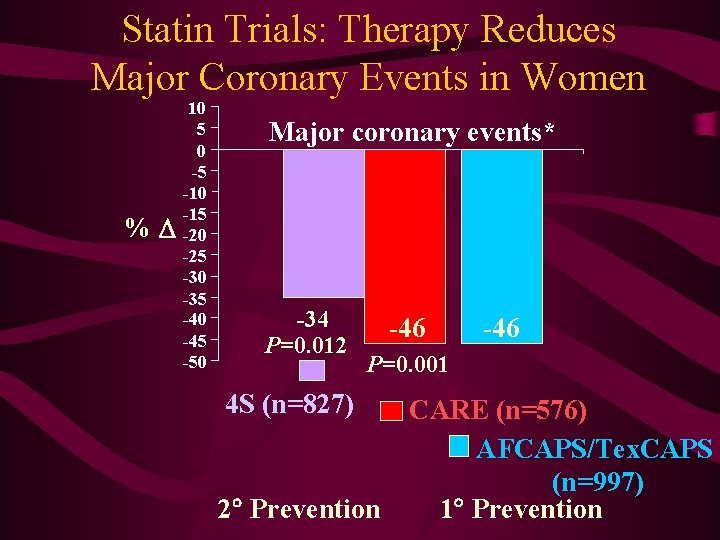
Statin Trials: Therapy Reduces Major Coronary Events in Women % 10 5 0 -5 -10 -15 -20 -25 -30 -35 -40 -45 -50 Major coronary events* -34 P=0. 012 -46 P=0. 001 4 S (n=827) 2 Prevention CARE (n=576) AFCAPS/Tex. CAPS (n=997) 1 Prevention

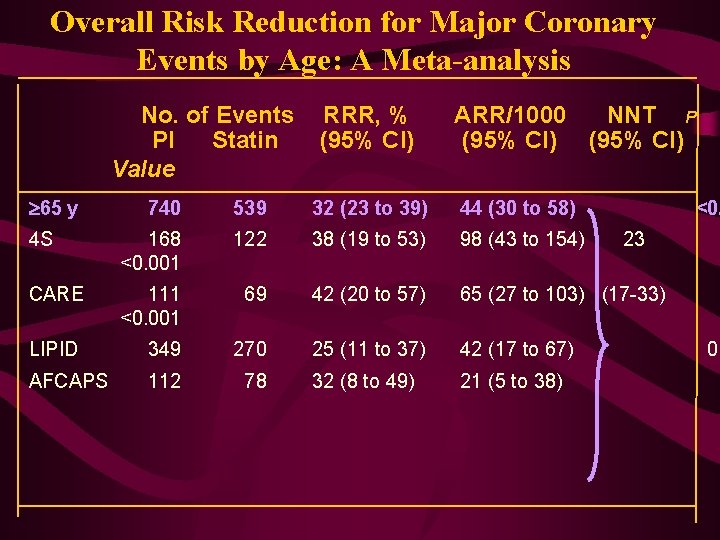
Overall Risk Reduction for Major Coronary Events by Age: A Meta-analysis No. of Events PI Statin Value 65 y RRR, % (95% CI) ARR/1000 NNT P (95% CI) 740 539 32 (23 to 39) 44 (30 to 58) <0. 4 S 168 <0. 001 122 38 (19 to 53) 98 (43 to 154) CARE 111 <0. 001 69 42 (20 to 57) 65 (27 to 103) (17 -33) LIPID 349 270 25 (11 to 37) 42 (17 to 67) AFCAPS 112 78 32 (8 to 49) 21 (5 to 38) 23 0. 0

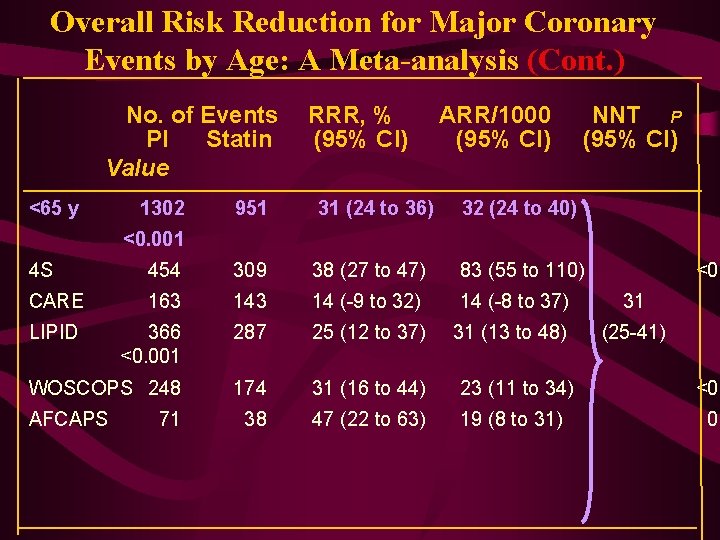
Overall Risk Reduction for Major Coronary Events by Age: A Meta-analysis (Cont. ) No. of Events PI Statin Value <65 y 1302 RRR, % (95% CI) ARR/1000 (95% CI) 951 31 (24 to 36) 32 (24 to 40) NNT P (95% CI) <0. 001 4 S 454 309 38 (27 to 47) 83 (55 to 110) CARE 163 14 (-9 to 32) 14 (-8 to 37) 31 LIPID 366 <0. 001 287 25 (12 to 37) 31 (13 to 48) (25 -41) WOSCOPS 248 174 31 (16 to 44) 23 (11 to 34) <0. 38 47 (22 to 63) 19 (8 to 31) 0. AFCAPS 71 <0. 0

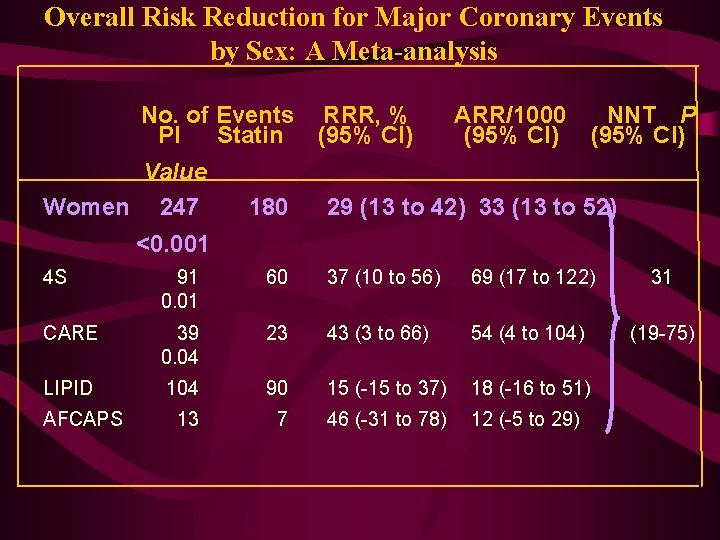
Overall Risk Reduction for Major Coronary Events by Sex: A Meta-analysis No. of Events RRR, % PI Statin (95% CI) Value Women 247 180 ARR/1000 (95% CI) NNT P (95% CI) 29 (13 to 42) 33 (13 to 52) <0. 001 4 S 91 0. 01 60 37 (10 to 56) 69 (17 to 122) 31 CARE 39 0. 04 23 43 (3 to 66) 54 (4 to 104) (19 -75) LIPID 104 90 15 (-15 to 37) 18 (-16 to 51) 13 7 46 (-31 to 78) 12 (-5 to 29) AFCAPS

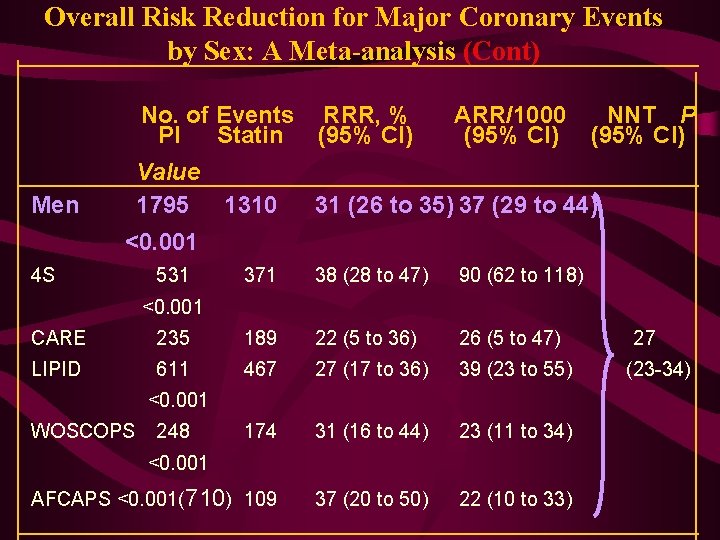
Overall Risk Reduction for Major Coronary Events by Sex: A Meta-analysis (Cont) No. of Events RRR, % PI Statin (95% CI) Value 1795 1310 Men ARR/1000 (95% CI) NNT P (95% CI) 31 (26 to 35) 37 (29 to 44) <0. 001 4 S 531 371 38 (28 to 47) 90 (62 to 118) <0. 001 CARE 235 189 22 (5 to 36) 26 (5 to 47) LIPID 611 467 27 (17 to 36) 39 (23 to 55) 174 31 (16 to 44) 23 (11 to 34) AFCAPS <0. 001(710) 109 37 (20 to 50) 22 (10 to 33) <0. 001 WOSCOPS 248 <0. 001 27 (23 -34)

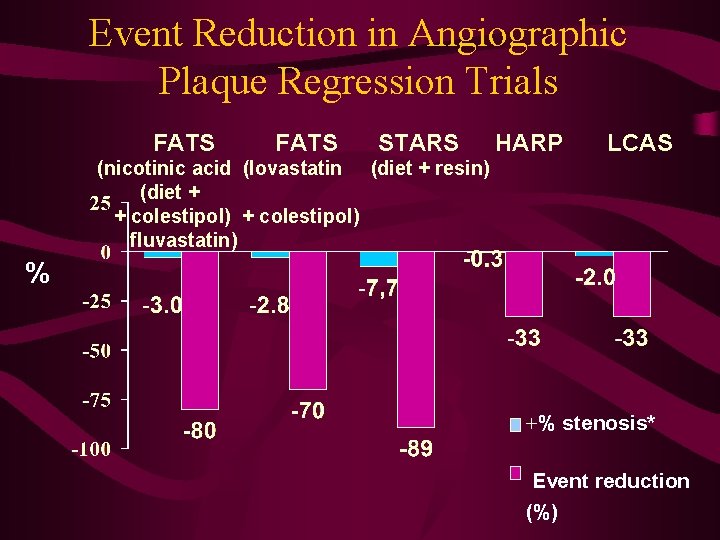
Event Reduction in Angiographic Plaque Regression Trials FATS STARS HARP LCAS (nicotinic acid (lovastatin (diet + resin) (diet + + colestipol) fluvastatin) % +% stenosis* Event reduction (%)

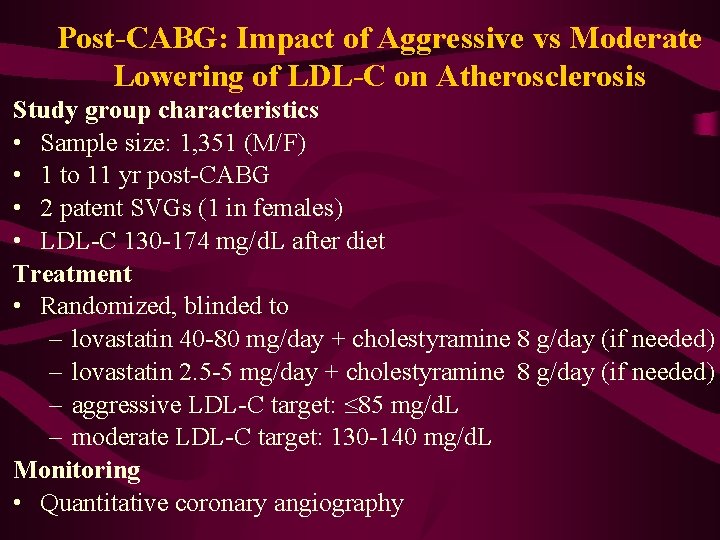
Post-CABG: Impact of Aggressive vs Moderate Lowering of LDL-C on Atherosclerosis Study group characteristics • Sample size: 1, 351 (M/F) • 1 to 11 yr post-CABG • 2 patent SVGs (1 in females) • LDL-C 130 -174 mg/d. L after diet Treatment • Randomized, blinded to – lovastatin 40 -80 mg/day + cholestyramine 8 g/day (if needed) – lovastatin 2. 5 -5 mg/day + cholestyramine 8 g/day (if needed) – aggressive LDL-C target: 85 mg/d. L – moderate LDL-C target: 130 -140 mg/d. L Monitoring • Quantitative coronary angiography

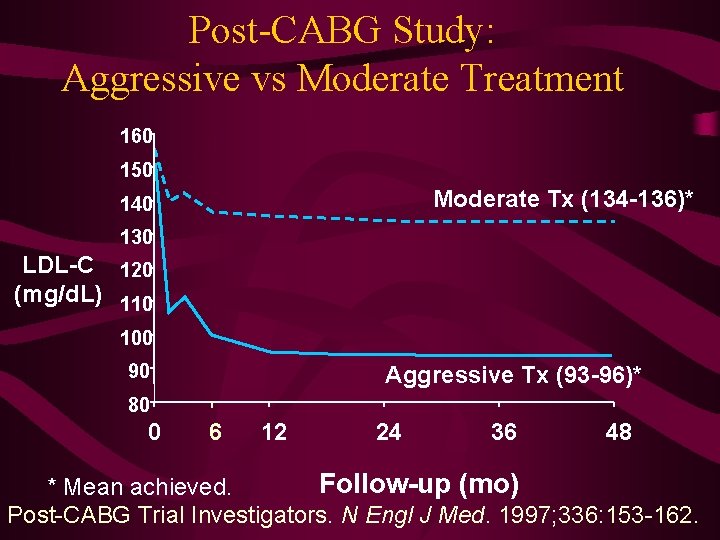
Post-CABG Study: Aggressive vs Moderate Treatment 160 150 Moderate Tx (134 -136)* 140 130 LDL-C 120 (mg/d. L) 110 100 90 Aggressive Tx (93 -96)* 80 0 6 12 24 36 48 Follow-up (mo) * Mean achieved. Post-CABG Trial Investigators. N Engl J Med. 1997; 336: 153 -162.

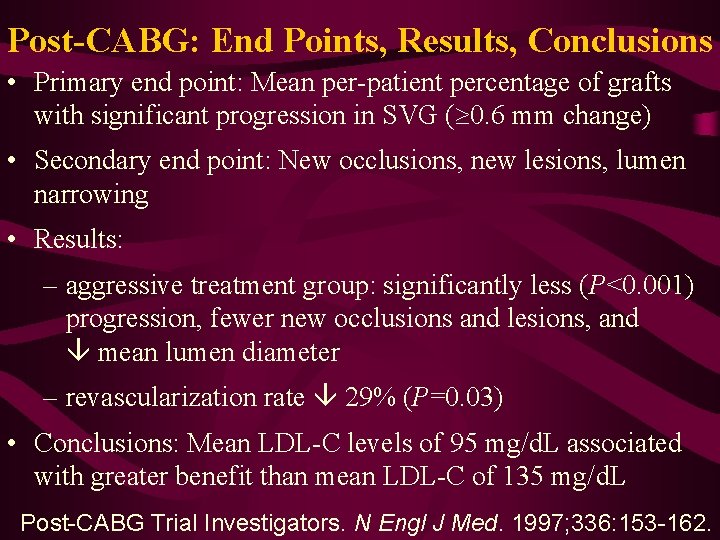
Post-CABG: End Points, Results, Conclusions • Primary end point: Mean per-patient percentage of grafts with significant progression in SVG (³ 0. 6 mm change) • Secondary end point: New occlusions, new lesions, lumen narrowing • Results: – aggressive treatment group: significantly less (P<0. 001) progression, fewer new occlusions and lesions, and mean lumen diameter – revascularization rate 29% (P=0. 03) • Conclusions: Mean LDL-C levels of 95 mg/d. L associated with greater benefit than mean LDL-C of 135 mg/d. L Post-CABG Trial Investigators. N Engl J Med. 1997; 336: 153 -162.

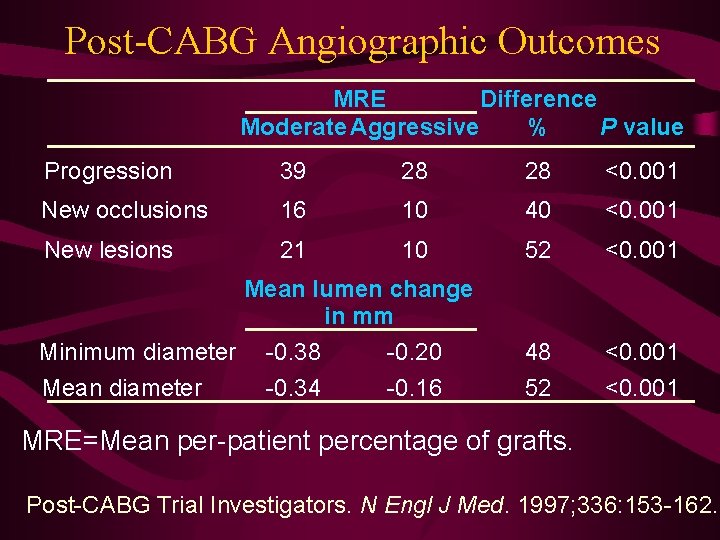
Post-CABG Angiographic Outcomes MRE Difference Moderate Aggressive % P value Progression 39 28 28 <0. 001 New occlusions 16 10 40 <0. 001 New lesions 21 10 52 <0. 001 48 52 <0. 001 Mean lumen change in mm Minimum diameter Mean diameter -0. 38 -0. 34 -0. 20 -0. 16 MRE=Mean per-patient percentage of grafts. Post-CABG Trial Investigators. N Engl J Med. 1997; 336: 153 -162.

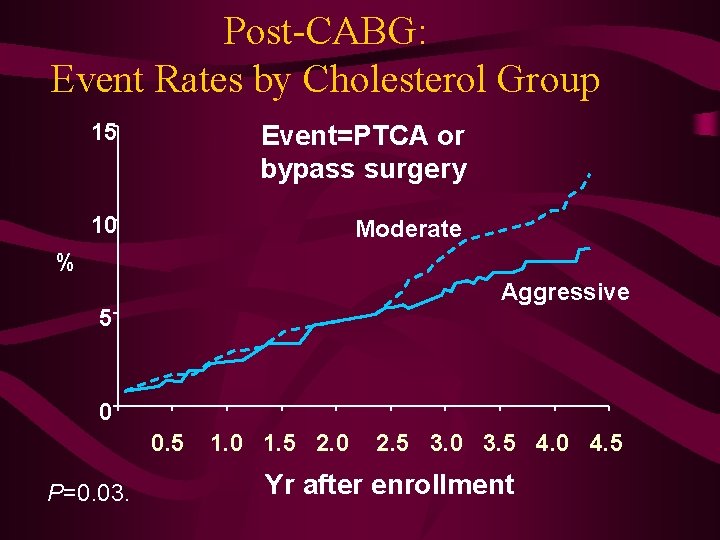
Post-CABG: Event Rates by Cholesterol Group 15 Event=PTCA or bypass surgery 10 Moderate % Aggressive 5 0 0. 5 P=0. 03. 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 4. 5 Yr after enrollment

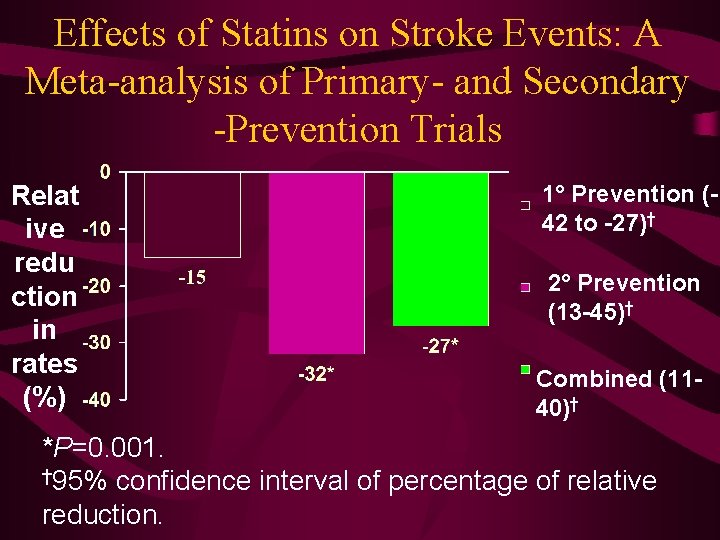
Effects of Statins on Stroke Events: A Meta-analysis of Primary- and Secondary -Prevention Trials Relat ive redu ction in rates (%) 1° Prevention (42 to -27)† 2° Prevention (13 -45)† Combined (1140)† *P=0. 001. † 95% confidence interval of percentage of relative reduction.

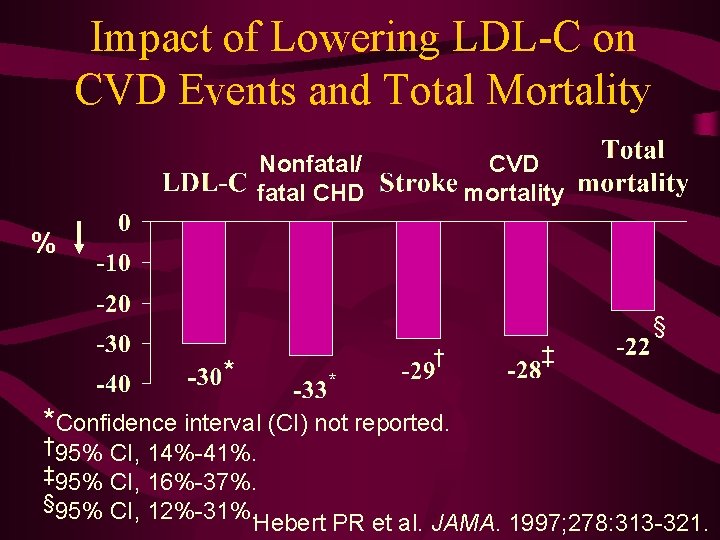
Impact of Lowering LDL-C on CVD Events and Total Mortality Nonfatal/ fatal CHD CVD mortality % § † * ‡ * *Confidence interval (CI) not reported. † 95% CI, 14%-41%. ‡ 95% CI, 16%-37%. § 95% CI, 12%-31%. Hebert PR et al. JAMA. 1997; 278: 313 -321.

Clinical Trial Findings: The Statins • Statins LDL-C by 25%-35% (achieved in clinical event trials and regression studies) • Benefits at various LDL-C levels; evident soon after therapy in some studies • in LDL-C required for in CHD morbidity/mortality • in all-cause mortality in 2° prevention and in cardiovascular mortality in 1° prevention • Studies support treatment in various patient groups – women – elderly – diabetics

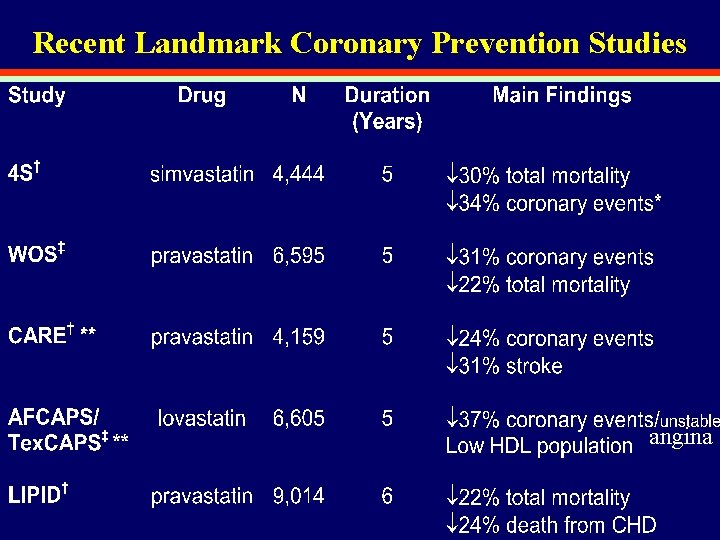
Recent Landmark Coronary Prevention Studies angina

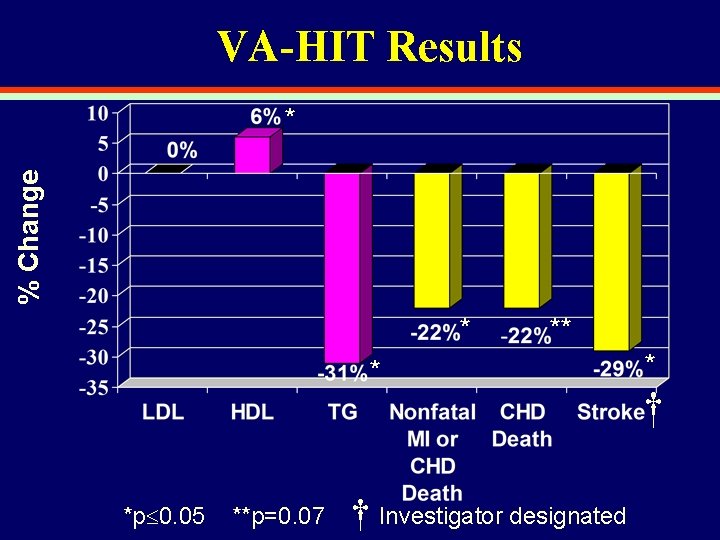
VA-HIT Results % Change * * *p 0. 05 **p=0. 07 † Investigator designated * †

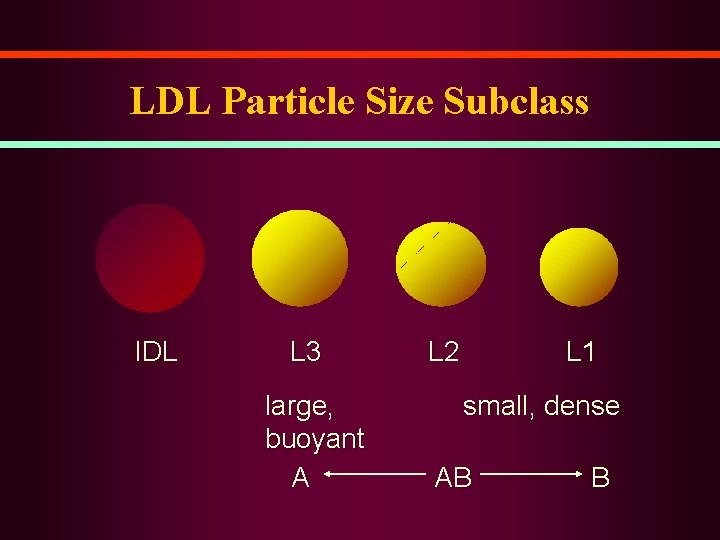
LDL Particle Size Subclass IDL L 3 large, buoyant A L 2 L 1 small, dense AB B

Significance of Small, Dense LDL • Low cholesterol content of LDL particles – particle number for given LDL-C level • Associated with levels of TG and LDL-C, and levels of HDL 2 • Marker for common genetic trait associated with risk of coronary disease (LDL subclass pattern B) • Possible mechanisms of atherogenicity – greater arterial uptake – uptake by macrophages – oxidation susceptibility

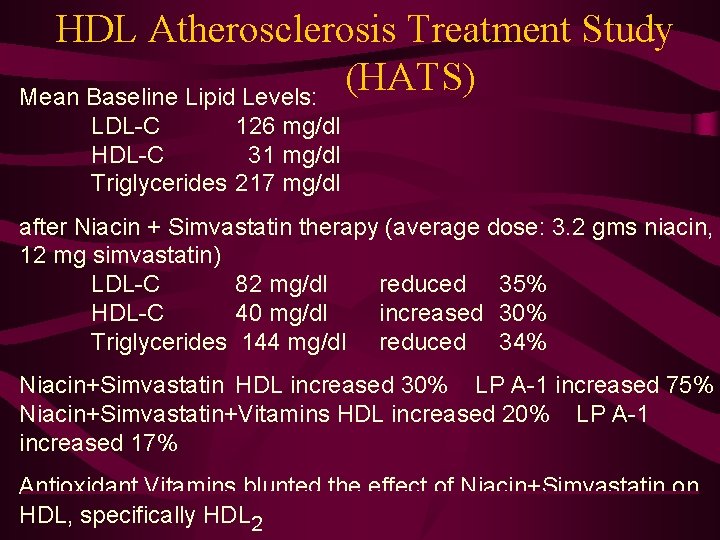
HDL Atherosclerosis Treatment Study (HATS) NIH-funded, the HDL Atherosclerosis Treatment Study is a double-blind, placebo controlled, factorial design, 3 -year angiographic trial. Patients (n=160) 15% had diabetes, 10% had impaired glucose tolerance. Patients randomized into four (4) treatment goups: 1. Niacin (2 -4 grams/day) + Simvastatin (10 -20 mg/day) plus Antioxidant Vitamins 2. Placebo (Niacin+Simvastatin) plus Antioxidant Vitamins 3. Niacin (2 -4 grams/day) + Simvastatin (10 -20 mg/day) plus Placebo (Vitamins) 4. Placebo (Niacin + Simvastatin) plus Placebo (Vitamins)

HDL Atherosclerosis Treatment Study (HATS) Mean Baseline Lipid Levels: LDL-C 126 mg/dl HDL-C 31 mg/dl Triglycerides 217 mg/dl after Niacin + Simvastatin therapy (average dose: 3. 2 gms niacin, 12 mg simvastatin) LDL-C 82 mg/dl reduced 35% HDL-C 40 mg/dl increased 30% Triglycerides 144 mg/dl reduced 34% Niacin+Simvastatin HDL increased 30% LP A-1 increased 75% Niacin+Simvastatin+Vitamins HDL increased 20% LP A-1 increased 17% Antioxidant Vitamins blunted the effect of Niacin+Simvastatin on HDL, specifically HDL 2

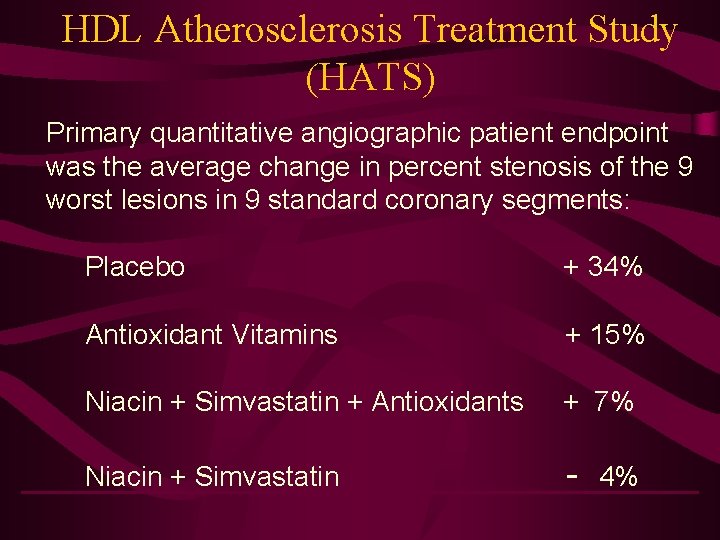
HDL Atherosclerosis Treatment Study (HATS) Primary quantitative angiographic patient endpoint was the average change in percent stenosis of the 9 worst lesions in 9 standard coronary segments: Placebo + 34% Antioxidant Vitamins + 15% Niacin + Simvastatin + Antioxidants + 7% Niacin + Simvastatin - 4%

HDL Atherosclerosis Treatment Study (HATS) Primary clinical endpoint was the Kaplan-Meier intention-to-treat time to first event: CAD death, MI, Stroke, Hospital-confirmed unstable ischemia + revascularization. 15% of the patients were diabetic while 10% of the patients had impaired glucose tolerance. Patients on Niacin + Simvastatin had clinical events reduced by 70%. The addition of Antioxidant Vitamins to Niacin + Simvastatin resulted in only a 15% reduction in clinical events.

Clinical Benefits of Cholesterol Reduction • A recent meta-analysis of 38 trials demonstrated that for every 10% reduction in TC – CHD mortality decreased by 15% (P<0. 001) – total mortality decreased by 11% (P<0. 001) • Decreases were similar for all treatment modalities • Cholesterol reduction did not increase non-CHD mortality Gould AL et al. Circulation. 1998; 97: 946 -952.

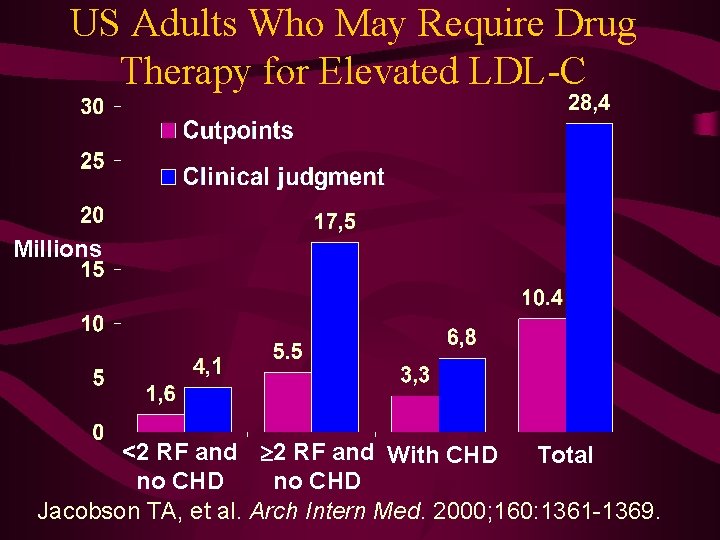
US Adults Who May Require Drug Therapy for Elevated LDL-C Millions <2 RF and With CHD Total no CHD Jacobson TA, et al. Arch Intern Med. 2000; 160: 1361 -1369.
 Gidl定義
Gidl定義 Why freezing point decreases on adding solute
Why freezing point decreases on adding solute Vapour pressure lowering
Vapour pressure lowering Lifting/lowering
Lifting/lowering Electrolytes and nonelectrolytes
Electrolytes and nonelectrolytes Vapor pressure lowering
Vapor pressure lowering Freezing point formula
Freezing point formula Vapor pressure lowering
Vapor pressure lowering Relocation and line lowering
Relocation and line lowering Relative lowering of vapour pressure
Relative lowering of vapour pressure Primary care secondary care tertiary care
Primary care secondary care tertiary care Western vs eastern values
Western vs eastern values Machiavellian personality
Machiavellian personality An individual's enduring tendency to feel
An individual's enduring tendency to feel A bit can have two values
A bit can have two values Classification of human values
Classification of human values Health and social care unit 2
Health and social care unit 2 Bohr's effect in respiration
Bohr's effect in respiration Founder effect vs bottleneck effect
Founder effect vs bottleneck effect Income effect graph
Income effect graph Duty of care outcome care certificate
Duty of care outcome care certificate Polii magnetului
Polii magnetului Palliative care vs hospice care
Palliative care vs hospice care Se inmultesc prin oua
Se inmultesc prin oua Care sunt simturile prin care sunt evocate
Care sunt simturile prin care sunt evocate Care certificate standard 9 answers
Care certificate standard 9 answers Hip fracture care clinical care standard
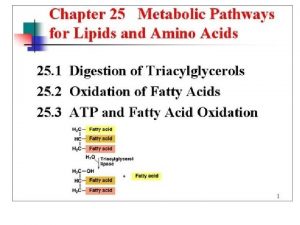
Hip fracture care clinical care standard Steroid hormones lipids
Steroid hormones lipids Storage of carbohydrates
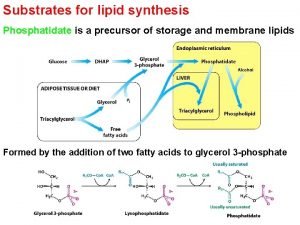
Storage of carbohydrates Triacylglycerol synthesis
Triacylglycerol synthesis Copper acetate test for lipids
Copper acetate test for lipids V
V