Atoms Studying Atoms Dalton proposed theory that all



- Slides: 23

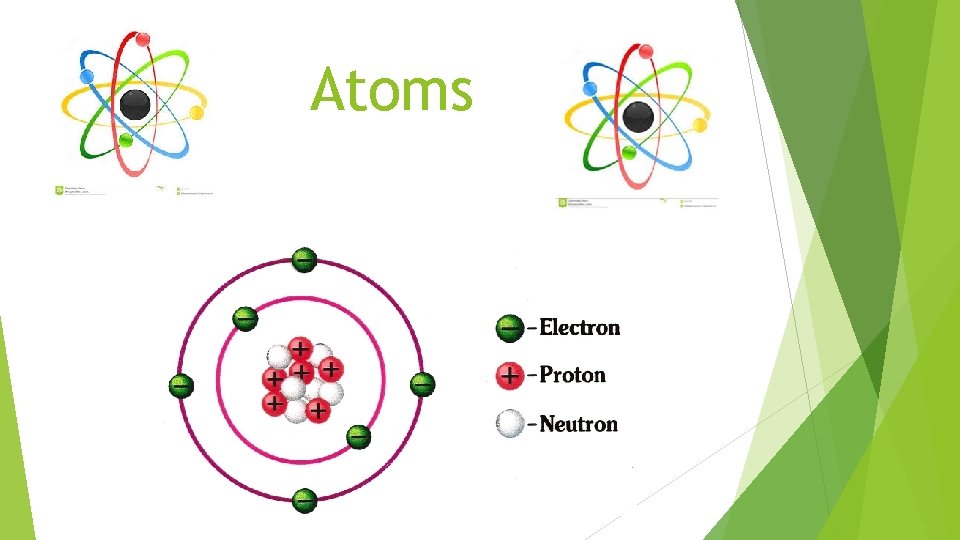
Atoms

Studying Atoms Dalton proposed theory that all matter is made up of individual particles called atoms, which cannot be divided based on those observations

Studying Atoms J. Thomson used an electric current to learn more about atoms based on the same idea of the activity

Studying Atoms Send a beam of light through a tube and placed plates on each side (1 positively charged and 1 negatively charged) The He beam of light bent to the positive side concluded the particles in the beam have a negative charge (+ and – attract) He hypothesized that the particles came from inside atoms

Studying Atoms Thomson’s experiment was the first evidence that atoms are made of even smaller particles Chocolate chip ice cream: vanilla ice cream is positive charge and chocolate chips are negative charges distributed throughout (plum pudding model)

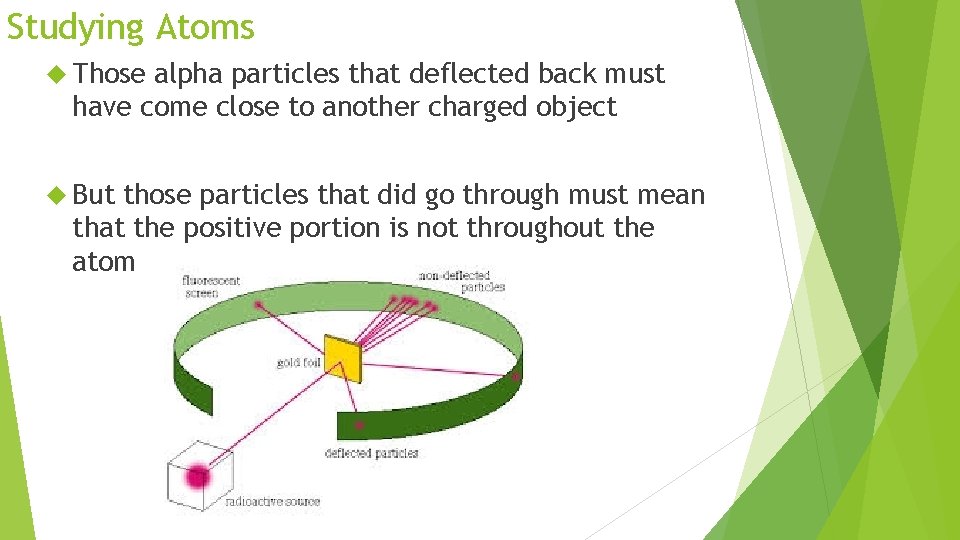
Studying Atoms Ernest Rutherford’s gold foil experiment His results were not the intended goal Those alpha particles that deflected back must have come close to another charged object But those particles that did go through must mean that the positive portion is not throughout the atom

Studying Atoms Those alpha particles that deflected back must have come close to another charged object But those particles that did go through must mean that the positive portion is not throughout the atom

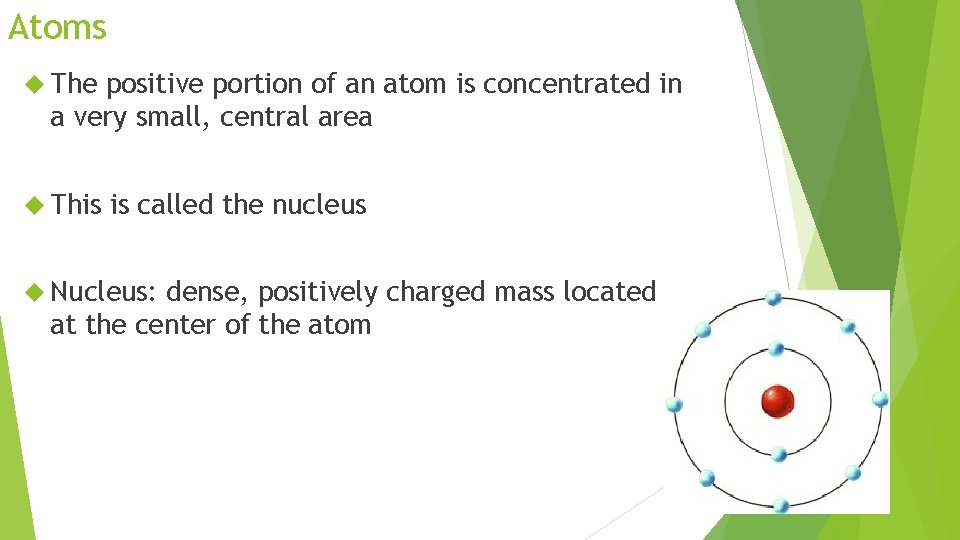
Atoms The positive portion of an atom is concentrated in a very small, central area This is called the nucleus Nucleus: dense, positively charged mass located at the center of the atom

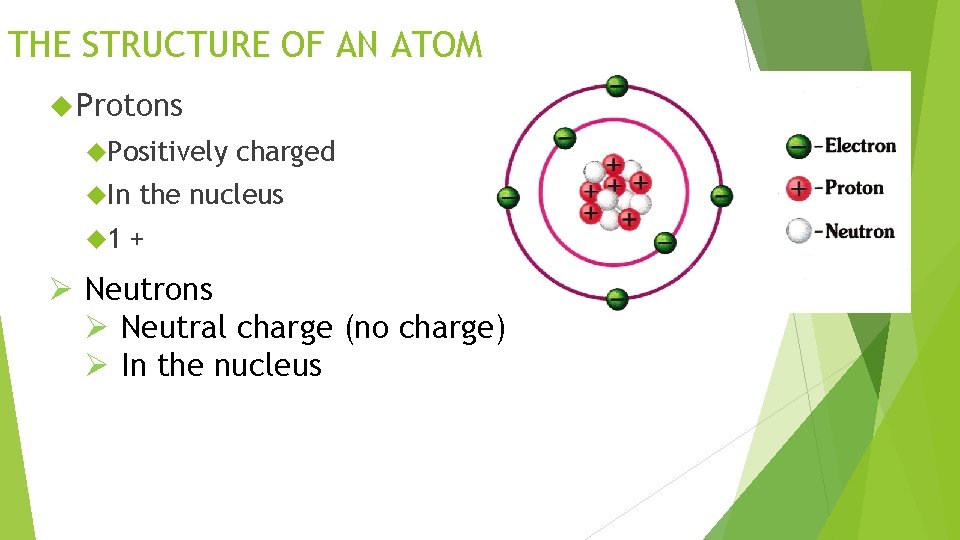
THE STRUCTURE OF AN ATOM Protons Positively In 1 charged the nucleus + Ø Neutrons Ø Neutral charge (no charge) Ø In the nucleus

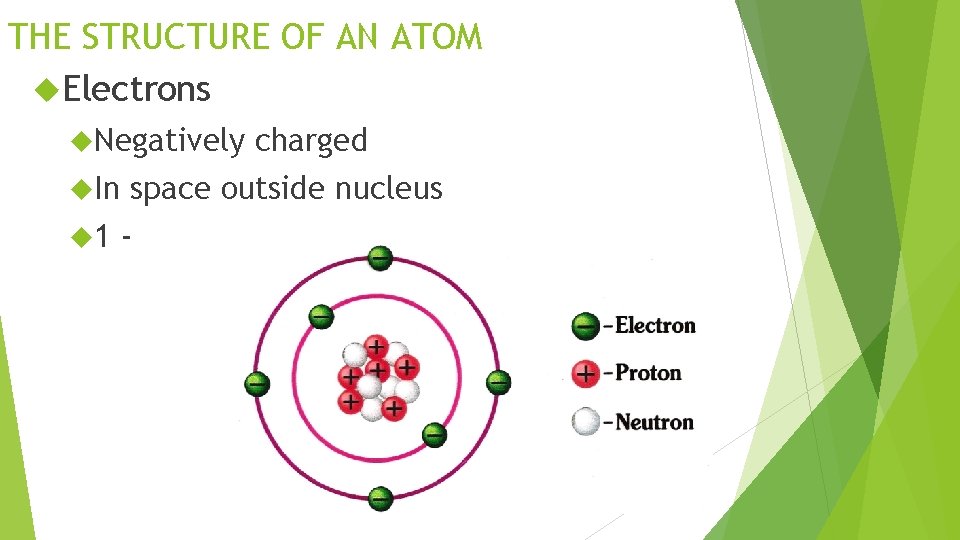
THE STRUCTURE OF AN ATOM Electrons Negatively In 1 charged space outside nucleus -

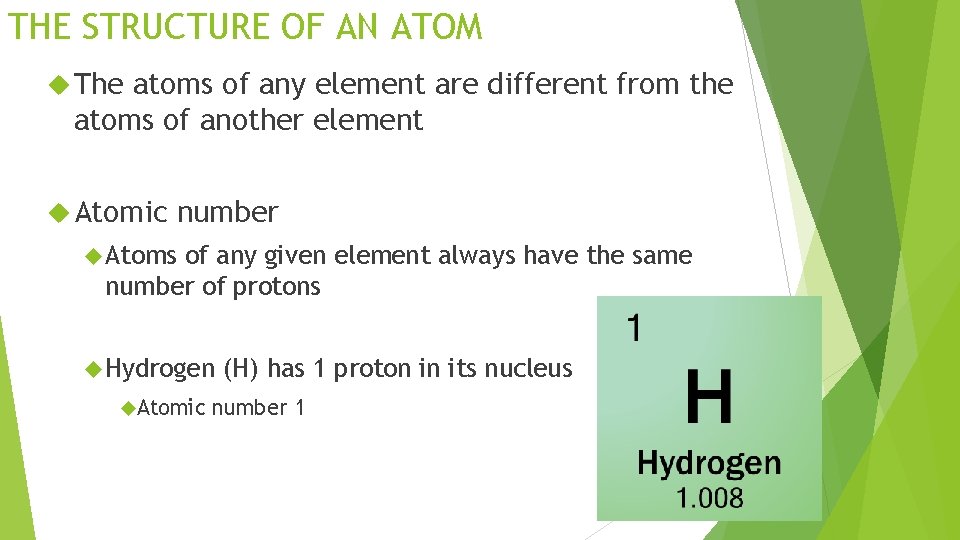
THE STRUCTURE OF AN ATOM The atoms of any element are different from the atoms of another element Atomic number Atoms of any given element always have the same number of protons Hydrogen Atomic (H) has 1 proton in its nucleus number 1

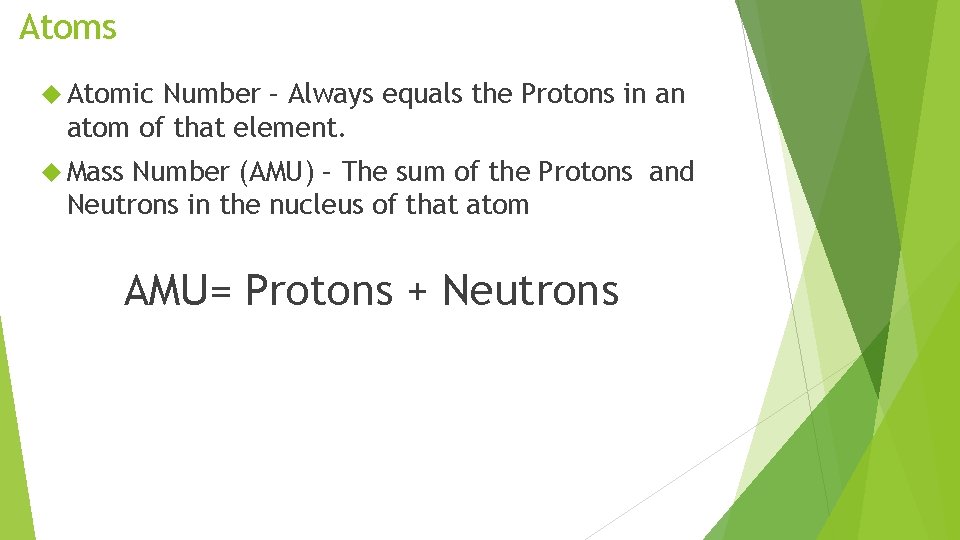
Atoms Atomic Number – Always equals the Protons in an atom of that element. Mass Number (AMU) – The sum of the Protons and Neutrons in the nucleus of that atom AMU= Protons + Neutrons

ATOMS Each positive charge (proton) is balanced with a negative charge (electron) and that means that atoms are neutral (no charge) Negative Charge means there are more ELECTRONS (anion) Positive Charge means there are more PROTONS (cation)

Modeling and Representing Atoms on Paper How to draw Bohr Diagrams and Electron Dot Diagrams (sometimes called Lewis Structures)

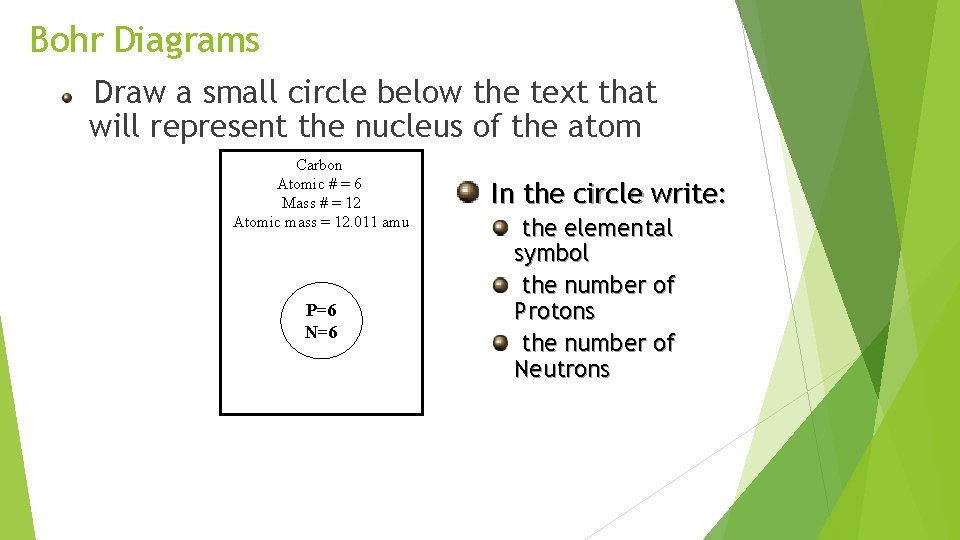
Bohr Diagrams Draw a small circle below the text that will represent the nucleus of the atom Carbon Atomic # = 6 Mass # = 12 Atomic mass = 12. 011 amu P=6 N=6 In the circle write: the elemental symbol the number of Protons the number of Neutrons

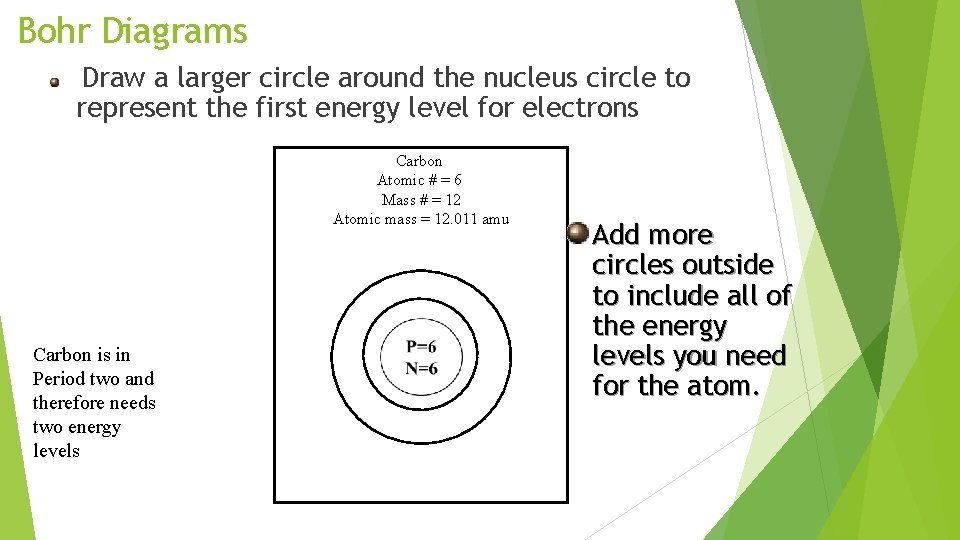
Bohr Diagrams Draw a larger circle around the nucleus circle to represent the first energy level for electrons Carbon Atomic # = 6 Mass # = 12 Atomic mass = 12. 011 amu Carbon is in Period two and therefore needs two energy levels Add more circles outside to include all of the energy levels you need for the atom.

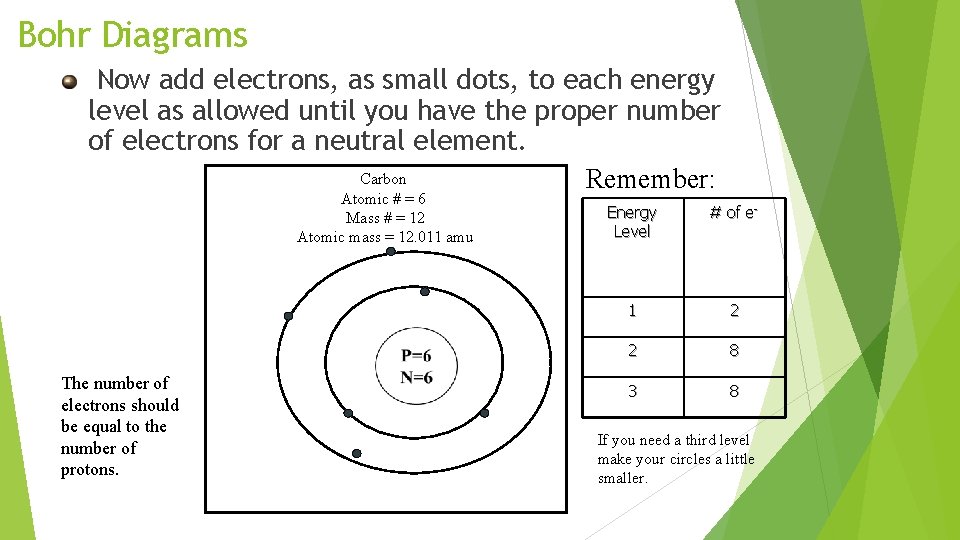
Bohr Diagrams Now add electrons, as small dots, to each energy level as allowed until you have the proper number of electrons for a neutral element. Carbon Remember: Atomic # = 6 Mass # = 12 Atomic mass = 12. 011 amu The number of electrons should be equal to the number of protons. Energy Level # of e- 1 2 2 8 3 8 If you need a third level make your circles a little smaller.

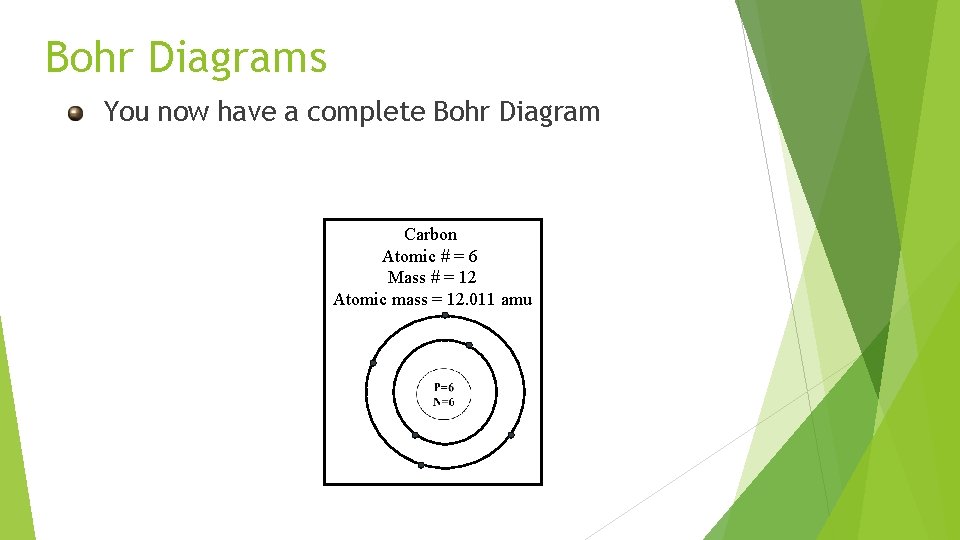
Bohr Diagrams You now have a complete Bohr Diagram Carbon Atomic # = 6 Mass # = 12 Atomic mass = 12. 011 amu

MODERN ATOMIC THEORY An electron in an atom can move from one energy level to another when the atom gains or loses energy Move up energy levels if e- gain energy Move down energy levels if e- loses energy

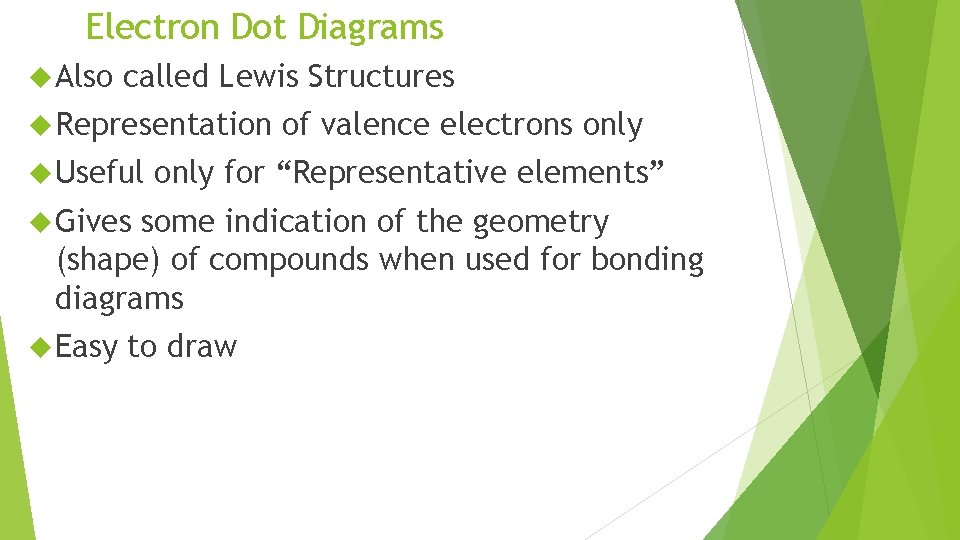
Electron Dot Diagrams Also called Lewis Structures Representation Useful of valence electrons only for “Representative elements” Gives some indication of the geometry (shape) of compounds when used for bonding diagrams Easy to draw

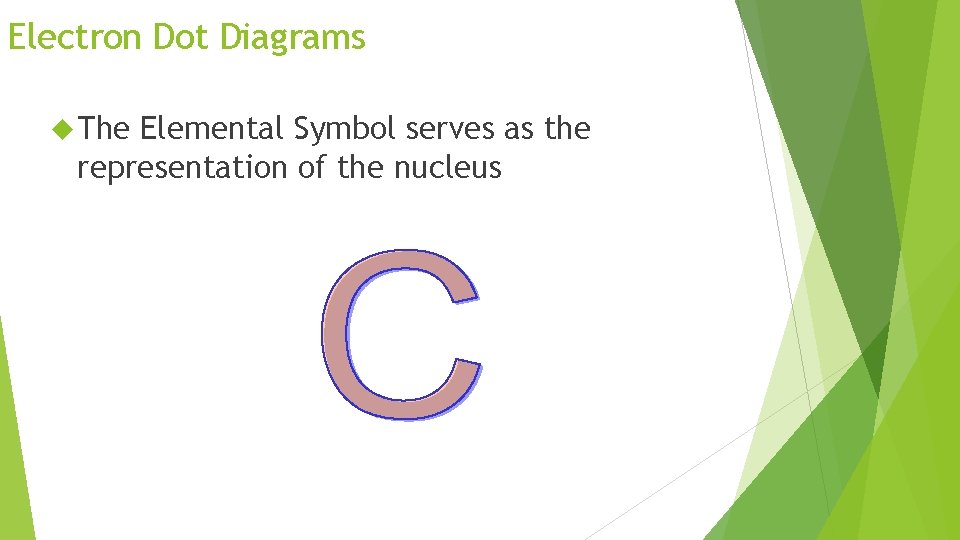
Electron Dot Diagrams The Elemental Symbol serves as the representation of the nucleus

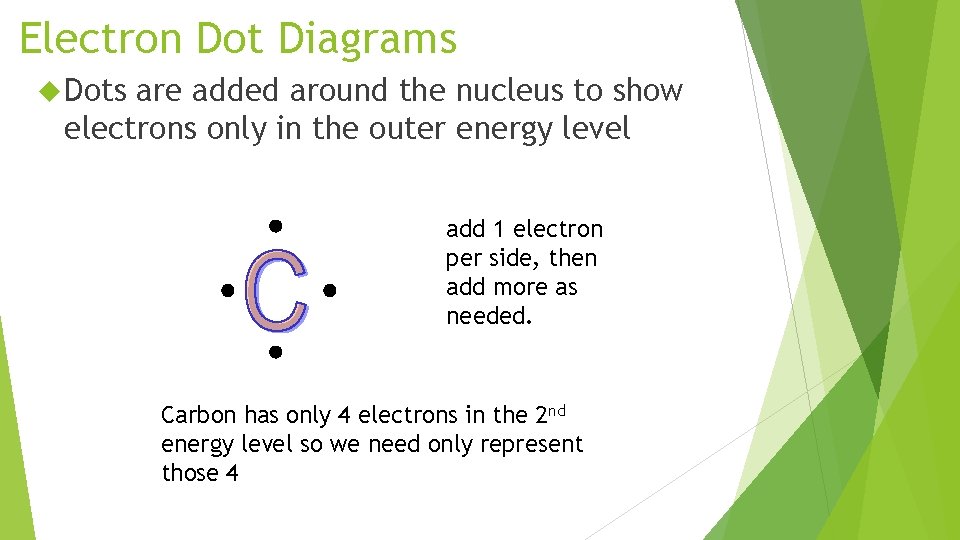
Electron Dot Diagrams Dots are added around the nucleus to show electrons only in the outer energy level add 1 electron per side, then add more as needed. Carbon has only 4 electrons in the 2 nd energy level so we need only represent those 4

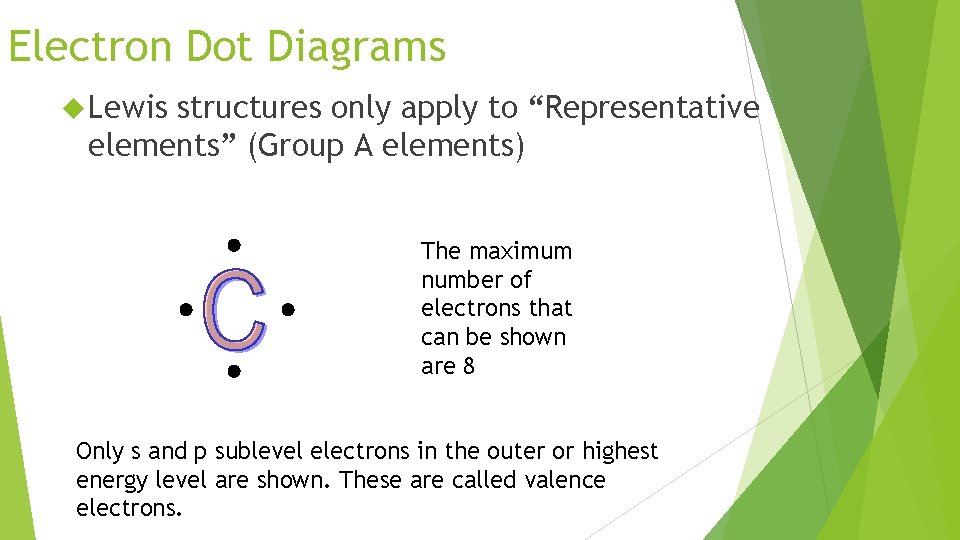
Electron Dot Diagrams Lewis structures only apply to “Representative elements” (Group A elements) The maximum number of electrons that can be shown are 8 Only s and p sublevel electrons in the outer or highest energy level are shown. These are called valence electrons.
 Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block av độ 1
Block av độ 1 Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Compared to atoms of metals, atoms of nonmetals generally
Compared to atoms of metals, atoms of nonmetals generally Status withdrawal theory is given by
Status withdrawal theory is given by Proposed the particle theory of light
Proposed the particle theory of light Tunica corpus theory
Tunica corpus theory Name a point that is collinear with the given points
Name a point that is collinear with the given points John daltons atom
John daltons atom Atomic theory models timeline
Atomic theory models timeline Dalton's atomic theory was accepted because
Dalton's atomic theory was accepted because Summarize dalton's atomic theory
Summarize dalton's atomic theory John dalton atomic theory
John dalton atomic theory Chapter 5 nutrition and your health
Chapter 5 nutrition and your health All about atoms
All about atoms Nuclear fission
Nuclear fission The lowest allowable energy state of an atom is called
The lowest allowable energy state of an atom is called