THE NATURE OF ATOMS ATOMIC THEORY John Dalton


- Slides: 13

THE NATURE OF ATOMS

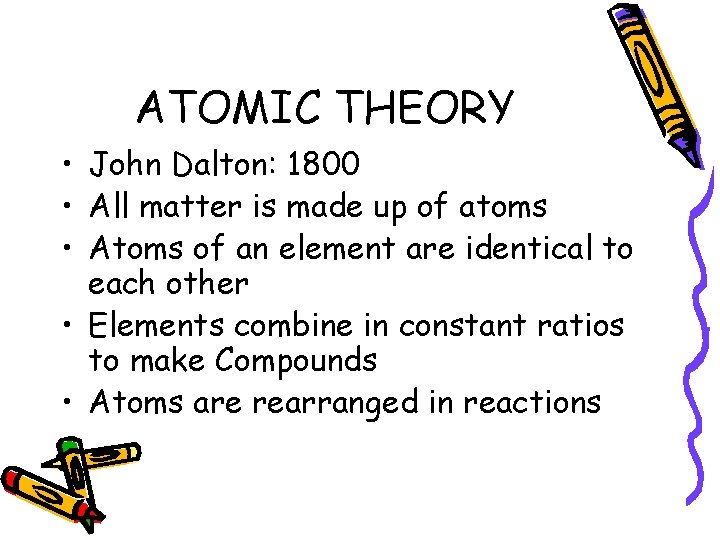
ATOMIC THEORY • John Dalton: 1800 • All matter is made up of atoms • Atoms of an element are identical to each other • Elements combine in constant ratios to make Compounds • Atoms are rearranged in reactions

Revised Atomic Theory 1. Atoms are the smallest particle of an element and are made of smaller subatomic particles. 2. Nuclear reactions allow conversion from one element to another. 3. Atoms of the same element have the same properties; these are different than atoms of other elements. 4. Compounds are formed by combining specific ratios of different atoms.

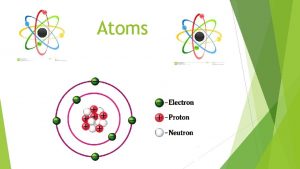
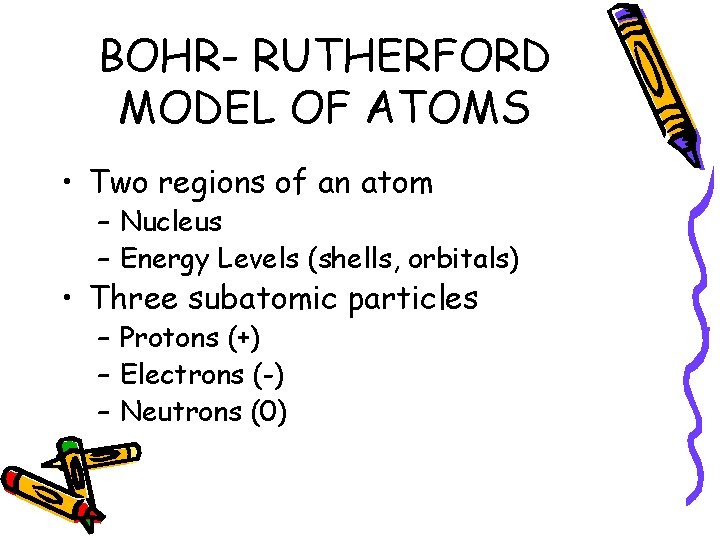
BOHR- RUTHERFORD MODEL OF ATOMS • Two regions of an atom – Nucleus – Energy Levels (shells, orbitals) • Three subatomic particles – Protons (+) – Electrons (-) – Neutrons (0)

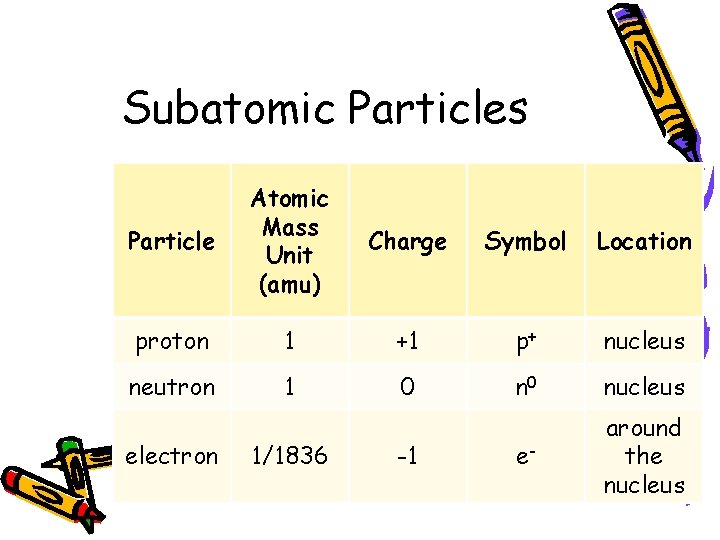
Subatomic Particles Particle Atomic Mass Unit (amu) Charge Symbol Location proton 1 +1 p+ nucleus neutron 1 0 nucleus e- around the nucleus electron 1/1836 -1

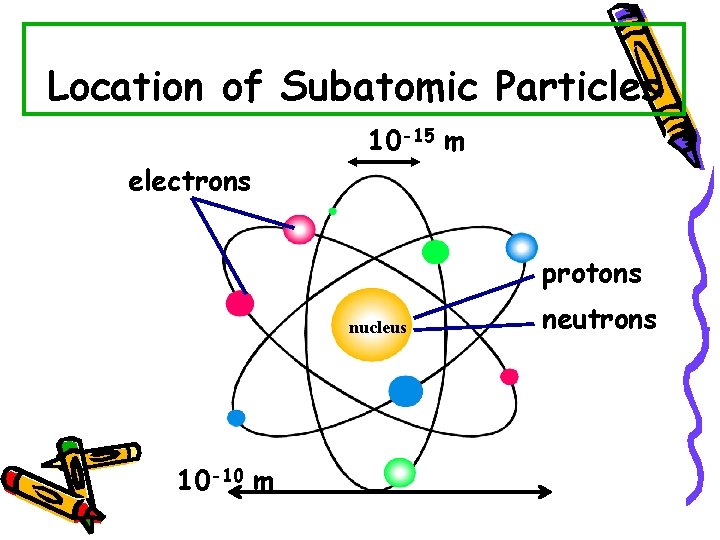
Location of Subatomic Particles 10 -15 m electrons protons nucleus 10 -10 m neutrons

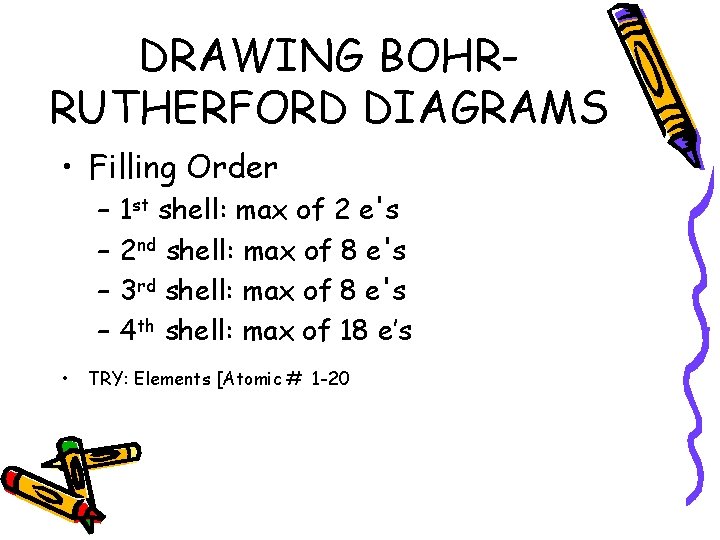
DRAWING BOHRRUTHERFORD DIAGRAMS • Filling Order – – • 1 st shell: max of 2 e's 2 nd shell: max of 8 e's 3 rd shell: max of 8 e's 4 th shell: max of 18 e’s TRY: Elements [Atomic # 1 -20

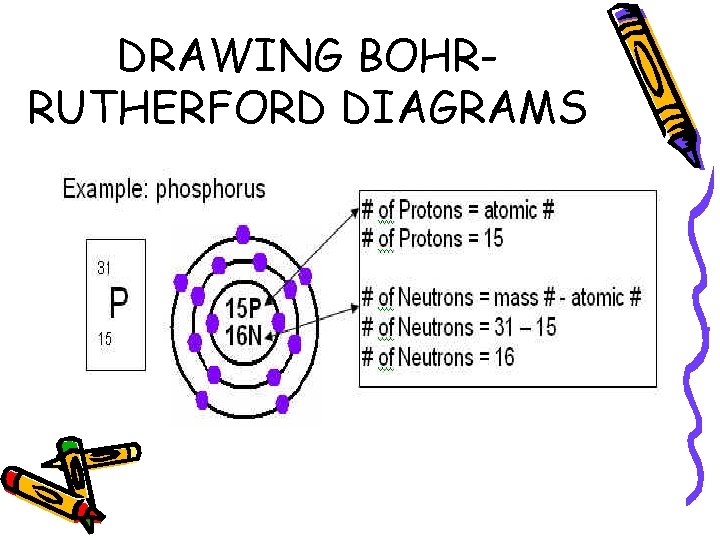
DRAWING BOHRRUTHERFORD DIAGRAMS

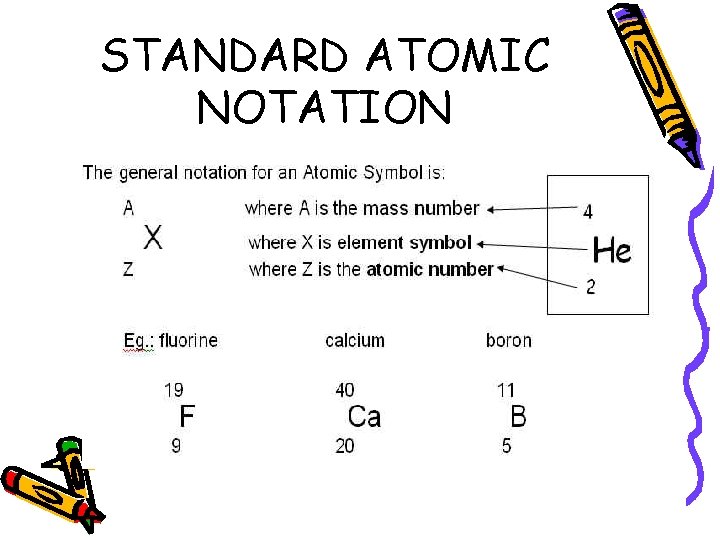
STANDARD ATOMIC NOTATION

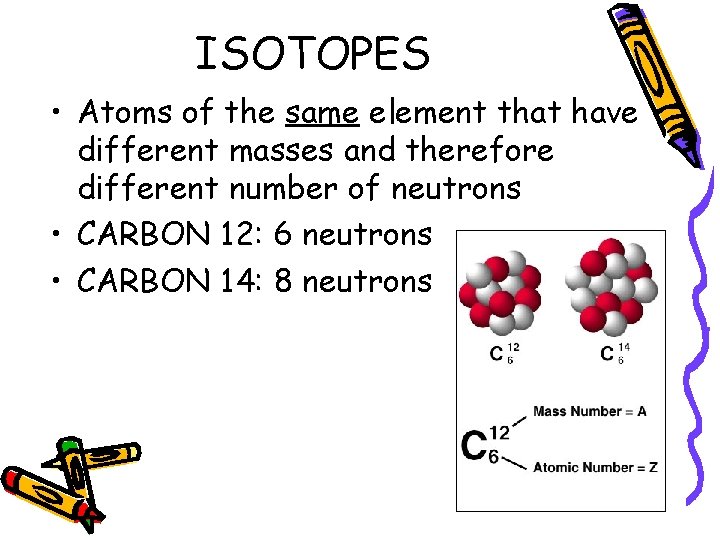
ISOTOPES • Atoms of the same element that have different masses and therefore different number of neutrons • CARBON 12: 6 neutrons • CARBON 14: 8 neutrons

Atoms Are all atoms of one element type equal to all others? Isotopes are atoms of the same element which differ only in the number of neutrons in the nucleus. All isotopes of the same element hold the same chemical property.

Atoms Isotopes may be stable or unstable. Unstable isotopes may spontaneously decay to release radioactivity and are called radioisotopes. Often, the instability is caused by excess energy in the nucleus. Upon release, different atoms are produced.
IONS • Charged atoms that have GAINED or LOST electrons • Positive ion Loses e's CATION • Negative ion Gains e's ANION
 Dalton atomic theory summary
Dalton atomic theory summary Atomic structure timeline
Atomic structure timeline John dalton atomic theory
John dalton atomic theory John dalton atomic model drawing
John dalton atomic model drawing Dalton's atomic theory was accepted because
Dalton's atomic theory was accepted because Summarize dalton's atomic theory
Summarize dalton's atomic theory At stp which substance is the best conductor of electricity
At stp which substance is the best conductor of electricity Dalton atomic model diagram
Dalton atomic model diagram The atoms family atomic math challenge answer key
The atoms family atomic math challenge answer key Period 7 group 2
Period 7 group 2 Atoms family song
Atoms family song Http sciencespot net
Http sciencespot net Matterville worksheet
Matterville worksheet The atoms family atomic math challenge
The atoms family atomic math challenge