Atoms Molecules and Ions John Dalton 1806 Atomic
- Slides: 15

Atoms, Molecules and Ions John Dalton 1806 Atomic Theory of Matter 1. Matter consists of indivisible atoms 2. All atoms of a given element have identical properties 3. Different elements have atoms that differ in mass 4. Atoms are indestructible and chemical reactions are a rearrangement of atoms 5. Compounds contain a definite and small number of atoms

1. Matter consists of indivisible atoms subatomic particles electrons protons neutrons 1. electrons 2. protons 3. neutrons charge = -1. 60 x 10 -19 C = -1 mass = 9. 1 x 10 -31 kg charge = +1 mass = 1. 673 x 10 -27 kg charge = 0 mass = 1. 675 x 10 -27 kg

1. Matter consists of indivisible atoms nucleus = protons + neutrons electrons move around the nucleus electron “cloud” = volume of atom average diameter of atom 10 -10 m = ångström (Å)

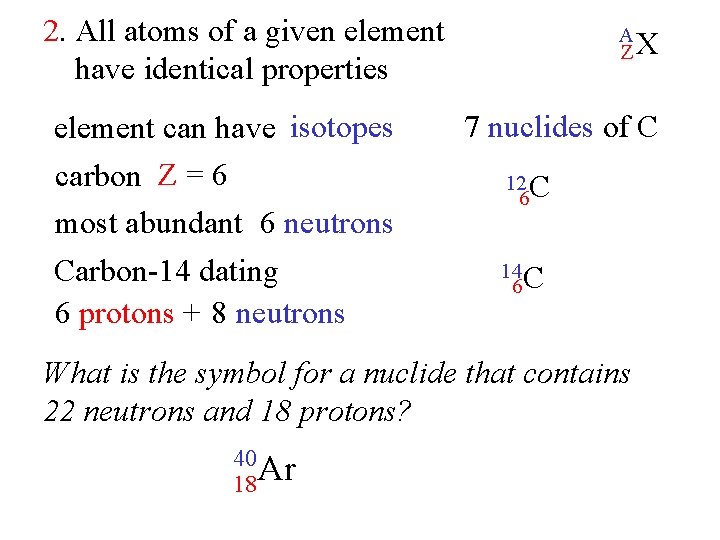
2. All atoms of a given element have identical properties ? not exactly A ZX atoms of an element = same number of protons atomic number = Z Z = 6 = C carbon Z = 30 = Zn zinc 6 C 30 Zn 6 e 30 e- elements neutral # protons = # eelements different # neutrons proton + neutron = A = mass number

2. All atoms of a given element have identical properties element can have isotopes carbon Z = 6 most abundant 6 neutrons Carbon-14 dating 6 protons + 8 neutrons A ZX 7 nuclides of C 12 C 6 14 C 6 What is the symbol for a nuclide that contains 22 neutrons and 18 protons? 40 18 Ar

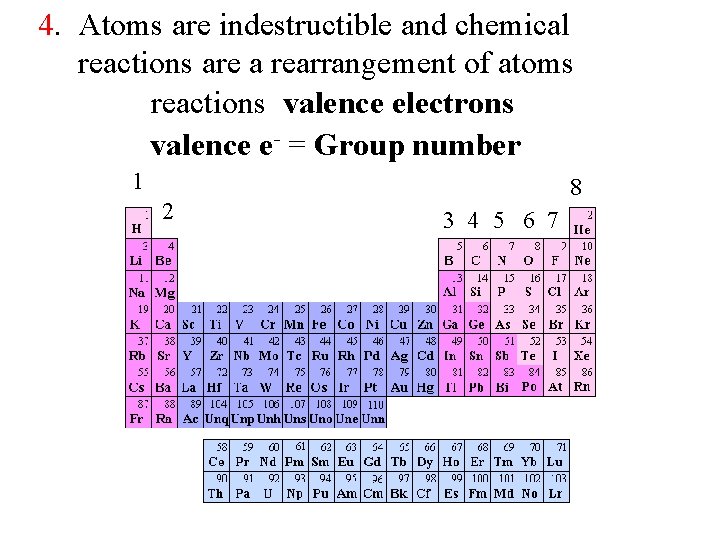
4. Atoms are indestructible and chemical reactions are a rearrangement of atoms reactions valence electrons valence e- = Group number 1 2 8 3 4 5 6 7

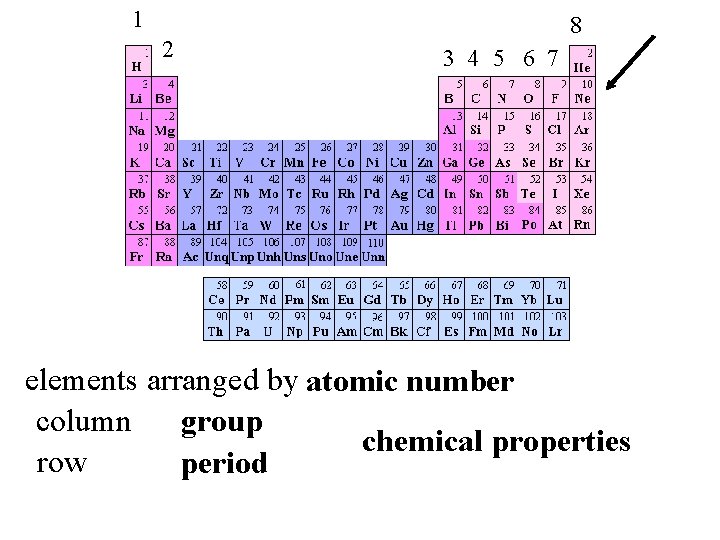
1 2 8 3 4 5 6 7 elements arranged by atomic number column group chemical properties row period

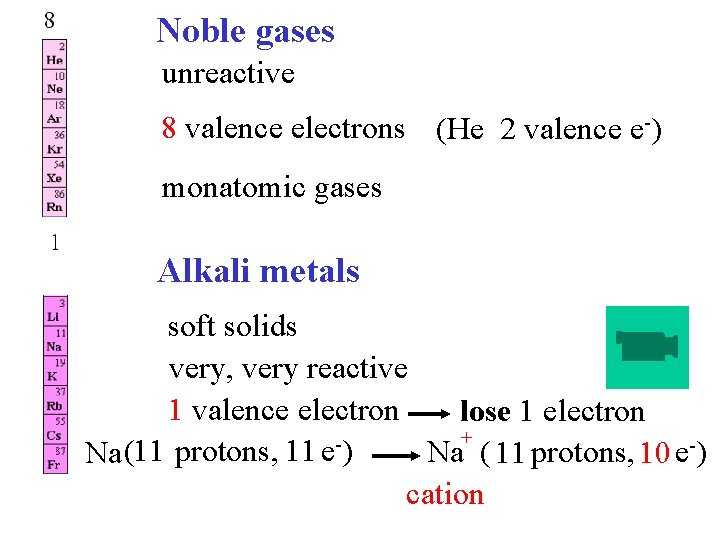
Noble gases unreactive 8 valence electrons (He 2 valence e-) monatomic gases Alkali metals soft solids very, very reactive 1 valence electron lose 1 electron + Na ( 11 protons, 10 e-) Na (11 protons, 11 e ) cation

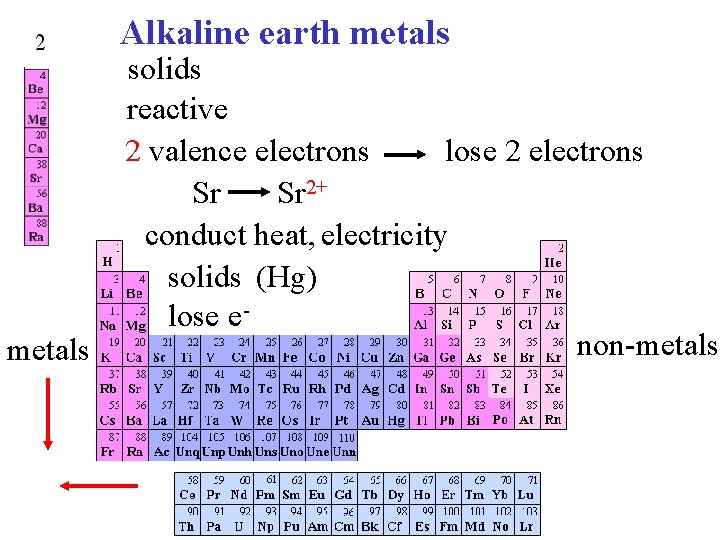
Alkaline earth metals solids reactive lose 2 electrons 2 valence electrons Sr Sr 2+ conduct heat, electricity solids (Hg) lose enon-metals

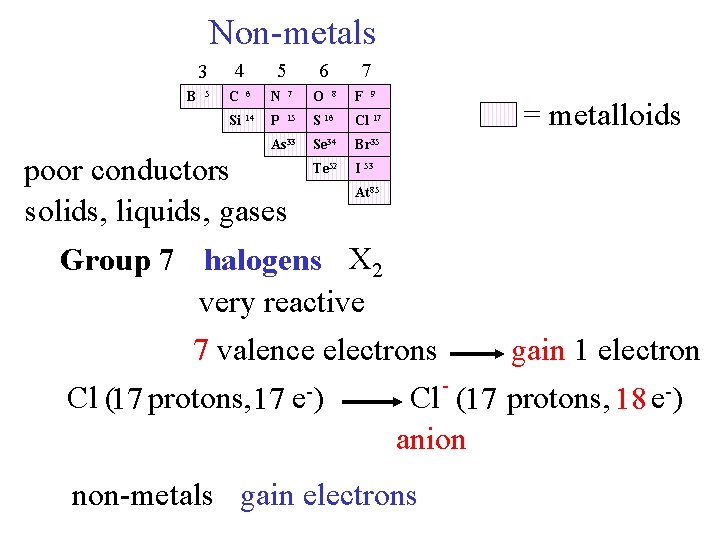
Non-metals 3 B 5 4 5 6 7 C 6 N 7 O Si 14 P 15 S 16 Cl 17 As 33 Se 34 Br 35 Te 52 I 53 poor conductors solids, liquids, gases 8 F 9 = metalloids At 85 Group 7 halogens X 2 very reactive 7 valence electrons gain 1 electron Cl (17 protons, 17 e-) Cl - (17 protons, 18 e-) anion non-metals gain electrons

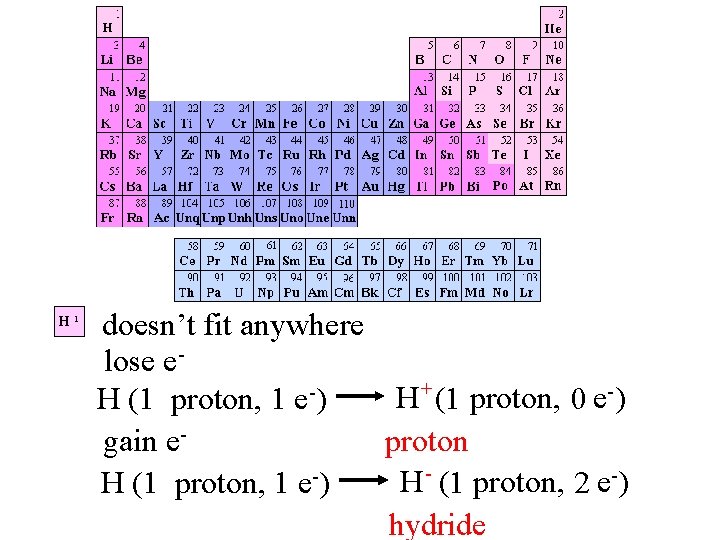
H 1 doesn’t fit anywhere lose e+ H (1 proton, 0 e-) H (1 proton, 1 e ) gain eproton H (1 proton, 2 e-) H (1 proton, 1 e ) hydride

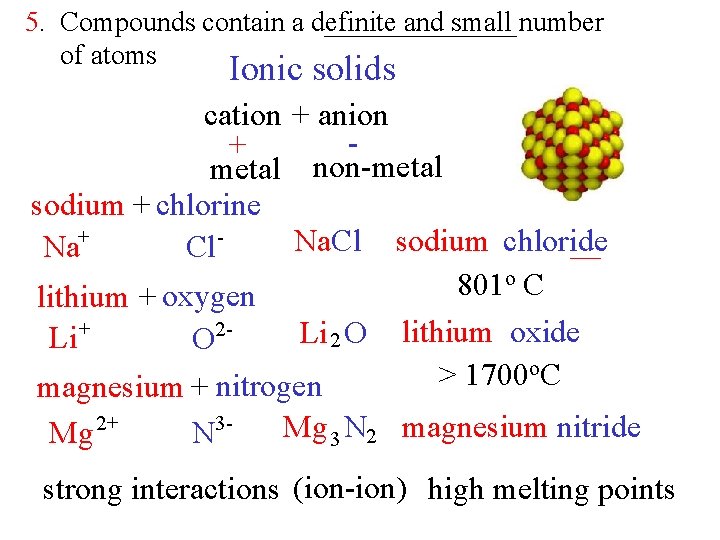
5. Compounds contain a definite and small number of atoms Ionic solids cation + anion + metal non-metal sodium + chlorine Na. Cl sodium chloride Na+ Cl o. C 801 lithium + oxygen 2 Li 2 O lithium oxide Li + O o. C > 1700 magnesium + nitrogen Mg 2+ N 3 - Mg 3 N 2 magnesium nitride strong interactions (ion-ion) high melting points

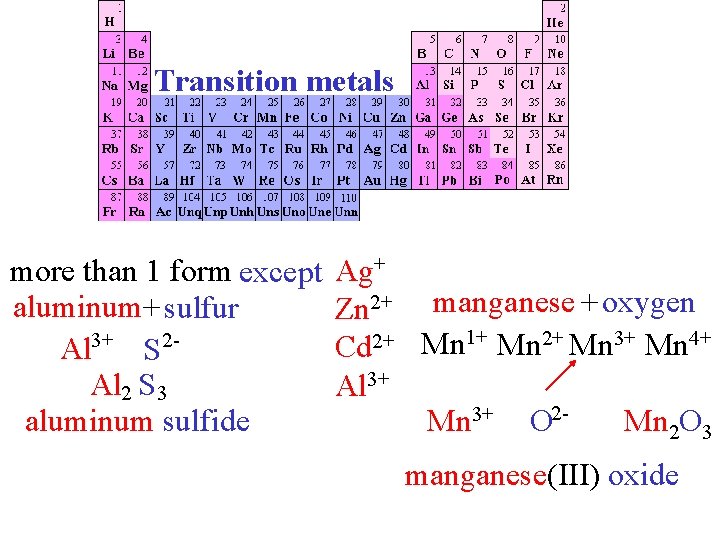
Transition metals more than 1 form except aluminum+ sulfur Al 3+ S 2 Al 2 S 3 aluminum sulfide Ag+ Zn 2+ manganese + oxygen Cd 2+ Mn 1+ Mn 2+ Mn 3+ Mn 4+ Al 3+ 23+ 4+ 2 Mn Mn 22 O 3 O Mn Mn. O O manganese(IV) manganese(III)oxide

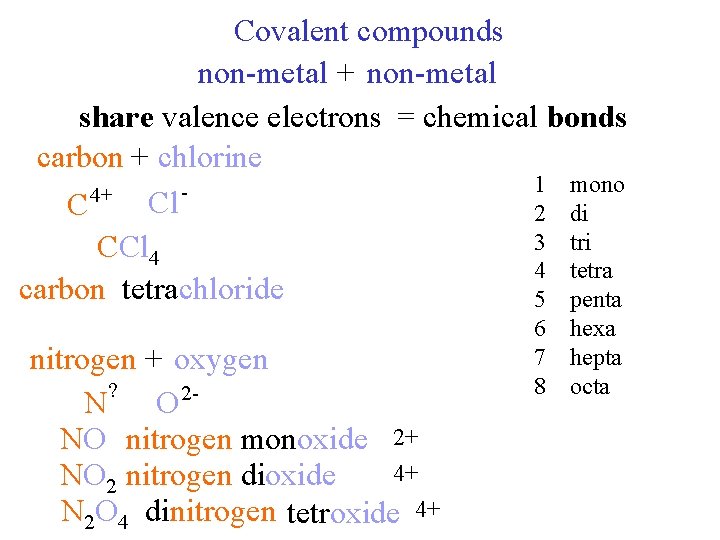
Covalent compounds non-metal + non-metal share valence electrons = chemical bonds carbon + chlorine 1 mono 4+ Cl C 2 di 3 tri CCl 4 4 tetra carbon tetrachloride 5 penta nitrogen + oxygen ? N O 2 NO nitrogen monoxide 2+ 4+ NO 2 nitrogen dioxide N 2 O 4 dinitrogen tetroxide 4+ 6 hexa 7 hepta 8 octa

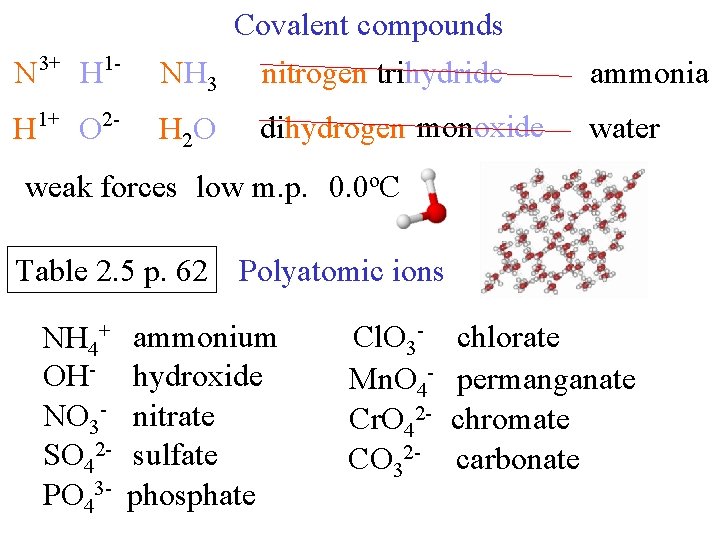
N 3+ H 1 - Covalent compounds NH 3 nitrogen trihydride H 1+ O 2 - H 2 O dihydrogen monoxide ammonia water weak forces low m. p. 0. 0 o. C Table 2. 5 p. 62 Polyatomic ions NH 4+ OHNO 3 SO 42 PO 43 - ammonium hydroxide nitrate sulfate phosphate Cl. O 3 Mn. O 4 Cr. O 42 CO 32 - chlorate permanganate chromate carbonate