ASPIRE KRd vs Rd in RRMM PFS OS






- Slides: 81

ASPIRE: KRd vs Rd in RRMM PFS, OS and Safety Subgroup analyses Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma SC-CH-CARFILZOMI-00140

Table of Contents - ASPIRE Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed MM KRd in RRMM (K 20/27 mg/m 2; D 1, 2, 8, 9, 15, 16; 18 cycles) Study Overview Primary Endpoint PFS Overall Survival q Endpoints q Study design q Inclusion/Exclusion criteria q Patient demographics q Dosing rationale q History of Nervous System Disorders q Patients with early progression during prior therapy Updated results q PFS by response category q Prior lines of therapy/type of previous treatment q PFS over time q Prior ASCT q PFS by peripheral neuropathy history q Cytogenetic risk q Early and late relapse q Age q Summary q Interim analysis q q Final OS analysis q OS in subgroups q Summary

Table of Contents – ASPIRE (continued) All studies Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed MM KRd in RRMM (K 20/27 mg/m 2; D 1, 2, 8, 9, 15, 16; 18 cycles) Overall Response Rate Quality of Life Adverse Events q Patients with early progression during prior therapy Prior lines of therapy/type of previous treatment q Early and late relapse patients q Prior ASCT q Response over time q Cytogenetic risk q Summary q Age q HRQo. L by response q Treatment group differences q HRQo. L by age q Longitudinal changes by treatment group q Summary q HRQo. L responder analysis q Safety population q Age q Cardiac events by treatment cycle q Patients with early progression during prior therapy q Safety population: Updated results q Early and late relapse patients q Prior lines of therapy q Rates of Peripheral Neuropathy q Prior ASCT q Summary q Cytogenetic risk q Interim analysis q

ASPIRE TOC All studies ASPIRE Study Overview Endpoints Inclusion/Exclusion Criteria Dosing Rationale Study Design Patient Demographics History of Nervous System Disorders

ASPIRE TOC Endpoints • Primary endpoint: Progression-free survival (PFS) • Secondary endpoints: Overall survival (OS), overall response rate (ORR), duration of response, healthrelated quality of life, safety Stewart AK, et al. N Engl J Med 2015; 372: 142 -152.

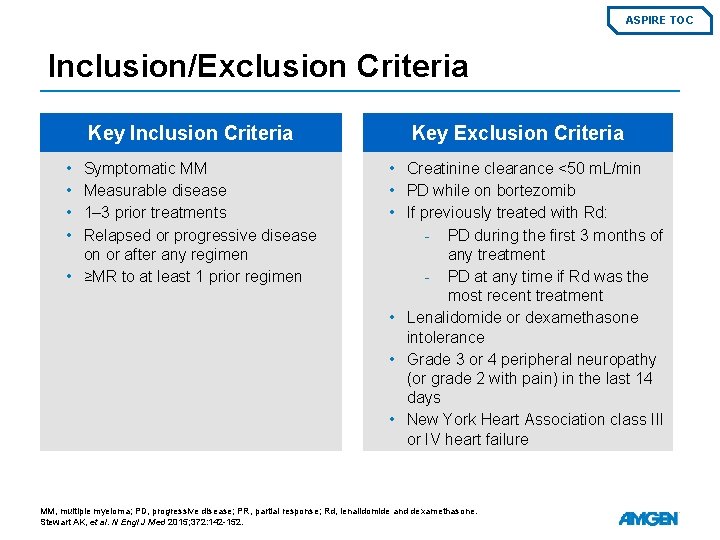
ASPIRE TOC Inclusion/Exclusion Criteria Key Inclusion Criteria • • Symptomatic MM Measurable disease 1– 3 prior treatments Relapsed or progressive disease on or after any regimen • ≥MR to at least 1 prior regimen 6 Key Exclusion Criteria • Creatinine clearance <50 m. L/min • PD while on bortezomib • If previously treated with Rd: - PD during the first 3 months of any treatment - PD at any time if Rd was the most recent treatment • Lenalidomide or dexamethasone intolerance • Grade 3 or 4 peripheral neuropathy (or grade 2 with pain) in the last 14 days • New York Heart Association class III or IV heart failure MM, multiple myeloma; PD, progressive disease; PR, partial response; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152.

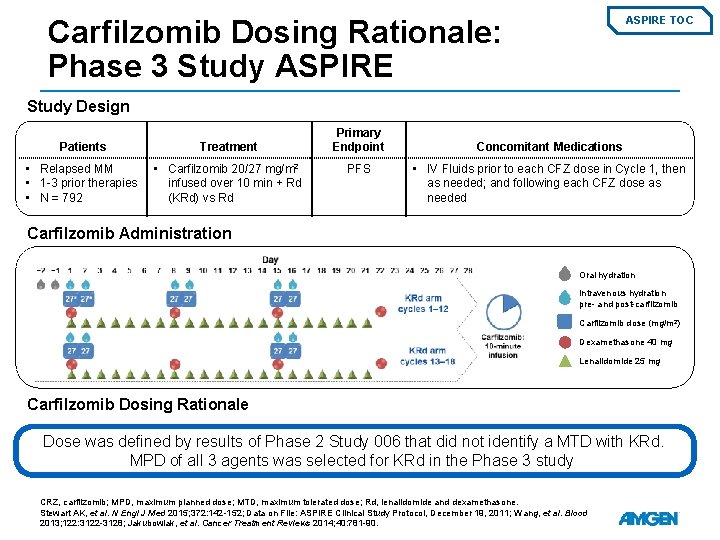
Carfilzomib Dosing Rationale: Phase 3 Study ASPIRE TOC Study Design Patients Treatment • Relapsed MM • 1 -3 prior therapies • N = 792 • Carfilzomib 20/27 mg/m 2 infused over 10 min + Rd (KRd) vs Rd Primary Endpoint PFS Concomitant Medications • IV Fluids prior to each CFZ dose in Cycle 1, then as needed; and following each CFZ dose as needed Carfilzomib Administration Oral hydration Intravenous hydration pre- and post-carfilzomib Carfilzomib dose (mg/m 2) Dexamethasone 40 mg Lenalidomide 25 mg Carfilzomib Dosing Rationale Dose was defined by results of Phase 2 Study 006 that did not identify a MTD with KRd. MPD of all 3 agents was selected for KRd in the Phase 3 study. CRZ, carfilzomib; MPD, maximum planned dose; MTD, maximum tolerated dose; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152; Data on File: ASPIRE Clinical Study Protocol, December 19, 2011; Wang, et al. Blood 2013; 122: 3122 -3128; Jakubowiak, et al. Cancer Treatment Reviews 2014; 40: 781 -90.

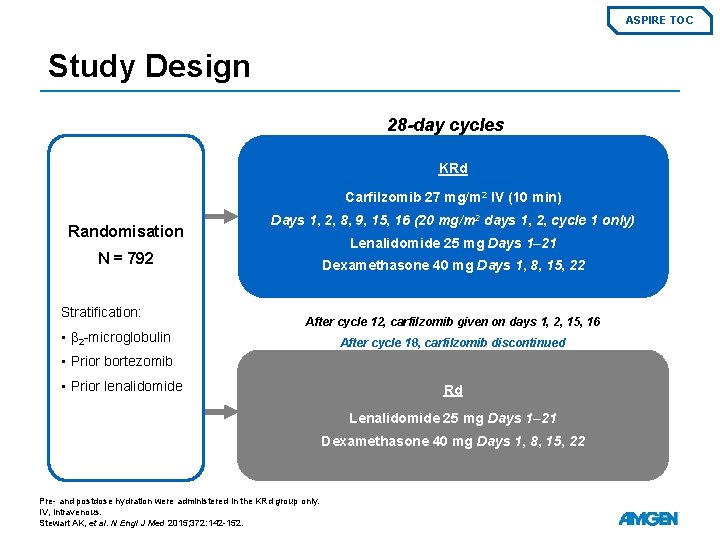
ASPIRE TOC Study Design 28 -day cycles KRd Carfilzomib 27 mg/m 2 IV (10 min) Randomisation Days 1, 2, 8, 9, 15, 16 (20 mg/m 2 days 1, 2, cycle 1 only) Lenalidomide 25 mg Days 1– 21 N = 792 Stratification: • β 2 -microglobulin Dexamethasone 40 mg Days 1, 8, 15, 22 After cycle 12, carfilzomib given on days 1, 2, 15, 16 After cycle 18, carfilzomib discontinued • Prior bortezomib • Prior lenalidomide Rd Lenalidomide 25 mg Days 1– 21 Dexamethasone 40 mg Days 1, 8, 15, 22 Pre- and postdose hydration were administered in the KRd group only. IV, intravenous. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152.

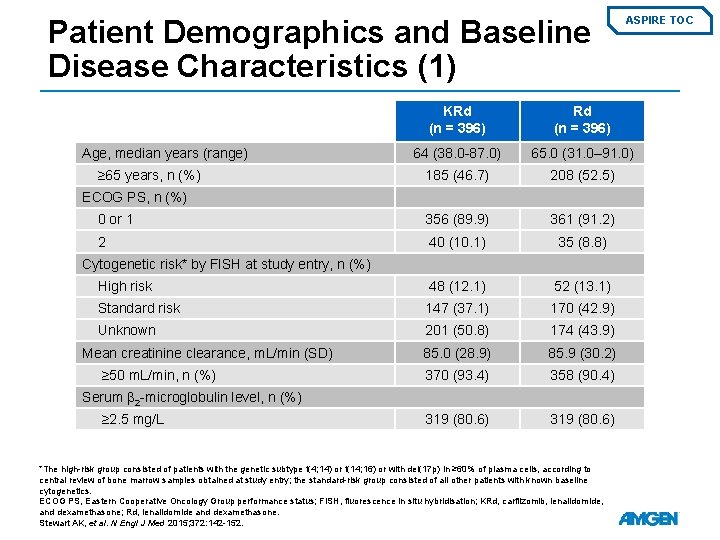
Patient Demographics and Baseline Disease Characteristics (1) ASPIRE TOC KRd (n = 396) 64 (38. 0 -87. 0) 65. 0 (31. 0– 91. 0) 185 (46. 7) 208 (52. 5) 0 or 1 356 (89. 9) 361 (91. 2) 2 40 (10. 1) 35 (8. 8) High risk 48 (12. 1) 52 (13. 1) Standard risk 147 (37. 1) 170 (42. 9) Unknown 201 (50. 8) 174 (43. 9) 85. 0 (28. 9) 85. 9 (30. 2) 370 (93. 4) 358 (90. 4) 319 (80. 6) Age, median years (range) ≥ 65 years, n (%) ECOG PS, n (%) Cytogenetic risk* by FISH at study entry, n (%) Mean creatinine clearance, m. L/min (SD) ≥ 50 m. L/min, n (%) Serum β 2 -microglobulin level, n (%) ≥ 2. 5 mg/L *The high-risk group consisted of patients with the genetic subtype t(4; 14) or t(14; 16) or with del(17 p) in ≥ 60% of plasma cells, according to central review of bone marrow samples obtained at study entry; the standard-risk group consisted of all other patients with known baseline cytogenetics. ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridisation; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152.

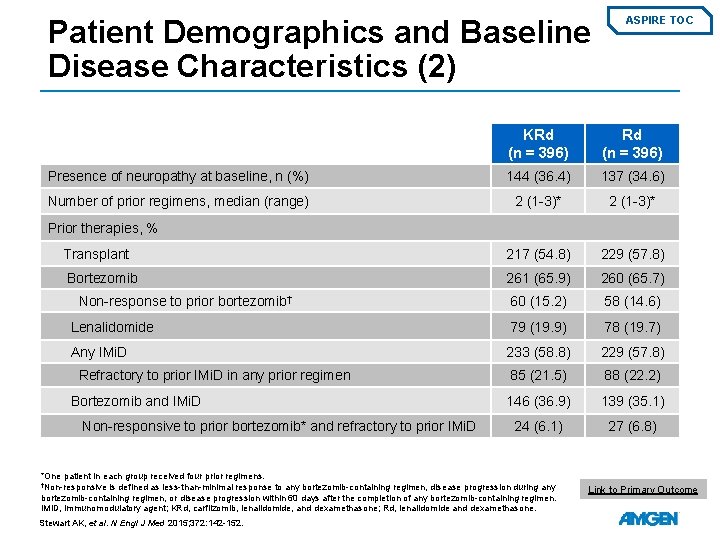
Patient Demographics and Baseline Disease Characteristics (2) ASPIRE TOC KRd (n = 396) Presence of neuropathy at baseline, n (%) 144 (36. 4) 137 (34. 6) Number of prior regimens, median (range) 2 (1 -3)* Transplant 217 (54. 8) 229 (57. 8) Bortezomib 261 (65. 9) 260 (65. 7) 60 (15. 2) 58 (14. 6) Lenalidomide 79 (19. 9) 78 (19. 7) Any IMi. D 233 (58. 8) 229 (57. 8) 85 (21. 5) 88 (22. 2) 146 (36. 9) 139 (35. 1) 24 (6. 1) 27 (6. 8) Prior therapies, % Non-response to prior bortezomib† Refractory to prior IMi. D in any prior regimen Bortezomib and IMi. D Non-responsive to prior bortezomib* and refractory to prior IMi. D *One patient in each group received four prior regimens. †Non-responsive is defined as less-than-minimal response to any bortezomib-containing regimen, disease progression during any bortezomib-containing regimen, or disease progression within 60 days after the completion of any bortezomib-containing regimen. IMi. D, immunomodulatory agent; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152. Link to Primary Outcome

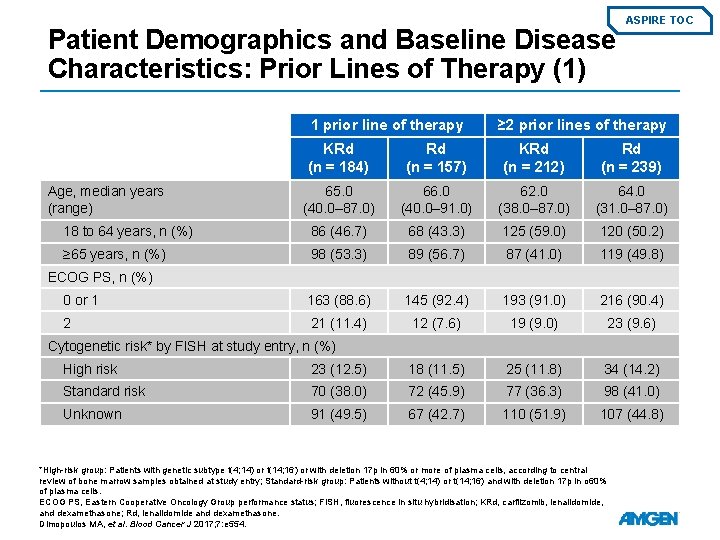
Patient Demographics and Baseline Disease Characteristics: Prior Lines of Therapy (1) 1 prior line of therapy ASPIRE TOC ≥ 2 prior lines of therapy KRd (n = 184) Rd (n = 157) KRd (n = 212) Rd (n = 239) 65. 0 (40. 0– 87. 0) 66. 0 (40. 0– 91. 0) 62. 0 (38. 0– 87. 0) 64. 0 (31. 0– 87. 0) 18 to 64 years, n (%) 86 (46. 7) 68 (43. 3) 125 (59. 0) 120 (50. 2) ≥ 65 years, n (%) 98 (53. 3) 89 (56. 7) 87 (41. 0) 119 (49. 8) 0 or 1 163 (88. 6) 145 (92. 4) 193 (91. 0) 216 (90. 4) 2 21 (11. 4) 12 (7. 6) 19 (9. 0) 23 (9. 6) Age, median years (range) ECOG PS, n (%) Cytogenetic risk* by FISH at study entry, n (%) High risk 23 (12. 5) 18 (11. 5) 25 (11. 8) 34 (14. 2) Standard risk 70 (38. 0) 72 (45. 9) 77 (36. 3) 98 (41. 0) Unknown 91 (49. 5) 67 (42. 7) 110 (51. 9) 107 (44. 8) *High-risk group: Patients with genetic subtype t(4; 14) or t(14; 16) or with deletion 17 p in 60% or more of plasma cells, according to central review of bone marrow samples obtained at study entry; Standard-risk group: Patients without t(4; 14) or t(14; 16) and with deletion 17 p in o 60% of plasma cells. ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridisation; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554.

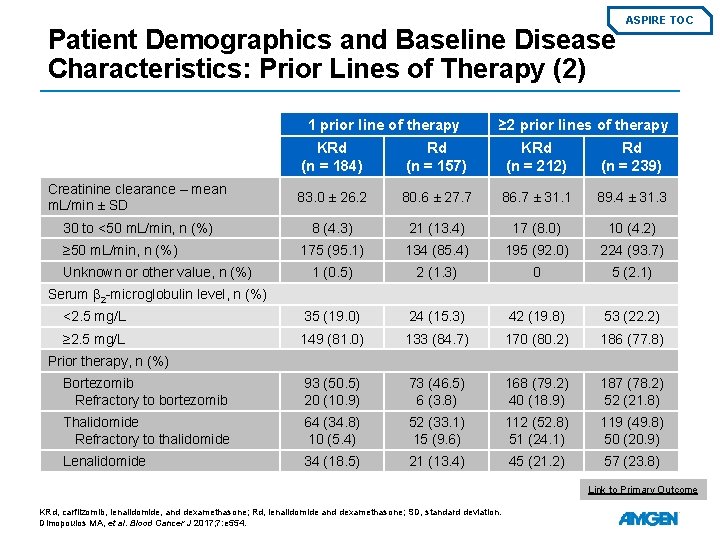
Patient Demographics and Baseline Disease Characteristics: Prior Lines of Therapy (2) 1 prior line of therapy ASPIRE TOC ≥ 2 prior lines of therapy KRd (n = 184) Rd (n = 157) KRd (n = 212) Rd (n = 239) Creatinine clearance – mean m. L/min ± SD 83. 0 ± 26. 2 80. 6 ± 27. 7 86. 7 ± 31. 1 89. 4 ± 31. 3 30 to <50 m. L/min, n (%) 8 (4. 3) 21 (13. 4) 17 (8. 0) 10 (4. 2) 175 (95. 1) 134 (85. 4) 195 (92. 0) 224 (93. 7) 1 (0. 5) 2 (1. 3) 0 5 (2. 1) <2. 5 mg/L 35 (19. 0) 24 (15. 3) 42 (19. 8) 53 (22. 2) ≥ 2. 5 mg/L 149 (81. 0) 133 (84. 7) 170 (80. 2) 186 (77. 8) Bortezomib Refractory to bortezomib 93 (50. 5) 20 (10. 9) 73 (46. 5) 6 (3. 8) 168 (79. 2) 40 (18. 9) 187 (78. 2) 52 (21. 8) Thalidomide Refractory to thalidomide 64 (34. 8) 10 (5. 4) 52 (33. 1) 15 (9. 6) 112 (52. 8) 51 (24. 1) 119 (49. 8) 50 (20. 9) Lenalidomide 34 (18. 5) 21 (13. 4) 45 (21. 2) 57 (23. 8) ≥ 50 m. L/min, n (%) Unknown or other value, n (%) Serum β 2 -microglobulin level, n (%) Prior therapy, n (%) Link to Primary Outcome KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; SD, standard deviation. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554.

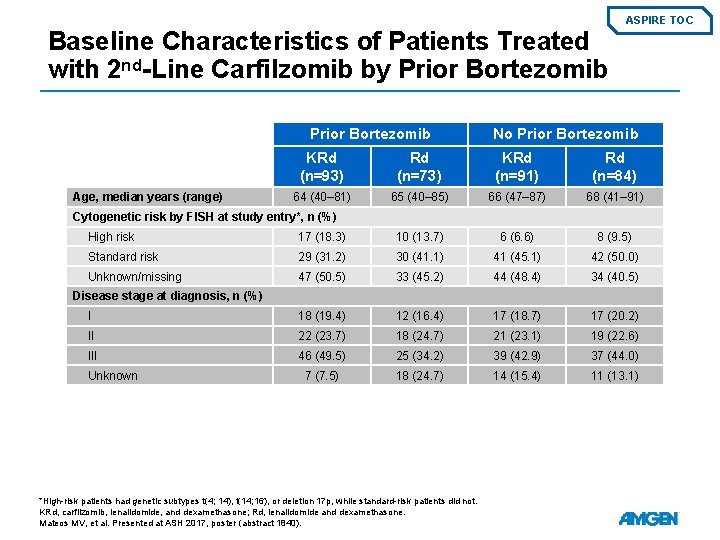
ASPIRE TOC Baseline Characteristics of Patients Treated with 2 nd-Line Carfilzomib by Prior Bortezomib Age, median years (range) No Prior Bortezomib KRd (n=93) Rd (n=73) KRd (n=91) Rd (n=84) 64 (40– 81) 65 (40– 85) 66 (47– 87) 68 (41– 91) Cytogenetic risk by FISH at study entry*, n (%) High risk 17 (18. 3) 10 (13. 7) 6 (6. 6) 8 (9. 5) Standard risk 29 (31. 2) 30 (41. 1) 41 (45. 1) 42 (50. 0) Unknown/missing 47 (50. 5) 33 (45. 2) 44 (48. 4) 34 (40. 5) I 18 (19. 4) 12 (16. 4) 17 (18. 7) 17 (20. 2) II 22 (23. 7) 18 (24. 7) 21 (23. 1) 19 (22. 6) III 46 (49. 5) 25 (34. 2) 39 (42. 9) 37 (44. 0) 7 (7. 5) 18 (24. 7) 14 (15. 4) 11 (13. 1) Disease stage at diagnosis, n (%) Unknown *High-risk patients had genetic subtypes t(4; 14), t(14; 16), or deletion 17 p, while standard-risk patients did not. KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Mateos MV, et al. Presented at ASH 2017, poster (abstract 1840).

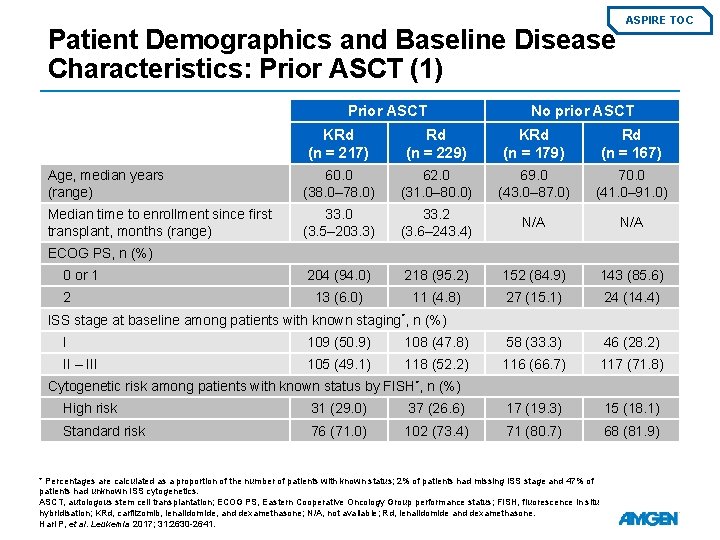
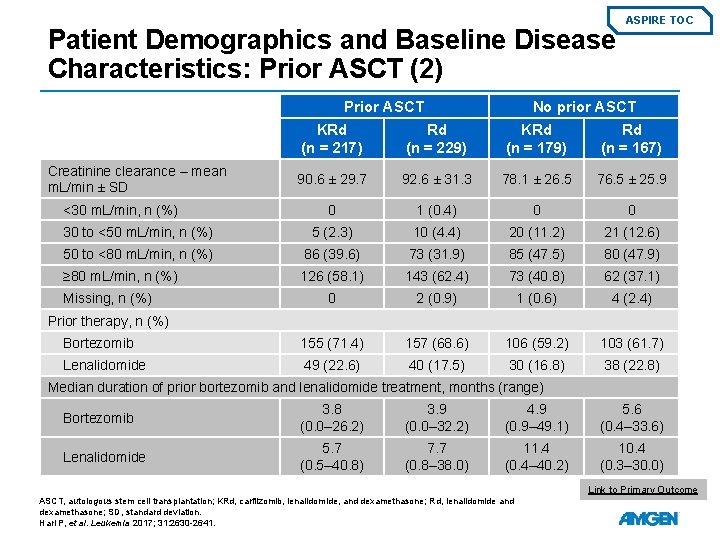
Patient Demographics and Baseline Disease Characteristics: Prior ASCT (1) Prior ASCT ASPIRE TOC No prior ASCT KRd (n = 217) Rd (n = 229) KRd (n = 179) Rd (n = 167) Age, median years (range) 60. 0 (38. 0– 78. 0) 62. 0 (31. 0– 80. 0) 69. 0 (43. 0– 87. 0) 70. 0 (41. 0– 91. 0) Median time to enrollment since first transplant, months (range) 33. 0 (3. 5– 203. 3) 33. 2 (3. 6– 243. 4) N/A 204 (94. 0) 218 (95. 2) 152 (84. 9) 143 (85. 6) 13 (6. 0) 11 (4. 8) 27 (15. 1) 24 (14. 4) ECOG PS, n (%) 0 or 1 2 ISS stage at baseline among patients with known staging*, n (%) I 109 (50. 9) 108 (47. 8) 58 (33. 3) 46 (28. 2) II – III 105 (49. 1) 118 (52. 2) 116 (66. 7) 117 (71. 8) Cytogenetic risk among patients with known status by FISH*, n (%) High risk 31 (29. 0) 37 (26. 6) 17 (19. 3) 15 (18. 1) Standard risk 76 (71. 0) 102 (73. 4) 71 (80. 7) 68 (81. 9) * Percentages are calculated as a proportion of the number of patients with known status; 2% of patients had missing ISS stage and 47% of patients had unknown ISS cytogenetics. ASCT, autologous stem cell transplantation; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridisation; KRd, carfilzomib, lenalidomide, and dexamethasone; N/A, not available; Rd, lenalidomide and dexamethasone. Hari P, et al. Leukemia 2017; 31: 2630 -2641.

Patient Demographics and Baseline Disease Characteristics: Prior ASCT (2) Prior ASCT ASPIRE TOC No prior ASCT KRd (n = 217) Rd (n = 229) KRd (n = 179) Rd (n = 167) 90. 6 ± 29. 7 92. 6 ± 31. 3 78. 1 ± 26. 5 76. 5 ± 25. 9 0 1 (0. 4) 0 0 30 to <50 m. L/min, n (%) 5 (2. 3) 10 (4. 4) 20 (11. 2) 21 (12. 6) 50 to <80 m. L/min, n (%) 86 (39. 6) 73 (31. 9) 85 (47. 5) 80 (47. 9) ≥ 80 m. L/min, n (%) 126 (58. 1) 143 (62. 4) 73 (40. 8) 62 (37. 1) 0 2 (0. 9) 1 (0. 6) 4 (2. 4) Bortezomib 155 (71. 4) 157 (68. 6) 106 (59. 2) 103 (61. 7) Lenalidomide 49 (22. 6) 40 (17. 5) 30 (16. 8) 38 (22. 8) Creatinine clearance – mean m. L/min ± SD <30 m. L/min, n (%) Missing, n (%) Prior therapy, n (%) Median duration of prior bortezomib and lenalidomide treatment, months (range) Bortezomib 3. 8 (0. 0– 26. 2) 3. 9 (0. 0– 32. 2) 4. 9 (0. 9– 49. 1) 5. 6 (0. 4– 33. 6) Lenalidomide 5. 7 (0. 5– 40. 8) 7. 7 (0. 8– 38. 0) 11. 4 (0. 4– 40. 2) 10. 4 (0. 3– 30. 0) Link to Primary Outcome ASCT, autologous stem cell transplantation; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; SD, standard deviation. Hari P, et al. Leukemia 2017; 31: 2630 -2641.

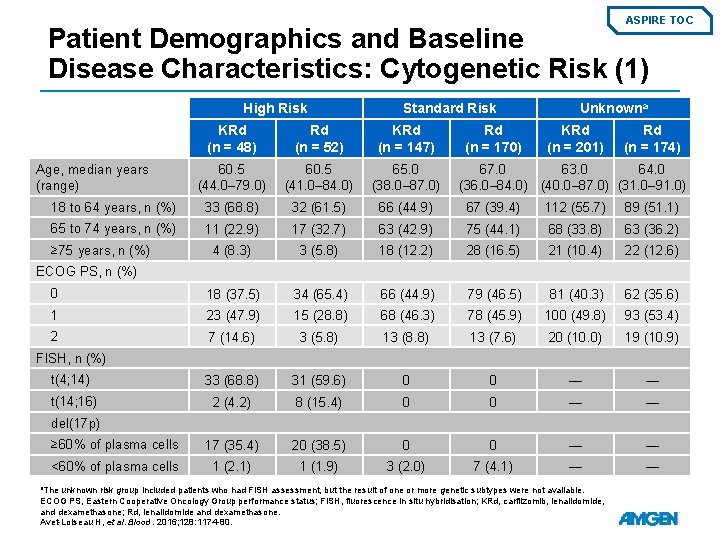
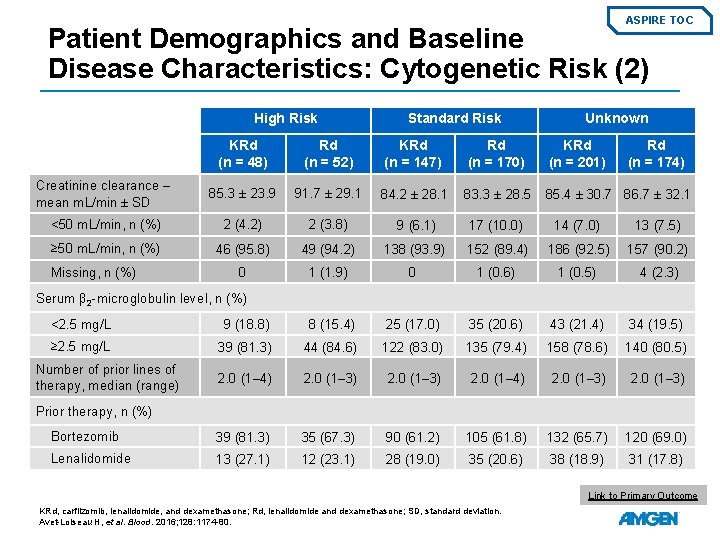
ASPIRE TOC Patient Demographics and Baseline Disease Characteristics: Cytogenetic Risk (1) High Risk Standard Risk KRd (n = 48) Rd (n = 52) KRd (n = 147) 60. 5 (44. 0– 79. 0) 60. 5 (41. 0– 84. 0) 65. 0 (38. 0– 87. 0) 18 to 64 years, n (%) 33 (68. 8) 32 (61. 5) 66 (44. 9) 67 (39. 4) 112 (55. 7) 89 (51. 1) 65 to 74 years, n (%) 11 (22. 9) 17 (32. 7) 63 (42. 9) 75 (44. 1) 68 (33. 8) 63 (36. 2) 4 (8. 3) 3 (5. 8) 18 (12. 2) 28 (16. 5) 21 (10. 4) 22 (12. 6) 0 18 (37. 5) 34 (65. 4) 66 (44. 9) 79 (46. 5) 81 (40. 3) 62 (35. 6) 1 23 (47. 9) 15 (28. 8) 68 (46. 3) 78 (45. 9) 100 (49. 8) 93 (53. 4) 2 7 (14. 6) 3 (5. 8) 13 (8. 8) 13 (7. 6) 20 (10. 0) 19 (10. 9) t(4; 14) 33 (68. 8) 31 (59. 6) 0 0 — — t(14; 16) 2 (4. 2) 8 (15. 4) 0 0 — — ≥ 60% of plasma cells 17 (35. 4) 20 (38. 5) 0 0 — — <60% of plasma cells 1 (2. 1) 1 (1. 9) 3 (2. 0) 7 (4. 1) — — Age, median years (range) ≥ 75 years, n (%) Rd (n = 170) Unknowna KRd (n = 201) Rd (n = 174) 67. 0 63. 0 64. 0 (36. 0– 84. 0) (40. 0– 87. 0) (31. 0– 91. 0) ECOG PS, n (%) FISH, n (%) del(17 p) a. The unknown risk group included patients who had FISH assessment, but the result of one or more genetic subtypes were not available. ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridisation; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Avet-Loiseau H, et al. Blood. 2016; 128: 1174 -80.

ASPIRE TOC Patient Demographics and Baseline Disease Characteristics: Cytogenetic Risk (2) High Risk Standard Risk Unknown KRd (n = 48) Rd (n = 52) KRd (n = 147) Rd (n = 170) KRd (n = 201) Creatinine clearance – mean m. L/min ± SD 85. 3 ± 23. 9 91. 7 ± 29. 1 84. 2 ± 28. 1 83. 3 ± 28. 5 85. 4 ± 30. 7 86. 7 ± 32. 1 <50 m. L/min, n (%) 2 (4. 2) 2 (3. 8) 9 (6. 1) 17 (10. 0) 14 (7. 0) 13 (7. 5) ≥ 50 m. L/min, n (%) 46 (95. 8) 49 (94. 2) 138 (93. 9) 152 (89. 4) 186 (92. 5) 157 (90. 2) 0 1 (1. 9) 0 1 (0. 6) 1 (0. 5) 4 (2. 3) Missing, n (%) Rd (n = 174) Serum β 2 -microglobulin level, n (%) <2. 5 mg/L 9 (18. 8) 8 (15. 4) 25 (17. 0) 35 (20. 6) 43 (21. 4) 34 (19. 5) ≥ 2. 5 mg/L 39 (81. 3) 44 (84. 6) 122 (83. 0) 135 (79. 4) 158 (78. 6) 140 (80. 5) 2. 0 (1– 4) 2. 0 (1– 3) Bortezomib 39 (81. 3) 35 (67. 3) 90 (61. 2) 105 (61. 8) 132 (65. 7) 120 (69. 0) Lenalidomide 13 (27. 1) 12 (23. 1) 28 (19. 0) 35 (20. 6) 38 (18. 9) 31 (17. 8) Number of prior lines of therapy, median (range) Prior therapy, n (%) Link to Primary Outcome KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; SD, standard deviation. Avet-Loiseau H, et al. Blood. 2016; 128: 1174 -80.

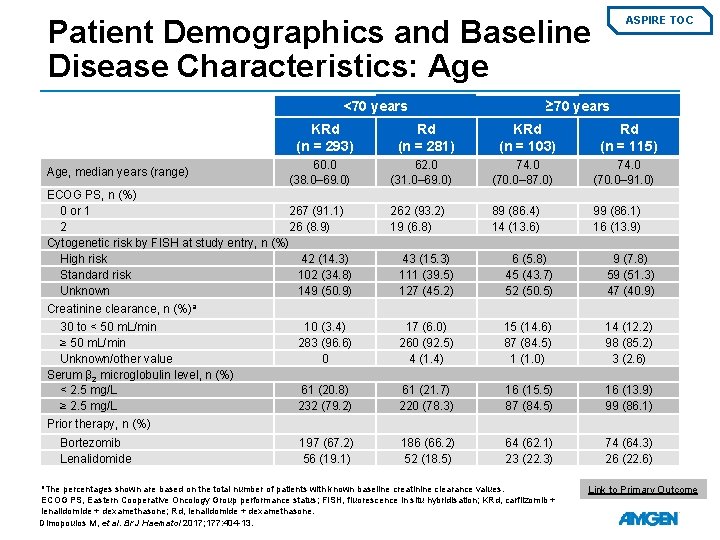
Patient Demographics and Baseline Disease Characteristics: Age <70 years Age, median years (range) ASPIRE TOC ≥ 70 years KRd (n = 293) Rd (n = 281) KRd (n = 103) Rd (n = 115) 60. 0 (38. 0– 69. 0) 62. 0 (31. 0– 69. 0) 74. 0 (70. 0– 87. 0) 74. 0 (70. 0– 91. 0) 262 (93. 2) 19 (6. 8) 89 (86. 4) 14 (13. 6) 99 (86. 1) 16 (13. 9) ECOG PS, n (%) 0 or 1 267 (91. 1) 2 26 (8. 9) Cytogenetic risk by FISH at study entry, n (%) High risk 42 (14. 3) Standard risk 102 (34. 8) Unknown 149 (50. 9) 43 (15. 3) 111 (39. 5) 127 (45. 2) 6 (5. 8) 45 (43. 7) 52 (50. 5) 9 (7. 8) 59 (51. 3) 47 (40. 9) 10 (3. 4) 283 (96. 6) 0 17 (6. 0) 260 (92. 5) 4 (1. 4) 15 (14. 6) 87 (84. 5) 1 (1. 0) 14 (12. 2) 98 (85. 2) 3 (2. 6) 61 (20. 8) 232 (79. 2) 61 (21. 7) 220 (78. 3) 16 (15. 5) 87 (84. 5) 16 (13. 9) 99 (86. 1) 197 (67. 2) 56 (19. 1) 186 (66. 2) 52 (18. 5) 64 (62. 1) 23 (22. 3) 74 (64. 3) 26 (22. 6) Creatinine clearance, n (%)a 30 to < 50 m. L/min ≥ 50 m. L/min Unknown/other value Serum β 2 microglobulin level, n (%) < 2. 5 mg/L ≥ 2. 5 mg/L Prior therapy, n (%) Bortezomib Lenalidomide a. The percentages shown are based on the total number of patients with known baseline creatinine clearance values. ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridisation; KRd, carfilzomib + lenalidomide + dexamethasone; Rd, lenalidomide + dexamethasone. Dimopoulos M, et al. Br J Haematol 2017; 177: 404 -13. Link to Primary Outcome

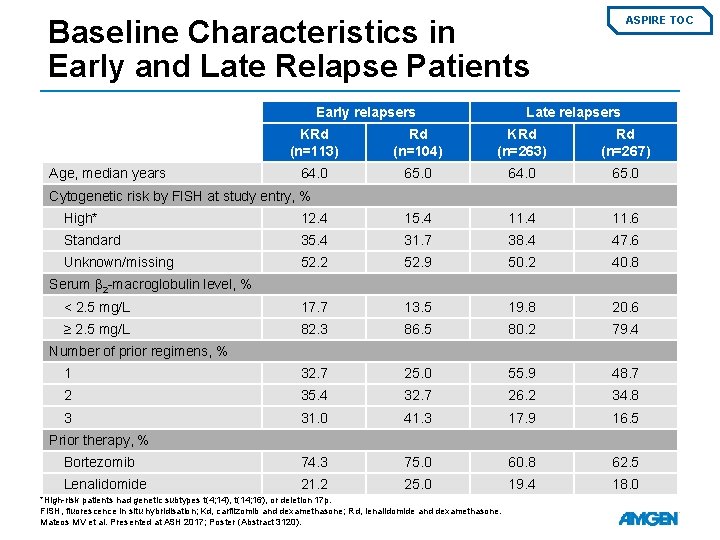
Baseline Characteristics in Early and Late Relapse Patients Early relapsers Age, median years ASPIRE TOC Late relapsers KRd (n=113) Rd (n=104) KRd (n=263) Rd (n=267) 64. 0 65. 0 Cytogenetic risk by FISH at study entry, % High* 12. 4 15. 4 11. 6 Standard 35. 4 31. 7 38. 4 47. 6 Unknown/missing 52. 2 52. 9 50. 2 40. 8 < 2. 5 mg/L 17. 7 13. 5 19. 8 20. 6 ≥ 2. 5 mg/L 82. 3 86. 5 80. 2 79. 4 1 32. 7 25. 0 55. 9 48. 7 2 35. 4 32. 7 26. 2 34. 8 3 31. 0 41. 3 17. 9 16. 5 Bortezomib 74. 3 75. 0 60. 8 62. 5 Lenalidomide 21. 2 25. 0 19. 4 18. 0 Serum β 2 -macroglobulin level, % Number of prior regimens, % Prior therapy, % *High-risk patients had genetic subtypes t(4; 14), t(14; 16), or deletion 17 p. FISH, fluorescence in situ hybridisation; Kd, carfilzomib and dexamethasone; Rd, lenalidomide and dexamethasone. Mateos MV et al. Presented at ASH 2017; Poster (Abstract 3120).

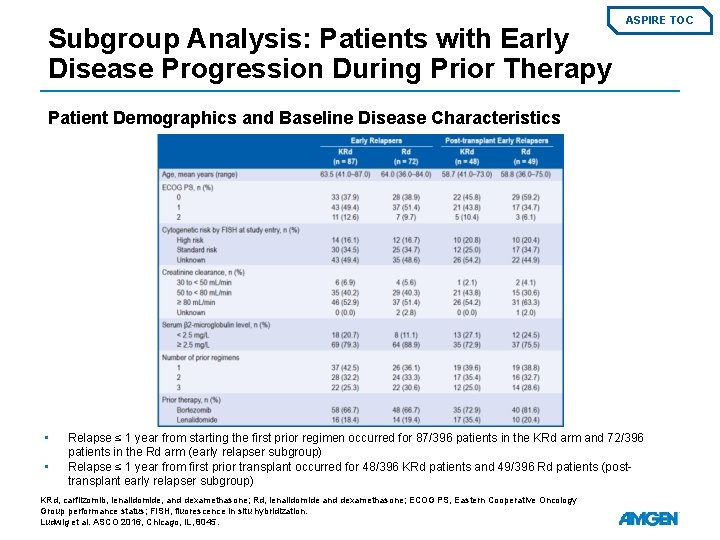
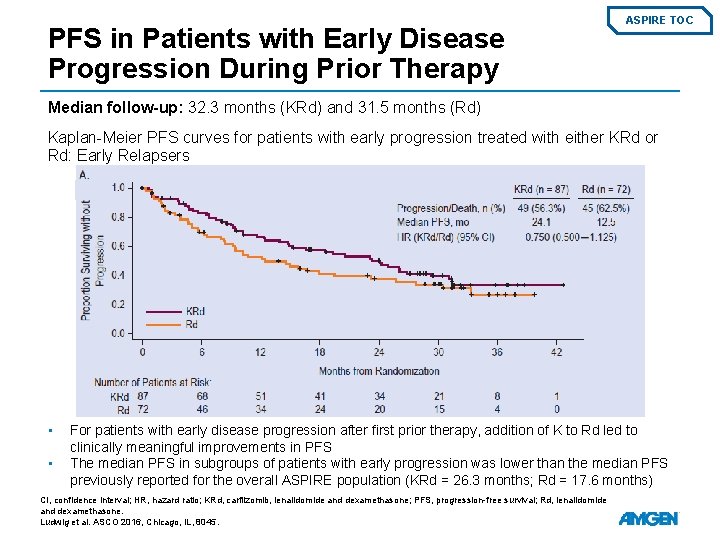
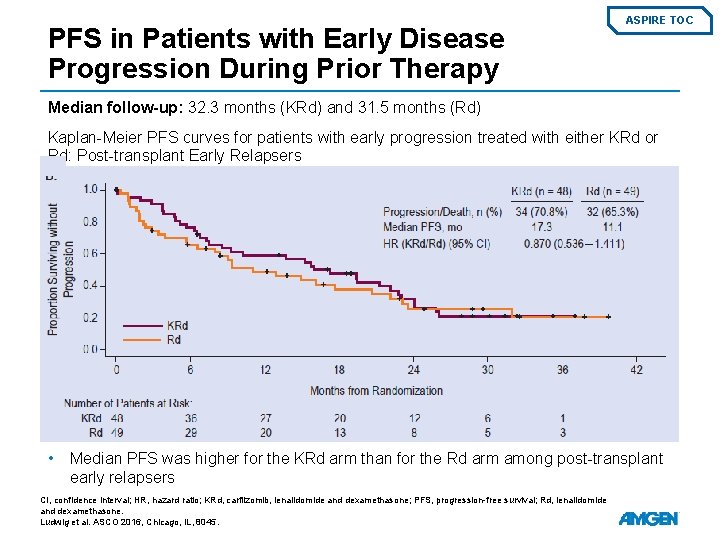
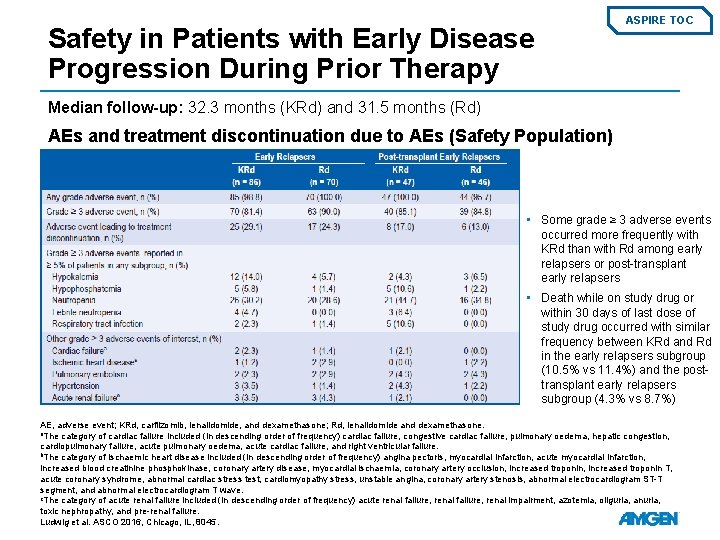
Subgroup Analysis: Patients with Early Disease Progression During Prior Therapy ASPIRE TOC Patient Demographics and Baseline Disease Characteristics • • Relapse ≤ 1 year from starting the first prior regimen occurred for 87/396 patients in the KRd arm and 72/396 patients in the Rd arm (early relapser subgroup) Relapse ≤ 1 year from first prior transplant occurred for 48/396 KRd patients and 49/396 Rd patients (posttransplant early relapser subgroup) KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization. Ludwig et al. ASCO 2016, Chicago, IL, 8045.

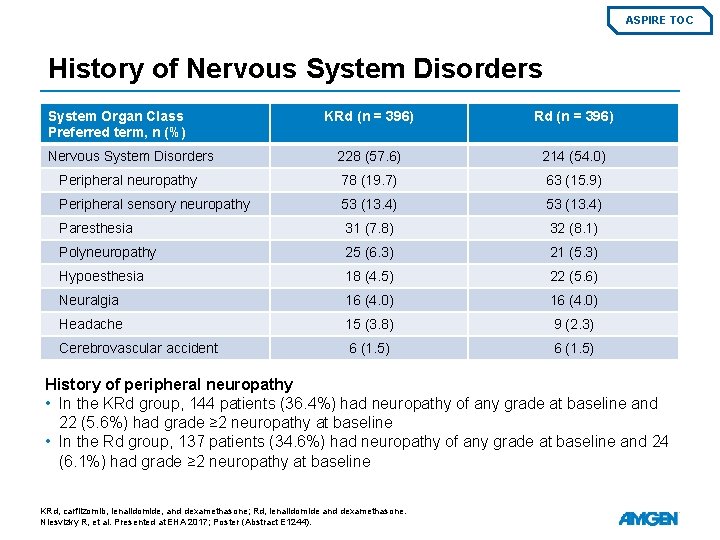
ASPIRE TOC History of Nervous System Disorders System Organ Class Preferred term, n (%) KRd (n = 396) Nervous System Disorders 228 (57. 6) 214 (54. 0) Peripheral neuropathy 78 (19. 7) 63 (15. 9) Peripheral sensory neuropathy 53 (13. 4) Paresthesia 31 (7. 8) 32 (8. 1) Polyneuropathy 25 (6. 3) 21 (5. 3) Hypoesthesia 18 (4. 5) 22 (5. 6) Neuralgia 16 (4. 0) Headache 15 (3. 8) 9 (2. 3) Cerebrovascular accident 6 (1. 5) History of peripheral neuropathy • In the KRd group, 144 patients (36. 4%) had neuropathy of any grade at baseline and 22 (5. 6%) had grade ≥ 2 neuropathy at baseline • In the Rd group, 137 patients (34. 6%) had neuropathy of any grade at baseline and 24 (6. 1%) had grade ≥ 2 neuropathy at baseline KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Niesvizky R, et al. Presented at EHA 2017; Poster (Abstract E 1244).

ASPIRE TOC All studies ASPIRE PFS – Primary Endpoint Interim Analysis PFS by Response Category Updated results PFS Over Time Prior Lines of Therapy/Type of previous treatment PFS by Peripheral Neuropathy History Prior ASCT PFS in Early vs Late Relapse Patients Cytogenetic Risk Summary Age Patients with Early Progression During Prior Therapy

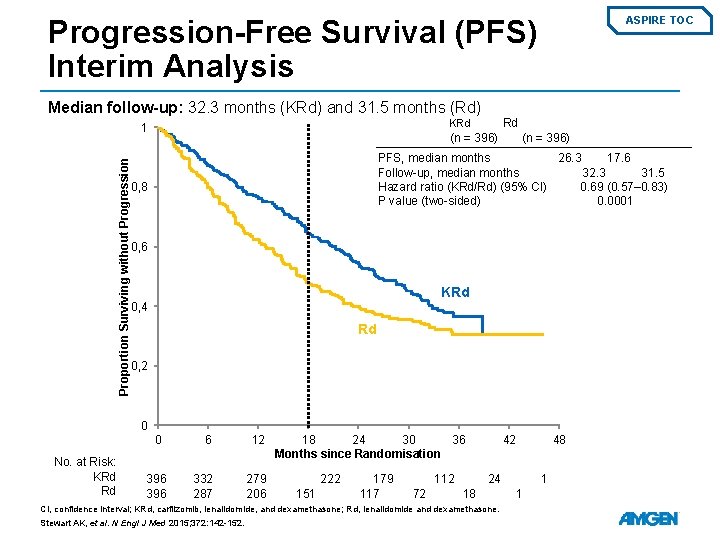
Progression-Free Survival (PFS) Interim Analysis ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Proportion Surviving without Progression Rd KRd 1 (n = 396) PFS, median months 26. 3 17. 6 Follow-up, median months 32. 3 31. 5 Hazard ratio (KRd/Rd) (95% CI) 0. 69 (0. 57– 0. 83) P value (two-sided) 0. 0001 0, 8 0, 6 KRd 0, 4 Rd 0, 2 0 0 No. at Risk: KRd Rd 396 6 12 332 287 279 206 18 24 30 36 Months since Randomisation 222 151 179 117 112 72 42 24 18 CI, confidence interval; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152. 48 1 1

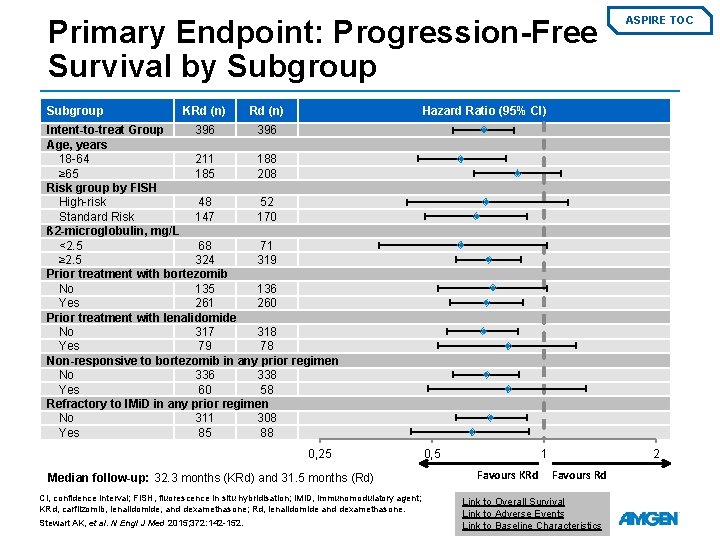
Primary Endpoint: Progression-Free Survival by Subgroup KRd (n) ASPIRE TOC Hazard Ratio (95% CI) Intent-to-treat Group 396 Age, years 18 -64 211 188 ≥ 65 185 208 Risk group by FISH High-risk 48 52 Standard Risk 147 170 ß 2 -microglobulin, mg/L <2. 5 68 71 ≥ 2. 5 324 319 Prior treatment with bortezomib No 135 136 Yes 261 260 Prior treatment with lenalidomide No 317 318 Yes 79 78 Non-responsive to bortezomib in any prior regimen No 336 338 Yes 60 58 Refractory to IMi. D in any prior regimen No 311 308 Yes 85 88 0, 25 Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) CI, confidence interval; FISH, fluorescence in situ hybridisation; IMi. D, immunomodulatory agent; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152. 0, 5 1 Favours KRd 2 Favours Rd Link to Overall Survival Link to Adverse Events Link to Baseline Characteristics

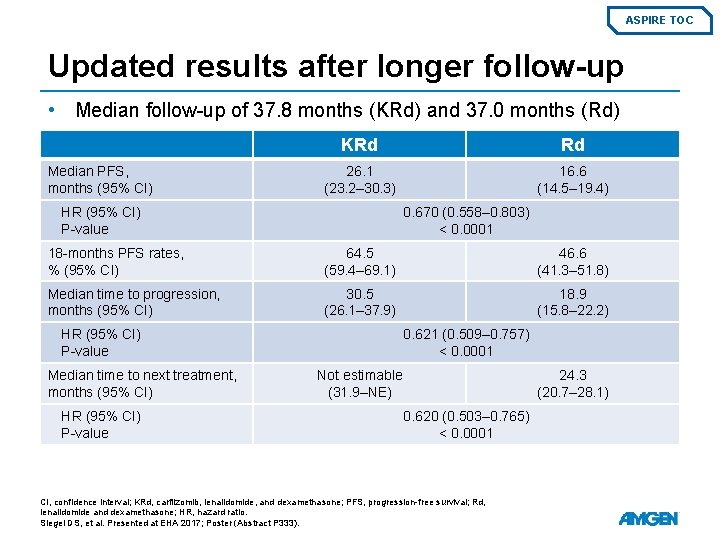
ASPIRE TOC Updated results after longer follow-up • Median follow-up of 37. 8 months (KRd) and 37. 0 months (Rd) Median PFS, months (95% CI) KRd Rd 26. 1 (23. 2– 30. 3) 16. 6 (14. 5– 19. 4) HR (95% CI) P-value 0. 670 (0. 558– 0. 803) < 0. 0001 18 -months PFS rates, % (95% CI) 64. 5 (59. 4– 69. 1) 46. 6 (41. 3– 51. 8) Median time to progression, months (95% CI) 30. 5 (26. 1– 37. 9) 18. 9 (15. 8– 22. 2) HR (95% CI) P-value Median time to next treatment, months (95% CI) HR (95% CI) P-value 0. 621 (0. 509– 0. 757) < 0. 0001 Not estimable (31. 9–NE) 24. 3 (20. 7– 28. 1) 0. 620 (0. 503– 0. 765) < 0. 0001 CI, confidence interval; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone; HR, hazard ratio. Siegel DS, et al. Presented at EHA 2017; Poster (Abstract P 333).

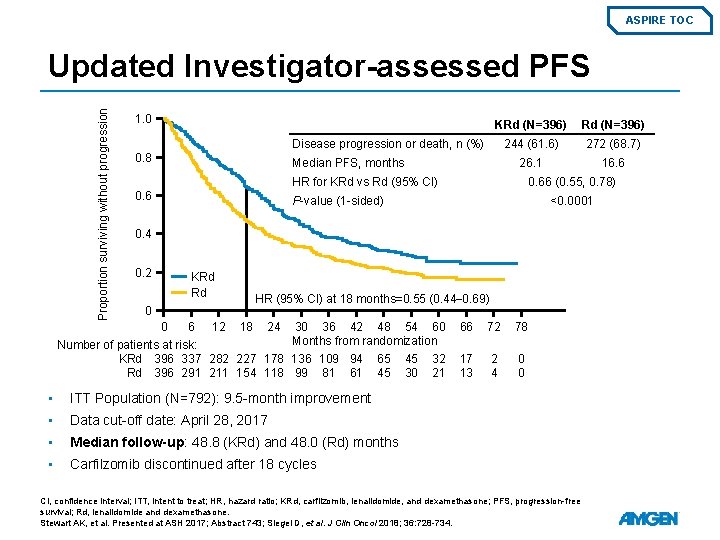
ASPIRE TOC Proportion surviving without progression Updated Investigator-assessed PFS 1. 0 KRd (N=396) 244 (61. 6) 272 (68. 7) 26. 1 16. 6 Disease progression or death, n (%) 0. 8 Median PFS, months HR for KRd vs Rd (95% CI) 0. 66 (0. 55, 0. 78) P-value (1 -sided) <0. 0001 0. 4 0. 2 KRd Rd HR (95% CI) at 18 months=0. 55 (0. 44– 0. 69) 0 0 6 12 18 24 30 36 42 48 54 60 Months from randomization Number of patients at risk: KRd 396 337 282 227 178 136 109 94 65 45 32 Rd 396 291 211 154 118 99 81 61 45 30 21 • ITT Population (N=792): 9. 5 -month improvement • Data cut-off date: April 28, 2017 • Median follow-up: 48. 8 (KRd) and 48. 0 (Rd) months • Carfilzomib discontinued after 18 cycles 66 72 78 17 13 2 4 0 0 CI, confidence interval; ITT, intent to treat; HR, hazard ratio; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Stewart AK, et al. Presented at ASH 2017; Abstract 743; Siegel D, et al. J Clin Oncol 2018; 36: 728 -734.

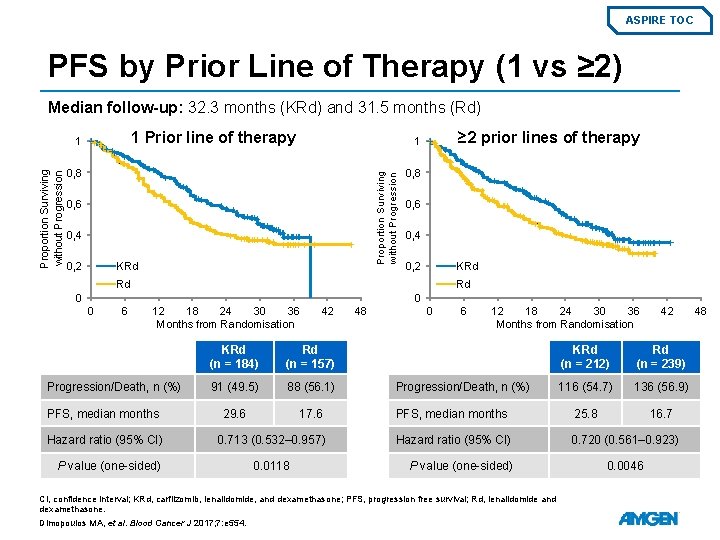
ASPIRE TOC PFS by Prior Line of Therapy (1 vs ≥ 2) Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) 1 Prior line of therapy 0, 8 0, 6 0, 4 KRd 0, 2 ≥ 2 prior lines of therapy 1 Proportion Surviving without Progression 1 0, 8 0, 6 0, 4 KRd 0, 2 Rd Rd 0 0 0 6 12 18 24 30 36 Months from Randomisation Progression/Death, n (%) PFS, median months Hazard ratio (95% CI) P value (one-sided) 42 KRd (n = 184) Rd (n = 157) 91 (49. 5) 88 (56. 1) 29. 6 17. 6 0. 713 (0. 532– 0. 957) 0. 0118 48 0 6 12 18 24 30 36 Months from Randomisation 42 KRd (n = 212) Rd (n = 239) 116 (54. 7) 136 (56. 9) PFS, median months 25. 8 16. 7 Hazard ratio (95% CI) 0. 720 (0. 561– 0. 923) Progression/Death, n (%) P value (one-sided) CI, confidence interval; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression free survival; Rd, lenalidomide and dexamethasone. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554. 0. 0046 48

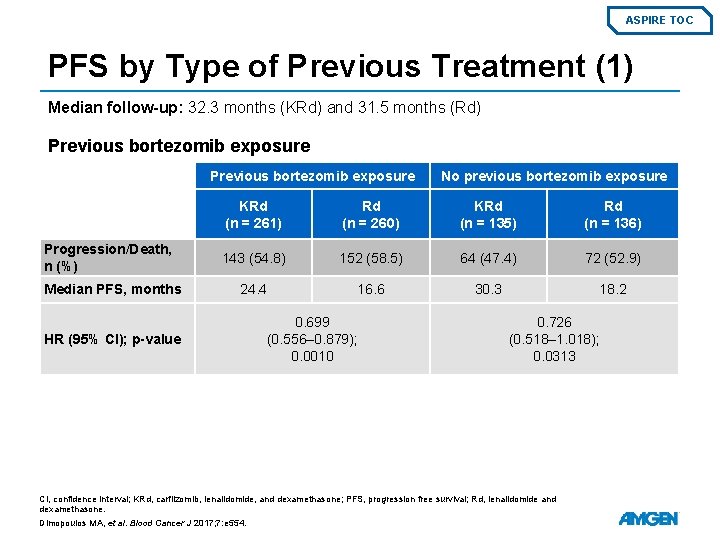
ASPIRE TOC PFS by Type of Previous Treatment (1) Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Previous bortezomib exposure Progression/Death, n (%) Median PFS, months HR (95% CI); p-value No previous bortezomib exposure KRd (n = 261) Rd (n = 260) KRd (n = 135) Rd (n = 136) 143 (54. 8) 152 (58. 5) 64 (47. 4) 72 (52. 9) 24. 4 16. 6 30. 3 18. 2 0. 699 (0. 556– 0. 879); 0. 0010 0. 726 (0. 518– 1. 018); 0. 0313 CI, confidence interval; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression free survival; Rd, lenalidomide and dexamethasone. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554.

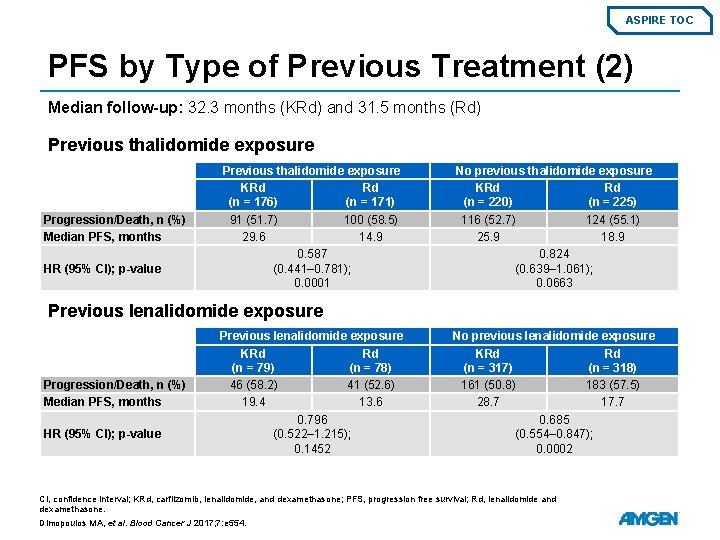
ASPIRE TOC PFS by Type of Previous Treatment (2) Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Previous thalidomide exposure Progression/Death, n (%) Median PFS, months HR (95% CI); p-value Previous thalidomide exposure KRd Rd (n = 176) (n = 171) 91 (51. 7) 100 (58. 5) 29. 6 14. 9 0. 587 (0. 441– 0. 781); 0. 0001 No previous thalidomide exposure KRd Rd (n = 220) (n = 225) 116 (52. 7) 124 (55. 1) 25. 9 18. 9 0. 824 (0. 639– 1. 061); 0. 0663 Previous lenalidomide exposure Progression/Death, n (%) Median PFS, months HR (95% CI); p-value Previous lenalidomide exposure KRd Rd (n = 79) (n = 78) 46 (58. 2) 41 (52. 6) 19. 4 13. 6 0. 796 (0. 522– 1. 215); 0. 1452 No previous lenalidomide exposure KRd Rd (n = 317) (n = 318) 161 (50. 8) 183 (57. 5) 28. 7 17. 7 0. 685 (0. 554– 0. 847); 0. 0002 CI, confidence interval; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression free survival; Rd, lenalidomide and dexamethasone. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554.

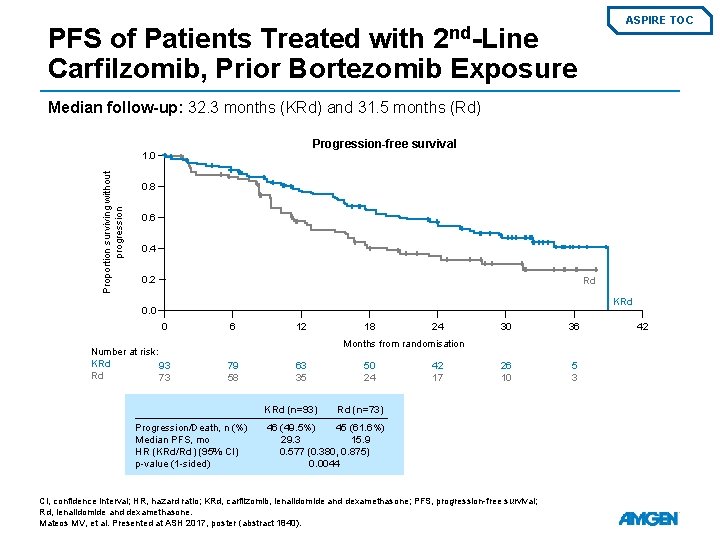
ASPIRE TOC 2 nd-Line PFS of Patients Treated with Carfilzomib, Prior Bortezomib Exposure Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Progression-free survival Proportion surviving without progression 1. 0 0. 8 0. 6 0. 4 0. 2 Rd KRd 0. 0 0 Number at risk: KRd 93 Rd 73 6 12 18 24 30 36 26 10 5 3 Months from randomisation 79 58 63 35 KRd (n=93) Progression/Death, n (%) Median PFS, mo HR (KRd/Rd) (95% CI) p-value (1 -sided) 50 24 42 17 Rd (n=73) 46 (49. 5%) 45 (61. 6%) 29. 3 15. 9 0. 577 (0. 380, 0. 875) 0. 0044 CI, confidence interval; HR, hazard ratio; KRd, carfilzomib, lenalidomide and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Mateos MV, et al. Presented at ASH 2017, poster (abstract 1840). 42

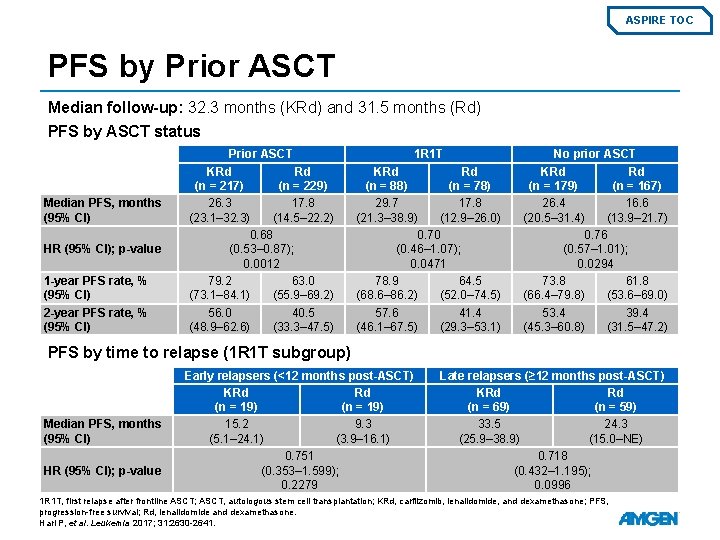
ASPIRE TOC PFS by Prior ASCT Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) PFS by ASCT status Median PFS, months (95% CI) HR (95% CI); p-value 1 -year PFS rate, % (95% CI) 2 -year PFS rate, % (95% CI) Prior ASCT KRd Rd (n = 217) (n = 229) 26. 3 17. 8 (23. 1– 32. 3) (14. 5– 22. 2) 0. 68 (0. 53– 0. 87); 0. 0012 79. 2 63. 0 (73. 1– 84. 1) (55. 9– 69. 2) 56. 0 40. 5 (48. 9– 62. 6) (33. 3– 47. 5) 1 R 1 T KRd Rd (n = 88) (n = 78) 29. 7 17. 8 (21. 3– 38. 9) (12. 9– 26. 0) 0. 70 (0. 46– 1. 07); 0. 0471 78. 9 64. 5 (68. 6– 86. 2) (52. 0– 74. 5) 57. 6 41. 4 (46. 1– 67. 5) (29. 3– 53. 1) No prior ASCT KRd Rd (n = 179) (n = 167) 26. 4 16. 6 (20. 5– 31. 4) (13. 9– 21. 7) 0. 76 (0. 57– 1. 01); 0. 0294 73. 8 61. 8 (66. 4– 79. 8) (53. 6– 69. 0) 53. 4 39. 4 (45. 3– 60. 8) (31. 5– 47. 2) PFS by time to relapse (1 R 1 T subgroup) Median PFS, months (95% CI) HR (95% CI); p-value Early relapsers (<12 months post-ASCT) KRd Rd (n = 19) 15. 2 9. 3 (5. 1– 24. 1) (3. 9– 16. 1) 0. 751 (0. 353– 1. 599); 0. 2279 Late relapsers (≥ 12 months post-ASCT) KRd Rd (n = 69) (n = 59) 33. 5 24. 3 (25. 9– 38. 9) (15. 0–NE) 0. 718 (0. 432– 1. 195); 0. 0996 1 R 1 T, first relapse after frontline ASCT; ASCT, autologous stem cell transplantation; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Hari P, et al. Leukemia 2017; 31: 2630 -2641.

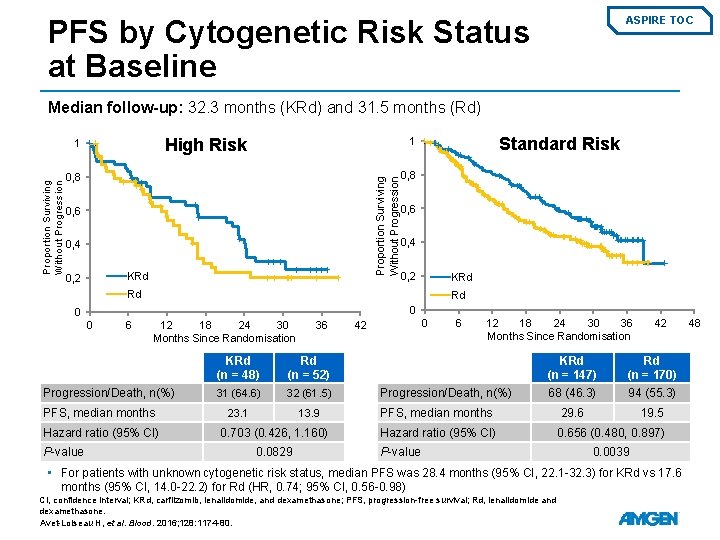
PFS by Cytogenetic Risk Status at Baseline ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) High Risk 0, 8 0, 6 0, 4 KRd 0, 2 Standard Risk 1 Proportion Surviving Without Progression 1 0, 8 0, 6 0, 4 0, 2 KRd Rd Rd 0 0 0 6 12 18 24 30 Months Since Randomisation Progression/Death, n(%) PFS, median months Hazard ratio (95% CI) P-value 36 KRd (n = 48) Rd (n = 52) 31 (64. 6) 32 (61. 5) 23. 1 13. 9 0. 703 (0. 426, 1. 160) 0. 0829 0 42 6 12 18 24 30 36 Months Since Randomisation Progression/Death, n(%) 42 KRd (n = 147) Rd (n = 170) 68 (46. 3) 94 (55. 3) 29. 6 19. 5 PFS, median months Hazard ratio (95% CI) P-value 0. 656 (0. 480, 0. 897) 0. 0039 • For patients with unknown cytogenetic risk status, median PFS was 28. 4 months (95% CI, 22. 1 -32. 3) for KRd vs 17. 6 months (95% CI, 14. 0 -22. 2) for Rd (HR, 0. 74; 95% CI, 0. 56 -0. 98) CI, confidence interval; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Avet-Loiseau H, et al. Blood. 2016; 128: 1174 -80. 48

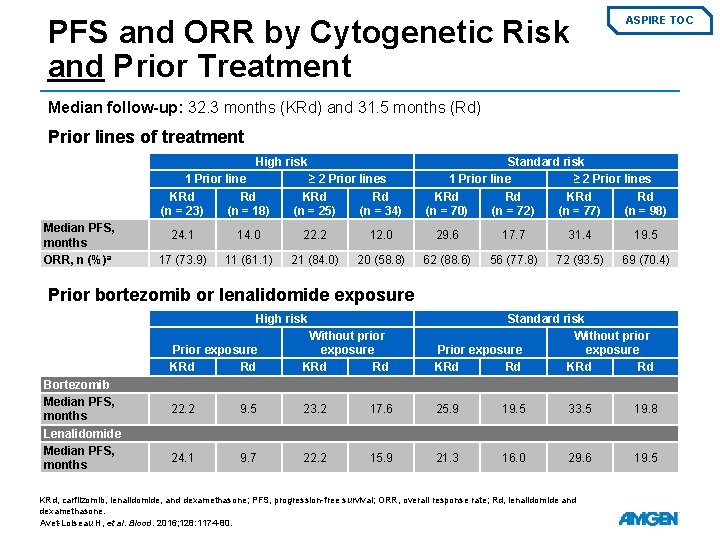
PFS and ORR by Cytogenetic Risk and Prior Treatment ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Prior lines of treatment High risk 1 Prior line KRd Rd (n = 23) (n = 18) Median PFS, months ORR, n (%)a ≥ 2 Prior lines KRd Rd (n = 25) (n = 34) Standard risk 1 Prior line ≥ 2 Prior lines KRd Rd (n = 70) (n = 72) (n = 77) (n = 98) 24. 1 14. 0 22. 2 12. 0 29. 6 17. 7 31. 4 19. 5 17 (73. 9) 11 (61. 1) 21 (84. 0) 20 (58. 8) 62 (88. 6) 56 (77. 8) 72 (93. 5) 69 (70. 4) Prior bortezomib or lenalidomide exposure High risk Bortezomib Median PFS, months Lenalidomide Median PFS, months Prior exposure KRd Rd Without prior exposure KRd Rd Standard risk Without prior Prior exposure KRd Rd 22. 2 9. 5 23. 2 17. 6 25. 9 19. 5 33. 5 19. 8 24. 1 9. 7 22. 2 15. 9 21. 3 16. 0 29. 6 19. 5 KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; ORR, overall response rate; Rd, lenalidomide and dexamethasone. Avet-Loiseau H, et al. Blood. 2016; 128: 1174 -80.

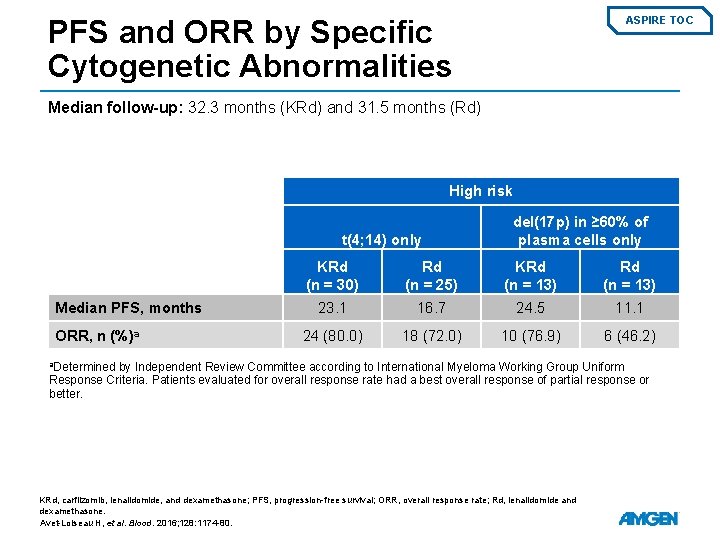
PFS and ORR by Specific Cytogenetic Abnormalities ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) High risk t(4; 14) only Median PFS, months ORR, n (%)a del(17 p) in ≥ 60% of plasma cells only KRd (n = 30) Rd (n = 25) KRd (n = 13) 23. 1 16. 7 24. 5 11. 1 24 (80. 0) 18 (72. 0) 10 (76. 9) 6 (46. 2) a. Determined by Independent Review Committee according to International Myeloma Working Group Uniform Response Criteria. Patients evaluated for overall response rate had a best overall response of partial response or better. KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; ORR, overall response rate; Rd, lenalidomide and dexamethasone. Avet-Loiseau H, et al. Blood. 2016; 128: 1174 -80.

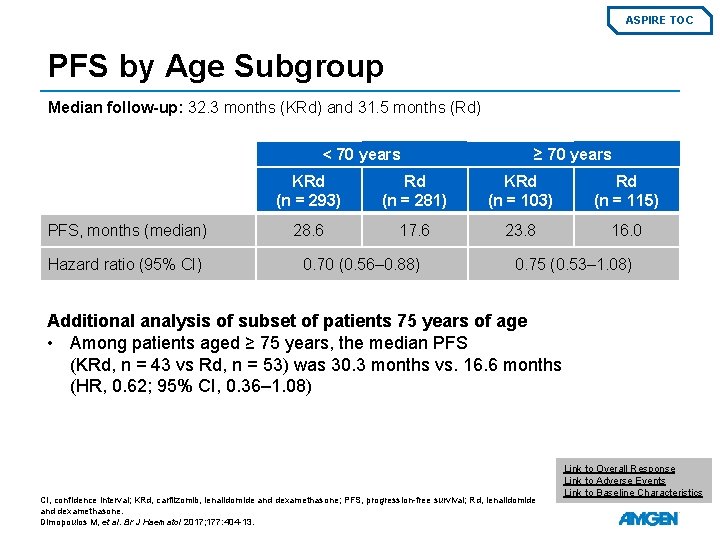
ASPIRE TOC PFS by Age Subgroup Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) < 70 years PFS, months (median) Hazard ratio (95% CI) ≥ 70 years KRd (n = 293) Rd (n = 281) KRd (n = 103) Rd (n = 115) 28. 6 17. 6 23. 8 16. 0 0. 70 (0. 56– 0. 88) 0. 75 (0. 53– 1. 08) Additional analysis of subset of patients 75 years of age • Among patients aged ≥ 75 years, the median PFS (KRd, n = 43 vs Rd, n = 53) was 30. 3 months vs. 16. 6 months (HR, 0. 62; 95% CI, 0. 36– 1. 08) CI, confidence interval; KRd, carfilzomib, lenalidomide and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Dimopoulos M, et al. Br J Haematol 2017; 177: 404 -13. Link to Overall Response Link to Adverse Events Link to Baseline Characteristics

PFS in Patients with Early Disease Progression During Prior Therapy ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Kaplan-Meier PFS curves for patients with early progression treated with either KRd or Rd: Early Relapsers • • For patients with early disease progression after first prior therapy, addition of K to Rd led to clinically meaningful improvements in PFS The median PFS in subgroups of patients with early progression was lower than the median PFS previously reported for the overall ASPIRE population (KRd = 26. 3 months; Rd = 17. 6 months) CI, confidence interval; HR, hazard ratio; KRd, carfilzomib, lenalidomide and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Ludwig et al. ASCO 2016, Chicago, IL, 8045.

PFS in Patients with Early Disease Progression During Prior Therapy ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Kaplan-Meier PFS curves for patients with early progression treated with either KRd or Rd: Post-transplant Early Relapsers • Median PFS was higher for the KRd arm than for the Rd arm among post-transplant early relapsers CI, confidence interval; HR, hazard ratio; KRd, carfilzomib, lenalidomide and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Ludwig et al. ASCO 2016, Chicago, IL, 8045.

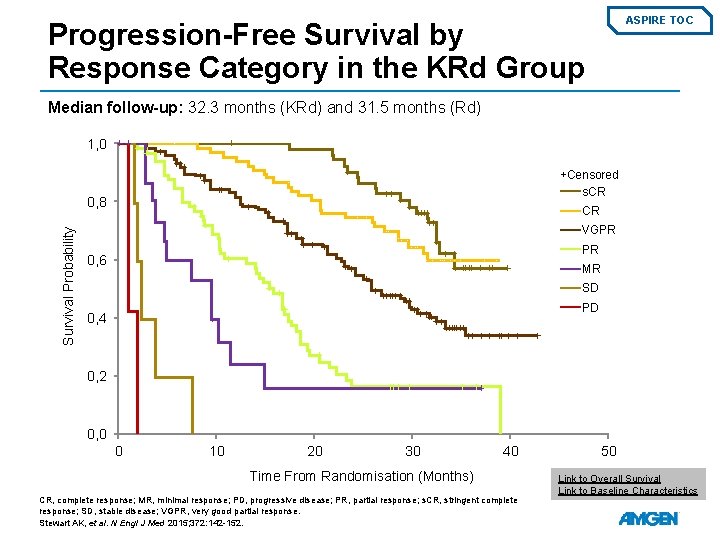
ASPIRE TOC Progression-Free Survival by Response Category in the KRd Group Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) 1, 0 +Censored s. CR Survival Probability 0, 8 CR VGPR PR 0, 6 MR SD PD 0, 4 0, 2 0, 0 0 10 20 30 40 Time From Randomisation (Months) CR, complete response; MR, minimal response; PD, progressive disease; PR, partial response; s. CR, stringent complete response; SD, stable disease; VGPR, very good partial response. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152. 50 Link to Overall Survival Link to Baseline Characteristics

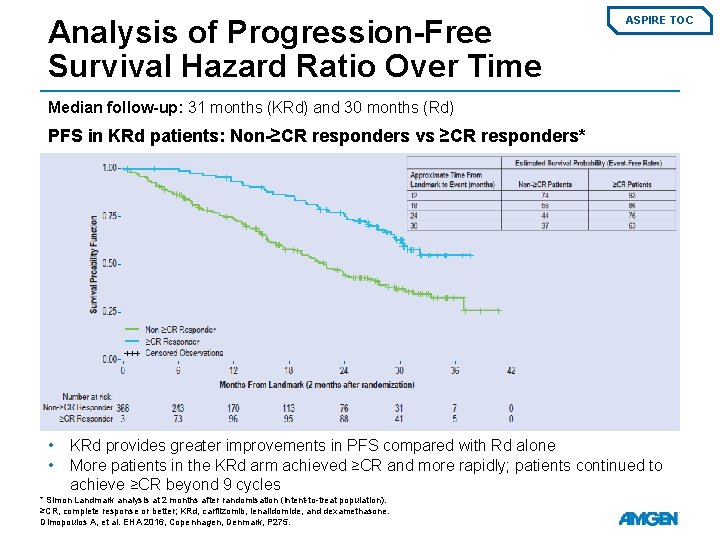
Analysis of Progression-Free Survival Hazard Ratio Over Time ASPIRE TOC Median follow-up: 31 months (KRd) and 30 months (Rd) PFS in KRd patients: Non-≥CR responders vs ≥CR responders* • • KRd provides greater improvements in PFS compared with Rd alone More patients in the KRd arm achieved ≥CR and more rapidly; patients continued to achieve ≥CR beyond 9 cycles * Simon Landmark analysis at 2 months after randomisation (intent-to-treat population). ≥CR, complete response or better; KRd, carfilzomib, lenalidomide, and dexamethasone. Dimopoulos A, et al. EHA 2016, Copenhagen, Denmark, P 275.

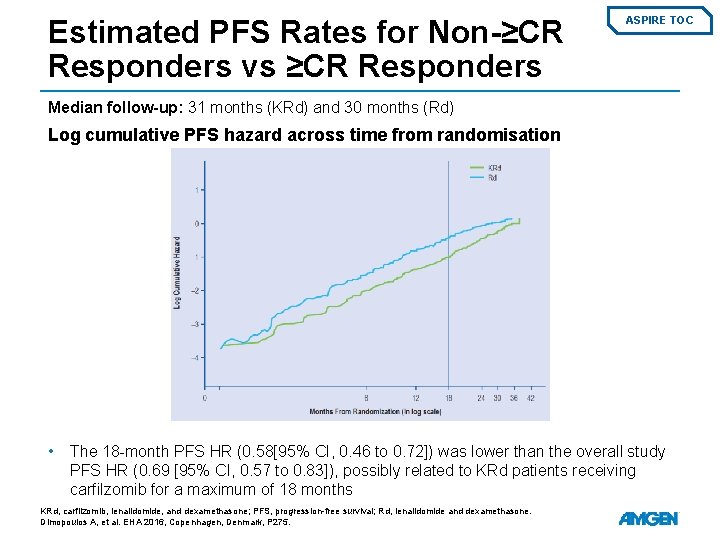
Estimated PFS Rates for Non-≥CR Responders vs ≥CR Responders ASPIRE TOC Median follow-up: 31 months (KRd) and 30 months (Rd) Log cumulative PFS hazard across time from randomisation • The 18 -month PFS HR (0. 58[95% CI, 0. 46 to 0. 72]) was lower than the overall study PFS HR (0. 69 [95% CI, 0. 57 to 0. 83]), possibly related to KRd patients receiving carfilzomib for a maximum of 18 months KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Dimopoulos A, et al. EHA 2016, Copenhagen, Denmark, P 275.

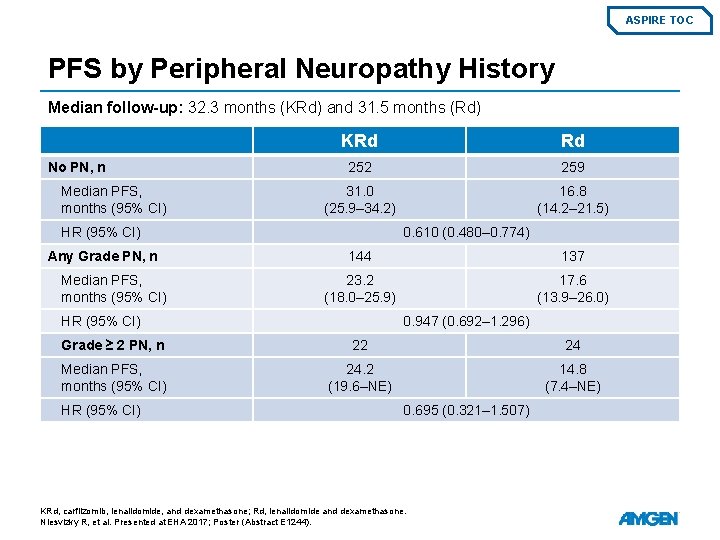
ASPIRE TOC PFS by Peripheral Neuropathy History Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) No PN, n Median PFS, months (95% CI) KRd Rd 252 259 31. 0 (25. 9– 34. 2) 16. 8 (14. 2– 21. 5) HR (95% CI) Any Grade PN, n Median PFS, months (95% CI) 0. 610 (0. 480– 0. 774) 144 137 23. 2 (18. 0– 25. 9) 17. 6 (13. 9– 26. 0) HR (95% CI) 0. 947 (0. 692– 1. 296) Grade ≥ 2 PN, n 22 24 Median PFS, months (95% CI) 24. 2 (19. 6–NE) 14. 8 (7. 4–NE) HR (95% CI) 0. 695 (0. 321– 1. 507) KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Niesvizky R, et al. Presented at EHA 2017; Poster (Abstract E 1244).

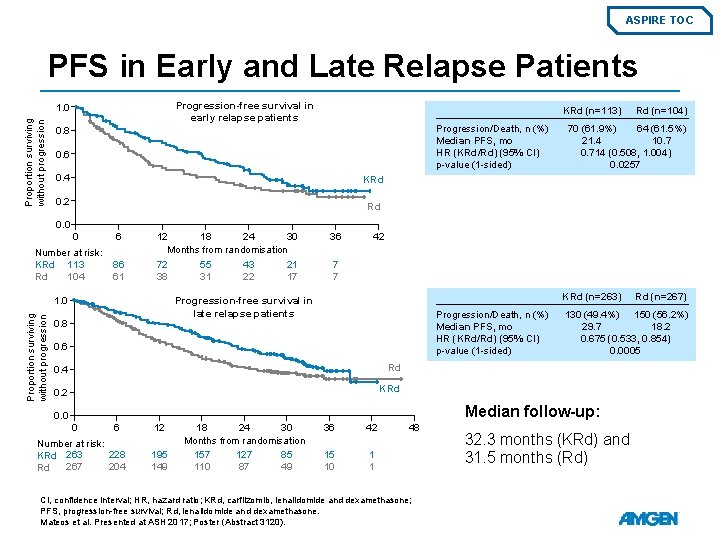
ASPIRE TOC PFS in Early and Late Relapse Patients Progression-free survival in early relapse patients Proportion surviving without progression 1. 0 KRd (n=113) Progression/Death, n (%) Median PFS, mo HR (KRd/Rd) (95% CI) p-value (1 -sided) 0. 8 0. 6 0. 4 Rd (n=104) 70 (61. 9%) 64 (61. 5%) 21. 4 10. 714 (0. 508, 1. 004) 0. 0257 KRd 0. 2 Rd 0. 0 0 Number at risk: KRd 113 104 Rd 6 86 61 12 18 24 30 Months from randomisation 72 55 43 21 38 31 22 17 42 7 7 KRd (n=263) Progression-free survival in late relapse patients 1. 0 Proportion surviving without progression 36 0. 8 Progression/Death, n (%) Median PFS, mo HR (KRd/Rd) (95% CI) p-value (1 -sided) 0. 6 0. 4 Rd 0. 2 KRd 130 (49. 4%) 150 (56. 2%) 29. 7 18. 2 0. 675 (0. 533, 0. 854) 0. 0005 Median follow-up: 0. 0 0 6 Number at risk: 228 KRd 263 267 204 Rd 12 195 149 18 24 30 Months from randomisation 157 127 85 110 87 49 36 42 15 10 1 1 48 CI, confidence interval; HR, hazard ratio; KRd, carfilzomib, lenalidomide and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Mateos et al. Presented at ASH 2017; Poster (Abstract 3120). Rd (n=267) 32. 3 months (KRd) and 31. 5 months (Rd)

ASPIRE TOC Summary: Primary Outcome Overall • PFS was significantly improved by 8. 7 months with KRd (HR, 0. 69; P-value=0. 0001) • KRd demonstrated a significantly improved median PFS of 26. 3 months compared to 17. 6 months for Rd • Results after additional follow-up show consistent results, with a median PFS of 26. 1 months in the KRd arm and 16. 6 months in the Rd arm (HR, 0. 66; P-value<0. 0001) Overall: Updated investigator-assessed results • PFS was improved by 9. 5 months with KRd vs Rd (HR, 0. 66) • Median follow-up: 48. 8 (KRd) and 48. 0 (Rd) months Prior Lines of therapy • The use of KRd led to improvements in PFS in patients with 1 or ≥ 2 prior lines of therapy • 12 -month improvement in median PFS vs Rd in patients with 1 prior line of therapy (HR, 0. 69) • 9 -month improvement in median PFS vs Rd in patients with ≥ 2 prior lines of therapy (HR, 0. 69) HR, hazard ratio; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone.

ASPIRE TOC Summary: Primary Outcome Second Line Carfilzomib by Prior Exposure to Bortezomib • Carfilzomib-based regimens were effective at first relapse compared to standard of care, regardless of prior exposure to bortezomib • KRd improved PFS vs Rd at first relapse after bortezomib-based frontline therapy, providing 13. 4 additional months without disease progression (29. 3 months vs 15. 9 months) Cytogenetic Risk • In the high-risk group, treatment with KRd resulted in a median PFS of nearly 2 years, which was a 9 -month improvement vs Rd (HR, 0. 7) • Treatment with KRd also led to a 10 -month improvement in median PFS vs Rd in patients with standard-risk cytogenetics (HR, 0. 66) • PFS improvement with KRd was observed regardless of cytogenetic risk status Age • KRd led to an 11 -month improvement in median PFS vs Rd in patients aged < 70 years (28. 6 months vs 17. 6 months, respectively; HR, 0. 67), and an 8 -month improvement in median PFS vs Rd in patients aged ≥ 70 years (23. 8 months vs 16. 0 months, respectively; HR, 0. 74) Early and Late Relapse • Both early and late relapse patients who received KRd had improved PFS compared to those who received Rd HR, hazard ratio; KRd, carfilzomib, lenalidomide, and dexamethasone; PFS, progression-free survival; Rd, lenalidomide and dexamethasone.

ASPIRE TOC All studies ASPIRE Secondary Endpoints Overall Survival Overall Response Rate Quality of Life

ASPIRE TOC All studies ASPIRE Secondary Endpoint: Overall Survival Final OS Analysis OS in subgroups Summary

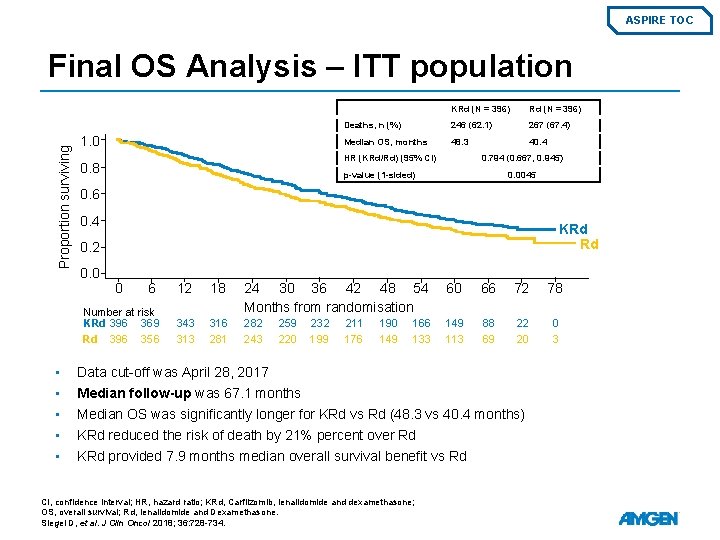
ASPIRE TOC Proportion surviving Final OS Analysis – ITT population 1. 0 Rd (N = 396) Deaths, n (%) 246 (62. 1) 267 (67. 4) Median OS, months 48. 3 40. 4 HR (KRd/Rd) (95% CI) 0. 8 0. 794 (0. 667, 0. 945) p-value (1 -sided) 0. 0045 0. 6 0. 4 KRd Rd 0. 2 0. 0 0 6 Number at risk KRd 396 369 Rd 396 356 • • • KRd (N = 396) 12 18 24 30 36 42 48 54 Months from randomisation 60 66 72 78 343 316 281 282 243 149 113 88 69 22 20 0 3 259 220 232 199 211 176 190 149 166 133 Data cut-off was April 28, 2017 Median follow-up was 67. 1 months Median OS was significantly longer for KRd vs Rd (48. 3 vs 40. 4 months) KRd reduced the risk of death by 21% percent over Rd KRd provided 7. 9 months median overall survival benefit vs Rd CI, confidence interval; HR, hazard ratio; KRd, Carfilzomib, lenalidomide and dexamethasone; OS, overall survival; Rd, lenalidomide and Dexamethasone. Siegel D, et al. J Clin Oncol 2018; 36: 728 -734.

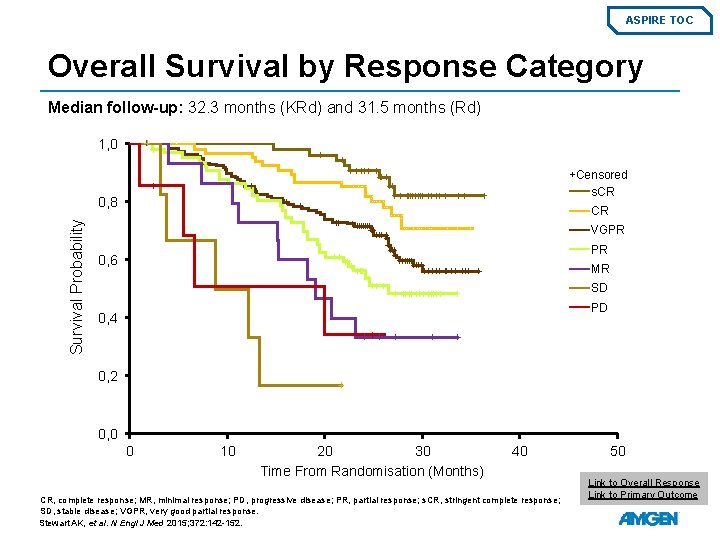
ASPIRE TOC Overall Survival by Response Category Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) 1, 0 +Censored s. CR Survival Probability 0, 8 CR VGPR PR 0, 6 MR SD PD 0, 4 0, 2 0, 0 0 10 20 30 Time From Randomisation (Months) 40 CR, complete response; MR, minimal response; PD, progressive disease; PR, partial response; s. CR, stringent complete response; SD, stable disease; VGPR, very good partial response. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152. 50 Link to Overall Response Link to Primary Outcome

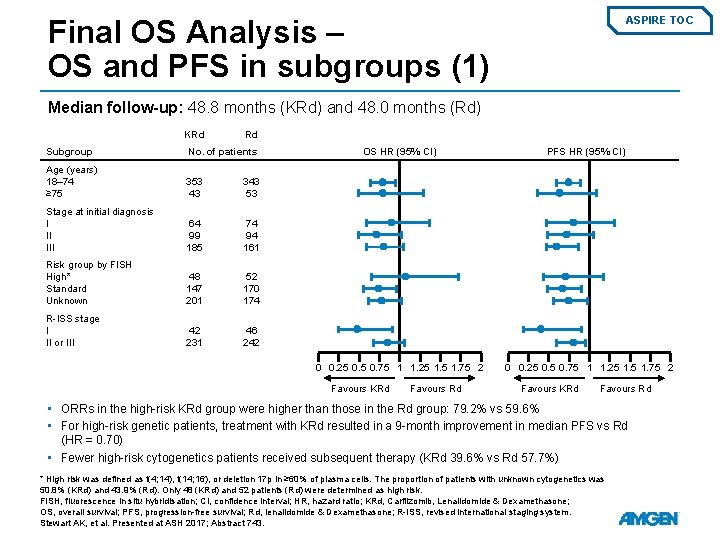
Final OS Analysis – OS and PFS in subgroups (1) ASPIRE TOC Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) KRd Rd Subgroup No. of patients Age (years) 18– 74 ≥ 75 353 43 343 53 Stage at initial diagnosis I II III 64 99 185 74 94 161 Risk group by FISH High* Standard Unknown 48 147 201 52 170 174 R-ISS stage I II or III 42 231 46 242 OS HR (95% CI) 0 0. 25 0. 75 1 1. 25 1. 75 2 Favours KRd Favours Rd PFS HR (95% CI) 0 0. 25 0. 75 1 1. 25 1. 75 2 Favours KRd Favours Rd • ORRs in the high-risk KRd group were higher than those in the Rd group: 79. 2% vs 59. 6% • For high-risk genetic patients, treatment with KRd resulted in a 9 -month improvement in median PFS vs Rd (HR = 0. 70) • Fewer high-risk cytogenetics patients received subsequent therapy (KRd 39. 6% vs Rd 57. 7%) * High risk was defined as t(4; 14), t(14; 16), or deletion 17 p in ≥ 60% of plasma cells. The proportion of patients with unknown cytogenetics was 50. 8% (KRd) and 43. 9% (Rd). Only 48 (KRd) and 52 patients (Rd) were determined as high risk. FISH, fluorescence in situ hybridisation; CI, confidence interval; HR, hazard ratio; KRd, Carfilzomib, Lenalidomide & Dexamethasone; OS, overall survival; PFS, progression-free survival; Rd, lenalidomide & Dexamethasone; R-ISS, revised international staging system. Stewart AK, et al. Presented at ASH 2017; Abstract 743.

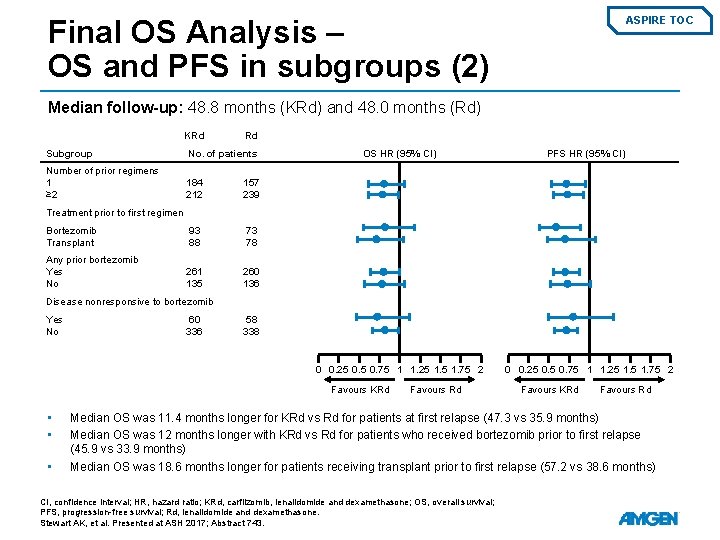
Final OS Analysis – OS and PFS in subgroups (2) ASPIRE TOC Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) KRd Rd Subgroup No. of patients Number of prior regimens 1 ≥ 2 184 212 157 239 Bortezomib Transplant 93 88 73 78 Any prior bortezomib Yes No 261 135 260 136 OS HR (95% CI) PFS HR (95% CI) Treatment prior to first regimen Disease nonresponsive to bortezomib Yes No 60 336 58 338 0 0. 25 0. 75 1 1. 25 1. 75 2 Favours KRd • • • Favours Rd 0 0. 25 0. 75 1 1. 25 1. 75 2 Favours KRd Favours Rd Median OS was 11. 4 months longer for KRd vs Rd for patients at first relapse (47. 3 vs 35. 9 months) Median OS was 12 months longer with KRd vs Rd for patients who received bortezomib prior to first relapse (45. 9 vs 33. 9 months) Median OS was 18. 6 months longer for patients receiving transplant prior to first relapse (57. 2 vs 38. 6 months) CI, confidence interval; HR, hazard ratio; KRd, carfilzomib, lenalidomide and dexamethasone; OS, overall survival; PFS, progression-free survival; Rd, lenalidomide and dexamethasone. Stewart AK, et al. Presented at ASH 2017; Abstract 743.

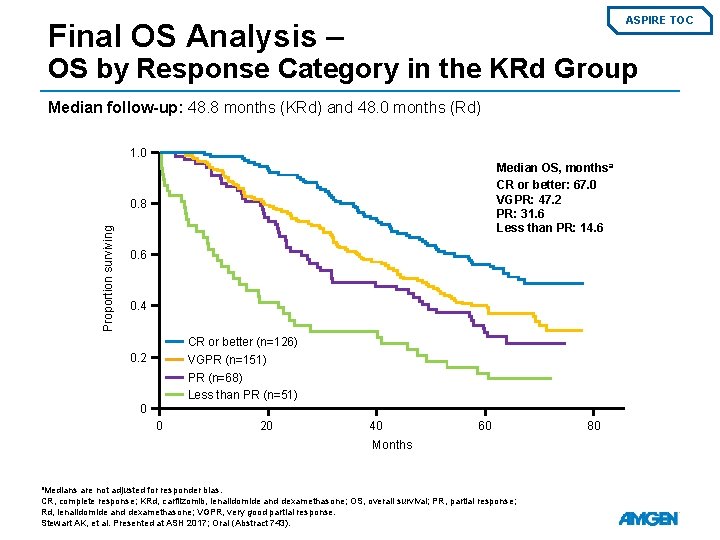
ASPIRE TOC Final OS Analysis – OS by Response Category in the KRd Group Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) 1. 0 Median OS, months a CR or better: 67. 0 VGPR: 47. 2 PR: 31. 6 Less than PR: 14. 6 Proportion surviving 0. 8 0. 6 0. 4 CR or better (n=126) VGPR (n=151) PR (n=68) Less than PR (n=51) 0. 2 0 0 20 40 60 Months a. Medians are not adjusted for responder bias. CR, complete response; KRd, carfilzomib, lenalidomide and dexamethasone; OS, overall survival; PR, partial response; Rd, lenalidomide and dexamethasone; VGPR, very good partial response. Stewart AK, et al. Presented at ASH 2017; Oral (Abstract 743). 80

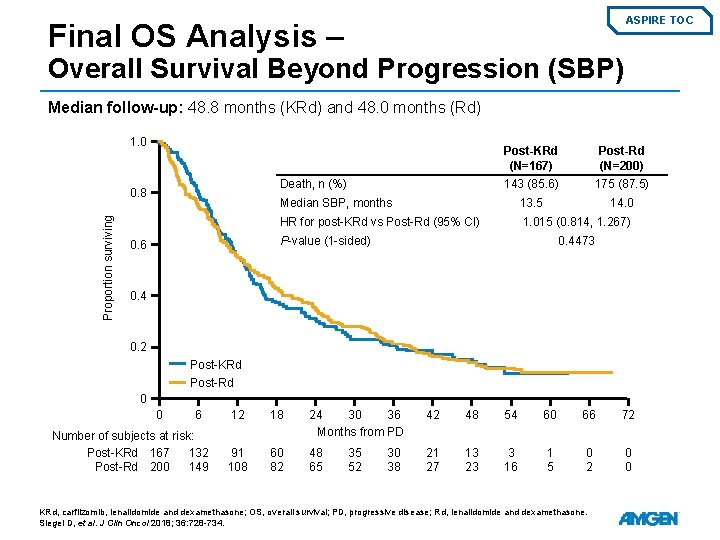
ASPIRE TOC Final OS Analysis – Overall Survival Beyond Progression (SBP) Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) 1. 0 Post-Rd (N=200) 143 (85. 6) 175 (87. 5) Median SBP, months 13. 5 14. 0 HR for post-KRd vs Post-Rd (95% CI) 1. 015 (0. 814, 1. 267) Death, n (%) 0. 8 Proportion surviving Post-KRd (N=167) P-value (1 -sided) 0. 6 0. 4473 0. 4 0. 2 Post-KRd Post-Rd 0 0 6 Number of subjects at risk: Post-KRd 167 132 Post-Rd 200 149 12 18 24 30 36 Months from PD 42 48 54 60 66 72 91 108 60 82 48 65 21 27 13 23 3 16 1 5 0 2 0 0 35 52 30 38 KRd, carfilzomib, lenalidomide and dexamethasone; OS, overall survival; PD, progressive disease; Rd, lenalidomide and dexamethasone. Siegel D, et al. J Clin Oncol 2018; 36: 728 -734.

ASPIRE TOC Summary: Overall Survival • Interim OS analysis showed trend in OS favouring KRd group; Kaplan-Meier 24 month OS rates 73. 3% (KRd) vs 65. 0% (Rd) • In the final OS analysis, KRd demonstrated a statistically significant and clinically meaningful reduction in the risk of death vs Rd, improving median OS by 7. 9 months (48. 3 vs 40. 4 months; HR, 0. 79, P=0. 0045) • KRd reduced the risk of death by 21% over Rd • The KRd efficacy advantage is most pronounced at first relapse, with an 11 -month improvement in median OS (47. 3 vs 35. 9 months; HR, 0. 81) • Treatment with KRd did not compromise OS after relapse KRd, carfilzomib, lenalidomide, and dexamethasone; OS, overall survival; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152; Siegel D, et al. J Clin Oncol 2018; 36: 728 -734.

ASPIRE TOC All studies ASPIRE Adverse Events Safety Population Cardiac Events by Treatment Cycle Safety Population: Updated Safety Prior Lines of Therapy Prior ASCT Cytogenetic Risk Age Patients with Early Progression During Prior Therapy Early and Late Relapse Patients Rates of Peripheral Neuropathy Time to First Onset of AEs Summary

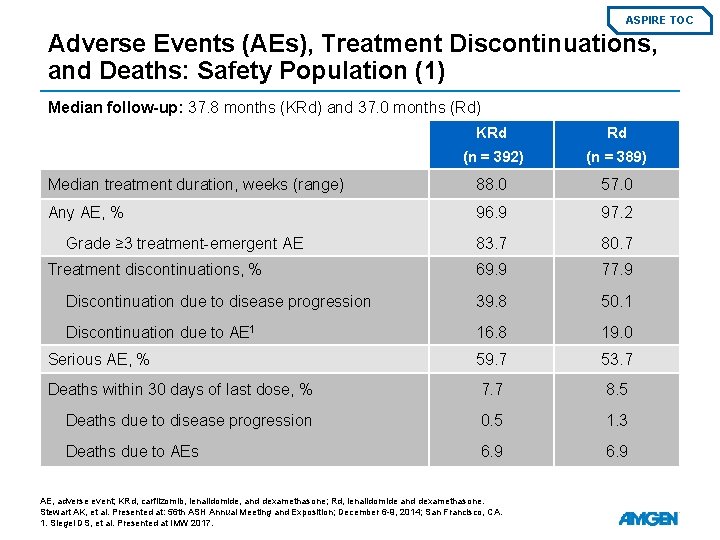
ASPIRE TOC Adverse Events (AEs), Treatment Discontinuations, and Deaths: Safety Population (1) Median follow-up: 37. 8 months (KRd) and 37. 0 months (Rd) KRd Rd (n = 392) (n = 389) Median treatment duration, weeks (range) 88. 0 57. 0 Any AE, % 96. 9 97. 2 83. 7 80. 7 69. 9 77. 9 Discontinuation due to disease progression 39. 8 50. 1 Discontinuation due to AE 1 16. 8 19. 0 Serious AE, % 59. 7 53. 7 Deaths within 30 days of last dose, % 7. 7 8. 5 Deaths due to disease progression 0. 5 1. 3 Deaths due to AEs 6. 9 Grade ≥ 3 treatment-emergent AE Treatment discontinuations, % AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Stewart AK, et al. Presented at: 56 th ASH Annual Meeting and Exposition; December 6 -9, 2014; San Francisco, CA. 1. Siegel DS, et al. Presented at IMW 2017.

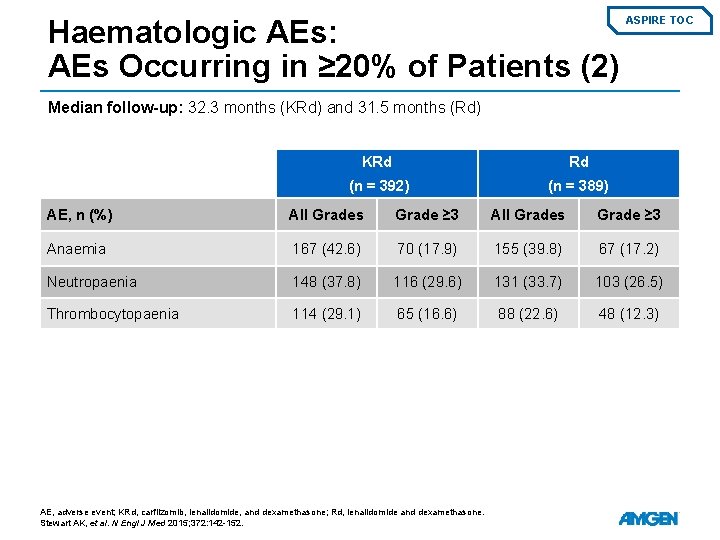
Haematologic AEs: AEs Occurring in ≥ 20% of Patients (2) ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) KRd Rd (n = 392) (n = 389) AE, n (%) All Grades Grade ≥ 3 Anaemia 167 (42. 6) 70 (17. 9) 155 (39. 8) 67 (17. 2) Neutropaenia 148 (37. 8) 116 (29. 6) 131 (33. 7) 103 (26. 5) Thrombocytopaenia 114 (29. 1) 65 (16. 6) 88 (22. 6) 48 (12. 3) AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152.

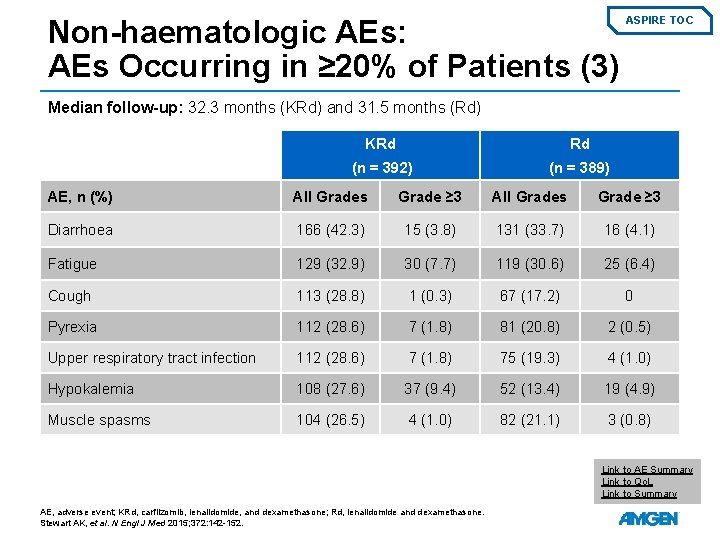
Non-haematologic AEs: AEs Occurring in ≥ 20% of Patients (3) ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) KRd Rd (n = 392) (n = 389) AE, n (%) All Grades Grade ≥ 3 Diarrhoea 166 (42. 3) 15 (3. 8) 131 (33. 7) 16 (4. 1) Fatigue 129 (32. 9) 30 (7. 7) 119 (30. 6) 25 (6. 4) Cough 113 (28. 8) 1 (0. 3) 67 (17. 2) 0 Pyrexia 112 (28. 6) 7 (1. 8) 81 (20. 8) 2 (0. 5) Upper respiratory tract infection 112 (28. 6) 7 (1. 8) 75 (19. 3) 4 (1. 0) Hypokalemia 108 (27. 6) 37 (9. 4) 52 (13. 4) 19 (4. 9) Muscle spasms 104 (26. 5) 4 (1. 0) 82 (21. 1) 3 (0. 8) Link to AE Summary Link to Qo. L Link to Summary AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Stewart AK, et al. N Engl J Med 2015; 372: 142 -152.

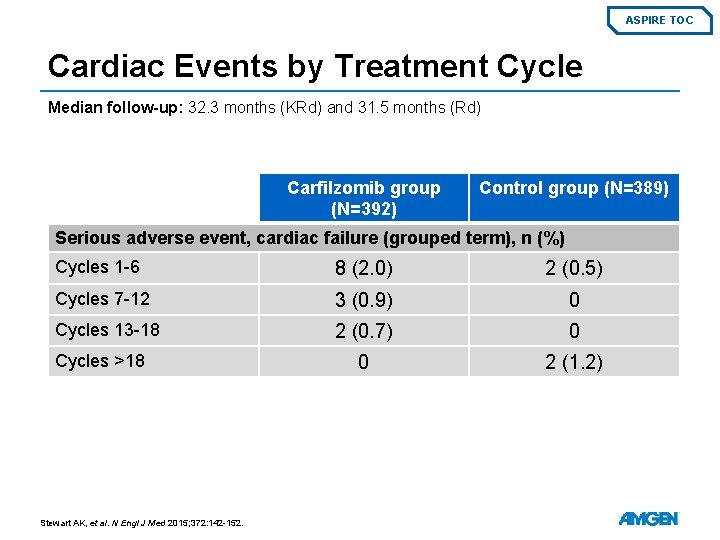
ASPIRE TOC Cardiac Events by Treatment Cycle Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Carfilzomib group (N=392) Control group (N=389) Serious adverse event, cardiac failure (grouped term), n (%) Cycles 1 -6 8 (2. 0) 2 (0. 5) Cycles 7 -12 3 (0. 9) 0 Cycles 13 -18 2 (0. 7) 0 0 2 (1. 2) Cycles >18 Stewart AK, et al. N Engl J Med 2015; 372: 142 -152.

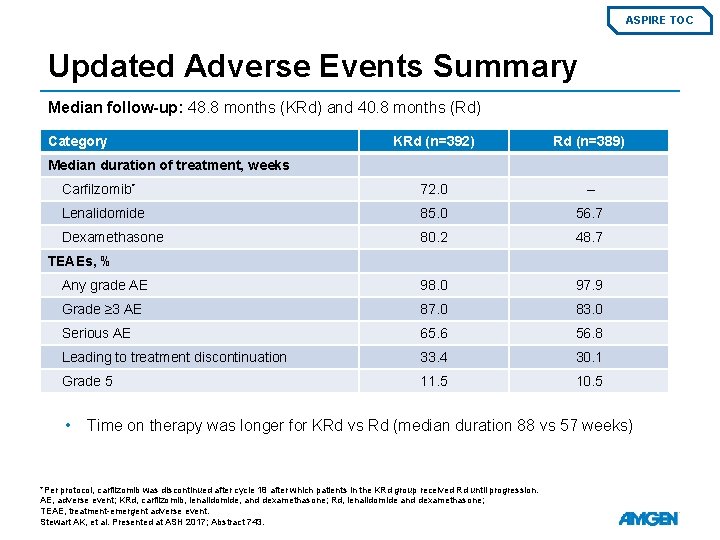
ASPIRE TOC Updated Adverse Events Summary Median follow-up: 48. 8 months (KRd) and 40. 8 months (Rd) Category KRd (n=392) Rd (n=389) Carfilzomib* 72. 0 – Lenalidomide 85. 0 56. 7 Dexamethasone 80. 2 48. 7 Any grade AE 98. 0 97. 9 Grade ≥ 3 AE 87. 0 83. 0 Serious AE 65. 6 56. 8 Leading to treatment discontinuation 33. 4 30. 1 Grade 5 11. 5 10. 5 Median duration of treatment, weeks TEAEs, % • Time on therapy was longer for KRd vs Rd (median duration 88 vs 57 weeks) *Per protocol, carfilzomib was discontinued after cycle 18 after which patients in the KRd group received Rd until progression. AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; TEAE, treatment-emergent adverse event. Stewart AK, et al. Presented at ASH 2017; Abstract 743.

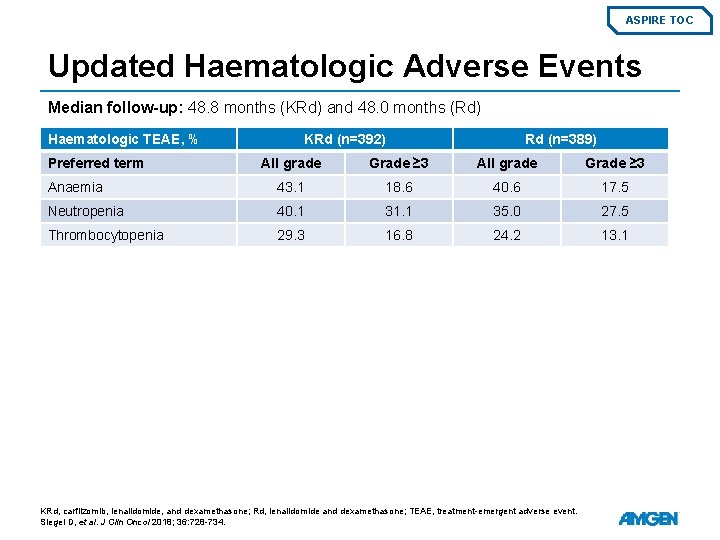
ASPIRE TOC Updated Haematologic Adverse Events Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) Haematologic TEAE, % Preferred term KRd (n=392) Rd (n=389) All grade Grade ≥ 3 Anaemia 43. 1 18. 6 40. 6 17. 5 Neutropenia 40. 1 31. 1 35. 0 27. 5 Thrombocytopenia 29. 3 16. 8 24. 2 13. 1 KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; TEAE, treatment-emergent adverse event. Siegel D, et al. J Clin Oncol 2018; 36: 728 -734.

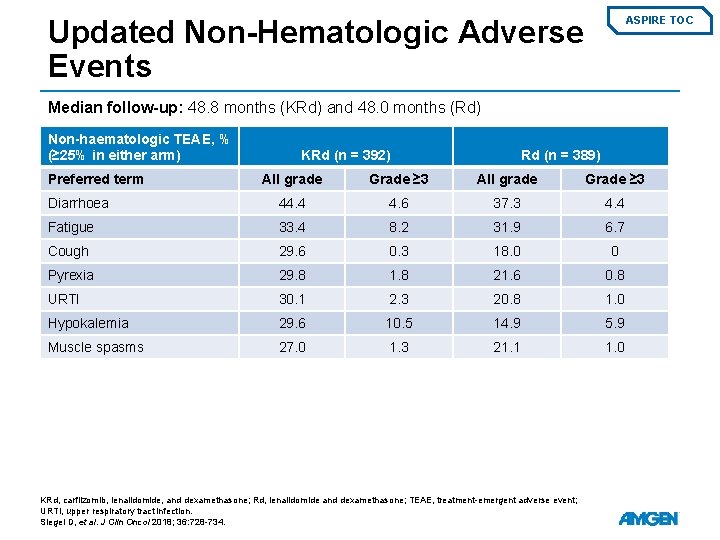
Updated Non-Hematologic Adverse Events ASPIRE TOC Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) Non-haematologic TEAE, % (≥ 25% in either arm) Preferred term KRd (n = 392) Rd (n = 389) All grade Grade ≥ 3 Diarrhoea 44. 4 4. 6 37. 3 4. 4 Fatigue 33. 4 8. 2 31. 9 6. 7 Cough 29. 6 0. 3 18. 0 0 Pyrexia 29. 8 1. 8 21. 6 0. 8 URTI 30. 1 2. 3 20. 8 1. 0 Hypokalemia 29. 6 10. 5 14. 9 5. 9 Muscle spasms 27. 0 1. 3 21. 1 1. 0 KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; TEAE, treatment-emergent adverse event; URTI, upper respiratory tract infection. Siegel D, et al. J Clin Oncol 2018; 36: 728 -734.

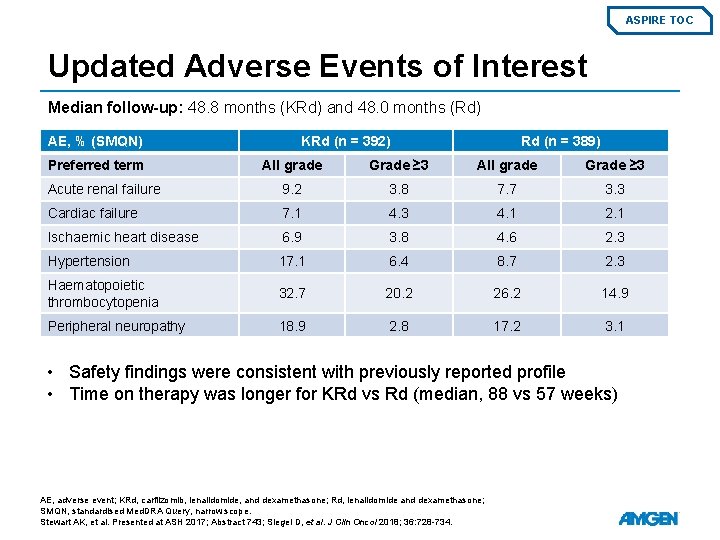
ASPIRE TOC Updated Adverse Events of Interest Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) AE, % (SMQN) Preferred term KRd (n = 392) Rd (n = 389) All grade Grade ≥ 3 Acute renal failure 9. 2 3. 8 7. 7 3. 3 Cardiac failure 7. 1 4. 3 4. 1 2. 1 Ischaemic heart disease 6. 9 3. 8 4. 6 2. 3 Hypertension 17. 1 6. 4 8. 7 2. 3 Haematopoietic thrombocytopenia 32. 7 20. 2 26. 2 14. 9 Peripheral neuropathy 18. 9 2. 8 17. 2 3. 1 • Safety findings were consistent with previously reported profile • Time on therapy was longer for KRd vs Rd (median, 88 vs 57 weeks) AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; SMQN, standardised Med. DRA Query, narrow scope. Stewart AK, et al. Presented at ASH 2017; Abstract 743; Siegel D, et al. J Clin Oncol 2018; 36: 728 -734.

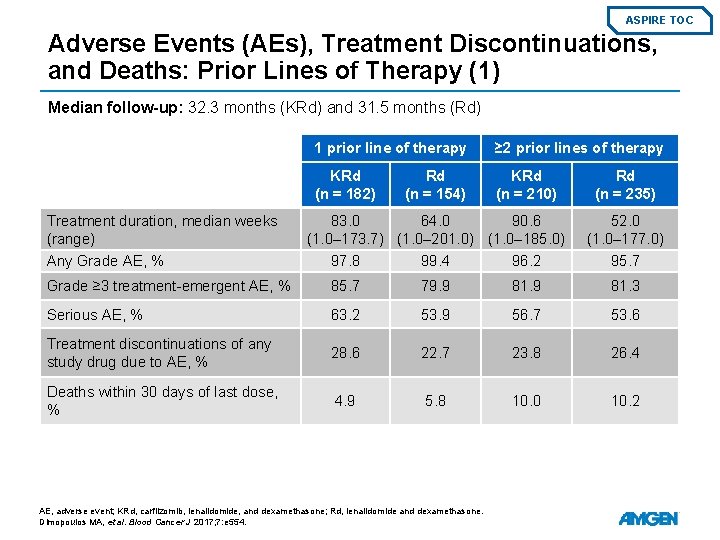
ASPIRE TOC Adverse Events (AEs), Treatment Discontinuations, and Deaths: Prior Lines of Therapy (1) Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Treatment duration, median weeks (range) 1 prior line of therapy ≥ 2 prior lines of therapy KRd (n = 182) KRd (n = 210) Rd (n = 154) 83. 0 64. 0 90. 6 (1. 0– 173. 7) (1. 0– 201. 0) (1. 0– 185. 0) Rd (n = 235) 52. 0 (1. 0– 177. 0) Any Grade AE, % 97. 8 99. 4 96. 2 95. 7 Grade ≥ 3 treatment-emergent AE, % 85. 7 79. 9 81. 3 Serious AE, % 63. 2 53. 9 56. 7 53. 6 Treatment discontinuations of any study drug due to AE, % 28. 6 22. 7 23. 8 26. 4 Deaths within 30 days of last dose, % 4. 9 5. 8 10. 0 10. 2 AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554.

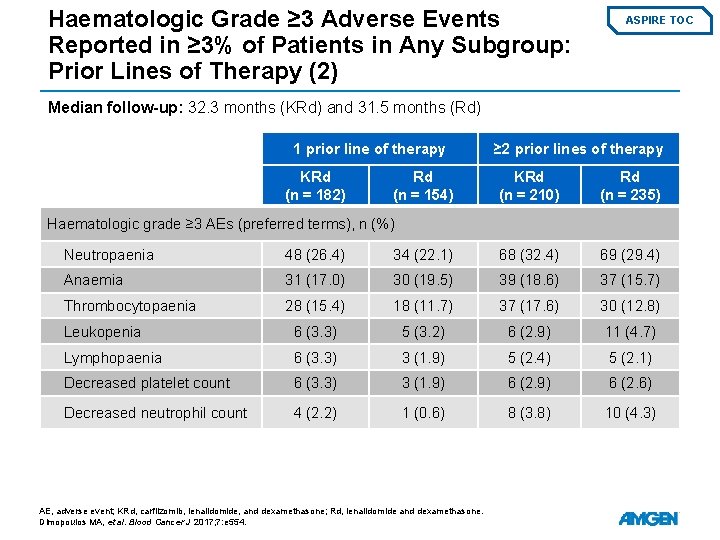
Haematologic Grade ≥ 3 Adverse Events Reported in ≥ 3% of Patients in Any Subgroup: Prior Lines of Therapy (2) ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) 1 prior line of therapy KRd (n = 182) Rd (n = 154) ≥ 2 prior lines of therapy KRd (n = 210) Rd (n = 235) Haematologic grade ≥ 3 AEs (preferred terms), n (%) Neutropaenia 48 (26. 4) 34 (22. 1) 68 (32. 4) 69 (29. 4) Anaemia 31 (17. 0) 30 (19. 5) 39 (18. 6) 37 (15. 7) Thrombocytopaenia 28 (15. 4) 18 (11. 7) 37 (17. 6) 30 (12. 8) Leukopenia 6 (3. 3) 5 (3. 2) 6 (2. 9) 11 (4. 7) Lymphopaenia 6 (3. 3) 3 (1. 9) 5 (2. 4) 5 (2. 1) Decreased platelet count 6 (3. 3) 3 (1. 9) 6 (2. 6) Decreased neutrophil count 4 (2. 2) 1 (0. 6) 8 (3. 8) 10 (4. 3) AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554.

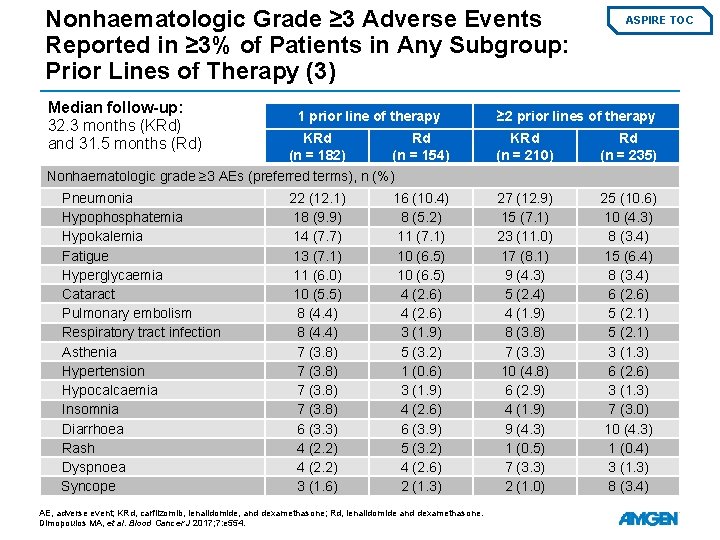
Nonhaematologic Grade ≥ 3 Adverse Events Reported in ≥ 3% of Patients in Any Subgroup: Prior Lines of Therapy (3) Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) 1 prior line of therapy KRd (n = 182) Rd (n = 154) ASPIRE TOC ≥ 2 prior lines of therapy KRd (n = 210) Rd (n = 235) 27 (12. 9) 15 (7. 1) 23 (11. 0) 17 (8. 1) 9 (4. 3) 5 (2. 4) 4 (1. 9) 8 (3. 8) 7 (3. 3) 10 (4. 8) 6 (2. 9) 4 (1. 9) 9 (4. 3) 1 (0. 5) 7 (3. 3) 2 (1. 0) 25 (10. 6) 10 (4. 3) 8 (3. 4) 15 (6. 4) 8 (3. 4) 6 (2. 6) 5 (2. 1) 3 (1. 3) 6 (2. 6) 3 (1. 3) 7 (3. 0) 10 (4. 3) 1 (0. 4) 3 (1. 3) 8 (3. 4) Nonhaematologic grade ≥ 3 AEs (preferred terms), n (%) Pneumonia Hypophosphatemia Hypokalemia Fatigue Hyperglycaemia Cataract Pulmonary embolism Respiratory tract infection Asthenia Hypertension Hypocalcaemia Insomnia Diarrhoea Rash Dyspnoea Syncope 22 (12. 1) 18 (9. 9) 14 (7. 7) 13 (7. 1) 11 (6. 0) 10 (5. 5) 8 (4. 4) 7 (3. 8) 6 (3. 3) 4 (2. 2) 3 (1. 6) 16 (10. 4) 8 (5. 2) 11 (7. 1) 10 (6. 5) 4 (2. 6) 3 (1. 9) 5 (3. 2) 1 (0. 6) 3 (1. 9) 4 (2. 6) 6 (3. 9) 5 (3. 2) 4 (2. 6) 2 (1. 3) AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554.

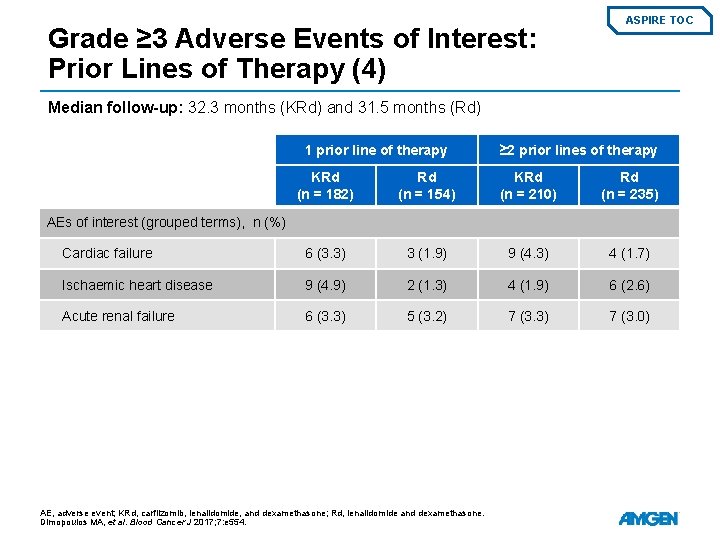
Grade ≥ 3 Adverse Events of Interest: Prior Lines of Therapy (4) ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) 1 prior line of therapy ≥ 2 prior lines of therapy KRd (n = 182) Rd (n = 154) KRd (n = 210) Rd (n = 235) Cardiac failure 6 (3. 3) 3 (1. 9) 9 (4. 3) 4 (1. 7) Ischaemic heart disease 9 (4. 9) 2 (1. 3) 4 (1. 9) 6 (2. 6) Acute renal failure 6 (3. 3) 5 (3. 2) 7 (3. 3) 7 (3. 0) AEs of interest (grouped terms), n (%) AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Dimopoulos MA, et al. Blood Cancer J 2017; 7: e 554.

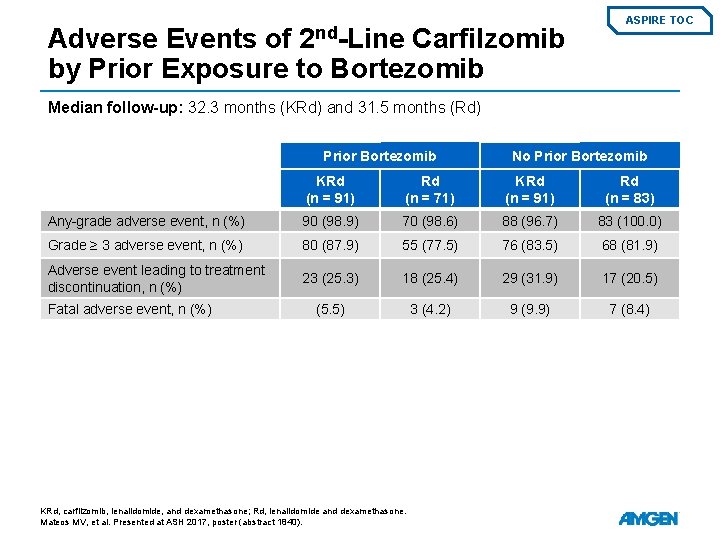
2 nd-Line Adverse Events of Carfilzomib by Prior Exposure to Bortezomib ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Prior Bortezomib No Prior Bortezomib KRd (n = 91) Rd (n = 71) KRd (n = 91) Rd (n = 83) Any-grade adverse event, n (%) 90 (98. 9) 70 (98. 6) 88 (96. 7) 83 (100. 0) Grade ≥ 3 adverse event, n (%) 80 (87. 9) 55 (77. 5) 76 (83. 5) 68 (81. 9) Adverse event leading to treatment discontinuation, n (%) 23 (25. 3) 18 (25. 4) 29 (31. 9) 17 (20. 5) (5. 5) 3 (4. 2) 9 (9. 9) 7 (8. 4) Fatal adverse event, n (%) KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Mateos MV, et al. Presented at ASH 2017, poster (abstract 1840).

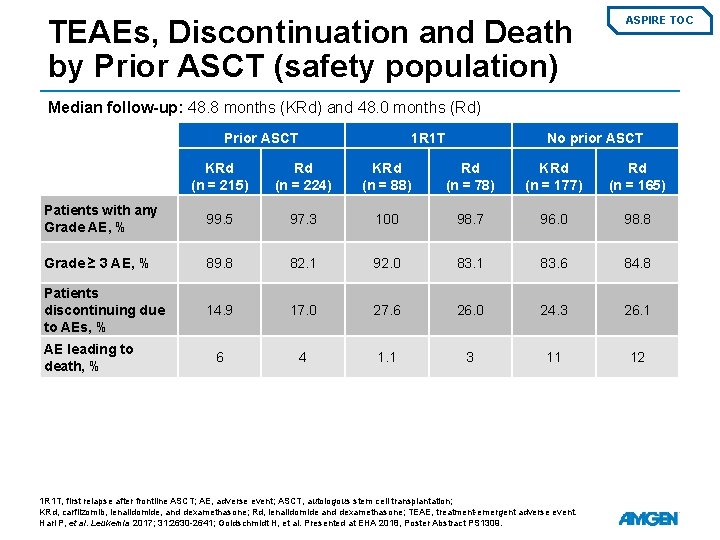
TEAEs, Discontinuation and Death by Prior ASCT (safety population) ASPIRE TOC Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) Prior ASCT 1 R 1 T No prior ASCT KRd (n = 215) Rd (n = 224) KRd (n = 88) Rd (n = 78) KRd (n = 177) Rd (n = 165) Patients with any Grade AE, % 99. 5 97. 3 100 98. 7 96. 0 98. 8 Grade ≥ 3 AE, % 89. 8 82. 1 92. 0 83. 1 83. 6 84. 8 Patients discontinuing due to AEs, % 14. 9 17. 0 27. 6 26. 0 24. 3 26. 1 6 4 1. 1 3 11 12 AE leading to death, % 1 R 1 T, first relapse after frontline ASCT; AE, adverse event; ASCT, autologous stem cell transplantation; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; TEAE, treatment-emergent adverse event. Hari P, et al. Leukemia 2017; 31: 2630 -2641; Goldschmidt H, et al. Presented at EHA 2018, Poster Abstract PS 1309.

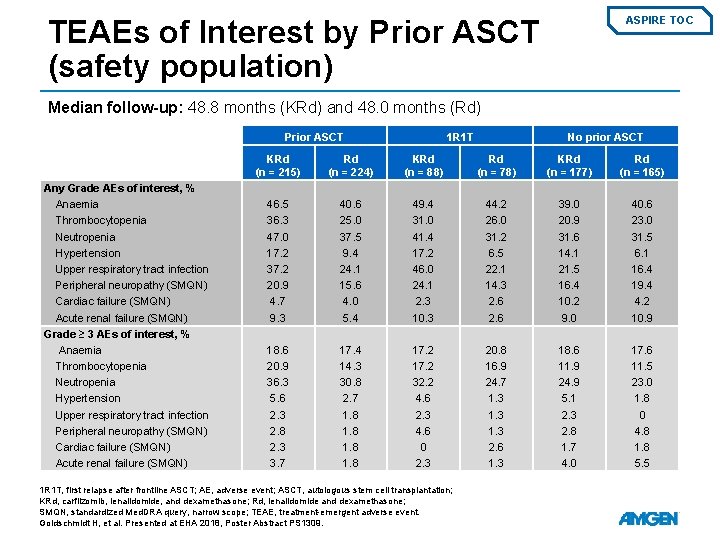
TEAEs of Interest by Prior ASCT (safety population) ASPIRE TOC Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) Prior ASCT Any Grade AEs of interest, % Anaemia Thrombocytopenia Neutropenia Hypertension Upper respiratory tract infection Peripheral neuropathy (SMQN) Cardiac failure (SMQN) Acute renal failure (SMQN) Grade ≥ 3 AEs of interest, % Anaemia Thrombocytopenia Neutropenia Hypertension Upper respiratory tract infection Peripheral neuropathy (SMQN) Cardiac failure (SMQN) Acute renal failure (SMQN) 1 R 1 T No prior ASCT KRd (n = 215) Rd (n = 224) KRd (n = 88) Rd (n = 78) KRd (n = 177) Rd (n = 165) 46. 5 36. 3 47. 0 17. 2 37. 2 20. 9 4. 7 9. 3 40. 6 25. 0 37. 5 9. 4 24. 1 15. 6 4. 0 5. 4 49. 4 31. 0 41. 4 17. 2 46. 0 24. 1 2. 3 10. 3 44. 2 26. 0 31. 2 6. 5 22. 1 14. 3 2. 6 39. 0 20. 9 31. 6 14. 1 21. 5 16. 4 10. 2 9. 0 40. 6 23. 0 31. 5 6. 1 16. 4 19. 4 4. 2 10. 9 18. 6 20. 9 36. 3 5. 6 2. 3 2. 8 2. 3 3. 7 17. 4 14. 3 30. 8 2. 7 1. 8 17. 2 32. 2 4. 6 2. 3 4. 6 0 2. 3 20. 8 16. 9 24. 7 1. 3 2. 6 1. 3 18. 6 11. 9 24. 9 5. 1 2. 3 2. 8 1. 7 4. 0 17. 6 11. 5 23. 0 1. 8 0 4. 8 1. 8 5. 5 1 R 1 T, first relapse after frontline ASCT; AE, adverse event; ASCT, autologous stem cell transplantation; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone; SMQN, standardized Med. DRA query, narrow scope; TEAE, treatment-emergent adverse event. Goldschmidt H, et al. Presented at EHA 2018, Poster Abstract PS 1309.

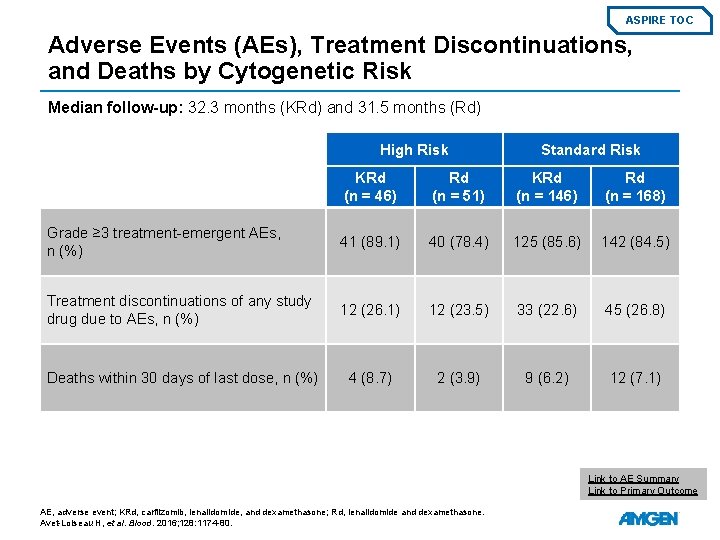
ASPIRE TOC Adverse Events (AEs), Treatment Discontinuations, and Deaths by Cytogenetic Risk Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) High Risk Standard Risk KRd (n = 46) Rd (n = 51) KRd (n = 146) Rd (n = 168) Grade ≥ 3 treatment-emergent AEs, n (%) 41 (89. 1) 40 (78. 4) 125 (85. 6) 142 (84. 5) Treatment discontinuations of any study drug due to AEs, n (%) 12 (26. 1) 12 (23. 5) 33 (22. 6) 45 (26. 8) Deaths within 30 days of last dose, n (%) 4 (8. 7) 2 (3. 9) 9 (6. 2) 12 (7. 1) Link to AE Summary Link to Primary Outcome AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Avet-Loiseau H, et al. Blood. 2016; 128: 1174 -80.

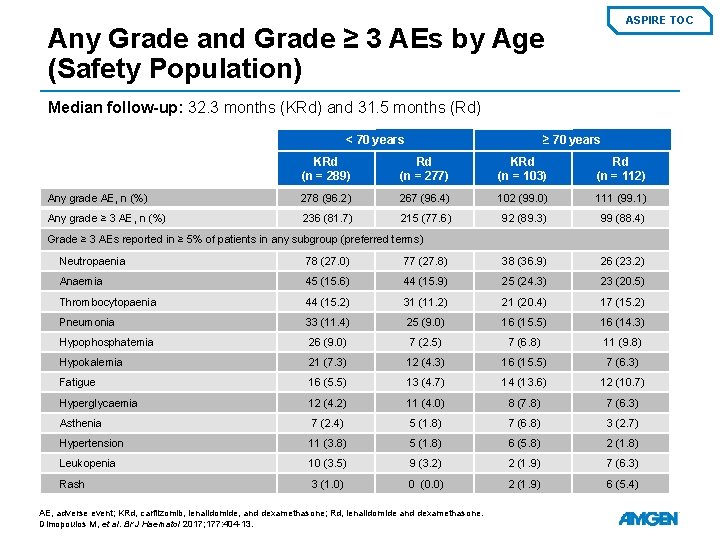
ASPIRE TOC Any Grade and Grade ≥ 3 AEs by Age (Safety Population) Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) < 70 years ≥ 70 years KRd (n = 289) Rd (n = 277) KRd (n = 103) Rd (n = 112) Any grade AE, n (%) 278 (96. 2) 267 (96. 4) 102 (99. 0) 111 (99. 1) Any grade ≥ 3 AE, n (%) 236 (81. 7) 215 (77. 6) 92 (89. 3) 99 (88. 4) Grade ≥ 3 AEs reported in ≥ 5% of patients in any subgroup (preferred terms) Neutropaenia 78 (27. 0) 77 (27. 8) 38 (36. 9) 26 (23. 2) Anaemia 45 (15. 6) 44 (15. 9) 25 (24. 3) 23 (20. 5) Thrombocytopaenia 44 (15. 2) 31 (11. 2) 21 (20. 4) 17 (15. 2) Pneumonia 33 (11. 4) 25 (9. 0) 16 (15. 5) 16 (14. 3) Hypophosphatemia 26 (9. 0) 7 (2. 5) 7 (6. 8) 11 (9. 8) Hypokalemia 21 (7. 3) 12 (4. 3) 16 (15. 5) 7 (6. 3) Fatigue 16 (5. 5) 13 (4. 7) 14 (13. 6) 12 (10. 7) Hyperglycaemia 12 (4. 2) 11 (4. 0) 8 (7. 8) 7 (6. 3) Asthenia 7 (2. 4) 5 (1. 8) 7 (6. 8) 3 (2. 7) Hypertension 11 (3. 8) 5 (1. 8) 6 (5. 8) 2 (1. 8) Leukopenia 10 (3. 5) 9 (3. 2) 2 (1. 9) 7 (6. 3) Rash 3 (1. 0) 0 (0. 0) 2 (1. 9) 6 (5. 4) AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. Dimopoulos M, et al. Br J Haematol 2017; 177: 404 -13.

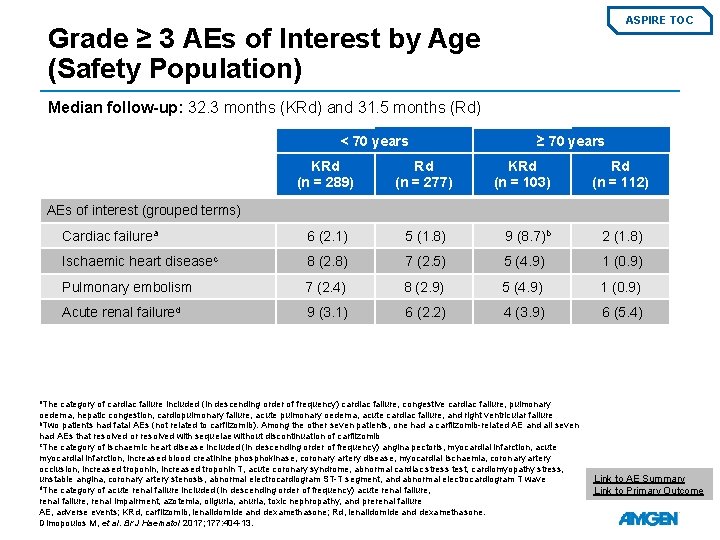
ASPIRE TOC Grade ≥ 3 AEs of Interest by Age (Safety Population) Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) < 70 years ≥ 70 years KRd (n = 289) Rd (n = 277) KRd (n = 103) Rd (n = 112) Cardiac failurea 6 (2. 1) 5 (1. 8) 9 (8. 7)b 2 (1. 8) Ischaemic heart diseasec 8 (2. 8) 7 (2. 5) 5 (4. 9) 1 (0. 9) Pulmonary embolism 7 (2. 4) 8 (2. 9) 5 (4. 9) 1 (0. 9) Acute renal failured 9 (3. 1) 6 (2. 2) 4 (3. 9) 6 (5. 4) AEs of interest (grouped terms) a. The category of cardiac failure included (in descending order of frequency) cardiac failure, congestive cardiac failure, pulmonary oedema, hepatic congestion, cardiopulmonary failure, acute pulmonary oedema, acute cardiac failure, and right ventricular failure b. Two patients had fatal AEs (not related to carfilzomib). Among the other seven patients, one had a carfilzomib-related AE and all seven had AEs that resolved or resolved with sequelae without discontinuation of carfilzomib c. The category of ischaemic heart disease included (in descending order of frequency) angina pectoris, myocardial infarction, acute myocardial infarction, increased blood creatinine phosphokinase, coronary artery disease, myocardial ischaemia, coronary artery occlusion, increased troponin T, acute coronary syndrome, abnormal cardiac stress test, cardiomyopathy stress, unstable angina, coronary artery stenosis, abnormal electrocardiogram ST-T segment, and abnormal electrocardiogram T wave d. The category of acute renal failure included (in descending order of frequency) acute renal failure, renal impairment, azotemia, oliguria, anuria, toxic nephropathy, and prerenal failure AE, adverse events; KRd, carfilzomib, lenalidomide and dexamethasone; Rd, lenalidomide and dexamethasone. Dimopoulos M, et al. Br J Haematol 2017; 177: 404 -13. Link to AE Summary Link to Primary Outcome

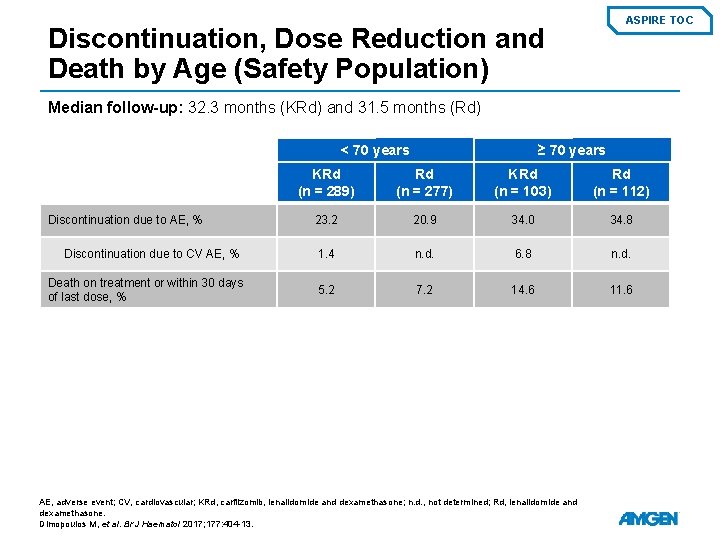
ASPIRE TOC Discontinuation, Dose Reduction and Death by Age (Safety Population) Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) < 70 years Discontinuation due to AE, % Discontinuation due to CV AE, % Death on treatment or within 30 days of last dose, % ≥ 70 years KRd (n = 289) Rd (n = 277) KRd (n = 103) Rd (n = 112) 23. 2 20. 9 34. 0 34. 8 1. 4 n. d. 6. 8 n. d. 5. 2 7. 2 14. 6 11. 6 AE, adverse event; CV, cardiovascular; KRd, carfilzomib, lenalidomide and dexamethasone; n. d. , not determined; Rd, lenalidomide and dexamethasone. Dimopoulos M, et al. Br J Haematol 2017; 177: 404 -13.

Safety in Patients with Early Disease Progression During Prior Therapy ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) AEs and treatment discontinuation due to AEs (Safety Population) • Some grade ≥ 3 adverse events occurred more frequently with KRd than with Rd among early relapsers or post-transplant early relapsers • Death while on study drug or within 30 days of last dose of study drug occurred with similar frequency between KRd and Rd in the early relapsers subgroup (10. 5% vs 11. 4%) and the posttransplant early relapsers subgroup (4. 3% vs 8. 7%) AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone. a. The category of cardiac failure included (in descending order of frequency) cardiac failure, congestive cardiac failure, pulmonary oedema, hepatic congestion, cardiopulmonary failure, acute pulmonary oedema, acute cardiac failure, and right ventricular failure. b. The category of ischaemic heart disease included (in descending order of frequency) angina pectoris, myocardial infarction, acute myocardial infarction, increased blood creatinine phosphokinase, coronary artery disease, myocardial ischaemia, coronary artery occlusion, increased troponin T, acute coronary syndrome, abnormal cardiac stress test, cardiomyopathy stress, unstable angina, coronary artery stenosis, abnormal electrocardiogram ST-T segment, and abnormal electrocardiogram T wave. c. The category of acute renal failure included (in descending order of frequency) acute renal failure, renal impairment, azotemia, oliguria, anuria, toxic nephropathy, and pre-renal failure. Ludwig et al. ASCO 2016, Chicago, IL, 8045.

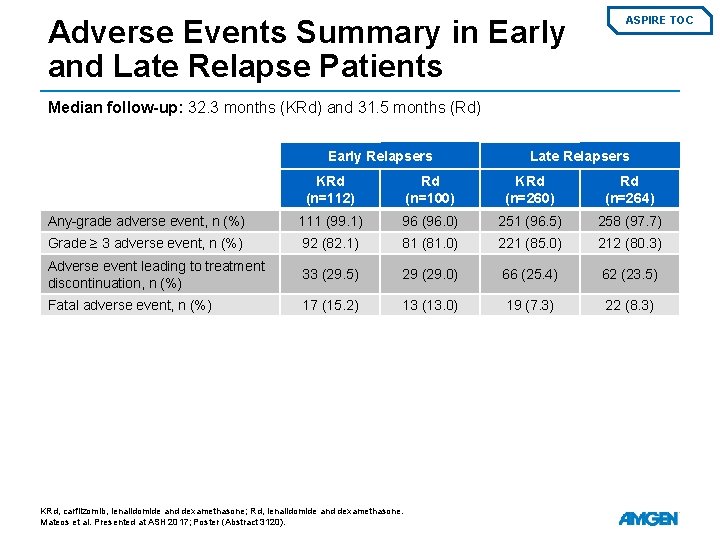
Adverse Events Summary in Early and Late Relapse Patients ASPIRE TOC Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Early Relapsers Late Relapsers KRd (n=112) Rd (n=100) KRd (n=260) Rd (n=264) Any-grade adverse event, n (%) 111 (99. 1) 96 (96. 0) 251 (96. 5) 258 (97. 7) Grade ≥ 3 adverse event, n (%) 92 (82. 1) 81 (81. 0) 221 (85. 0) 212 (80. 3) Adverse event leading to treatment discontinuation, n (%) 33 (29. 5) 29 (29. 0) 66 (25. 4) 62 (23. 5) Fatal adverse event, n (%) 17 (15. 2) 13 (13. 0) 19 (7. 3) 22 (8. 3) KRd, carfilzomib, lenalidomide and dexamethasone; Rd, lenalidomide and dexamethasone. Mateos et al. Presented at ASH 2017; Poster (Abstract 3120).

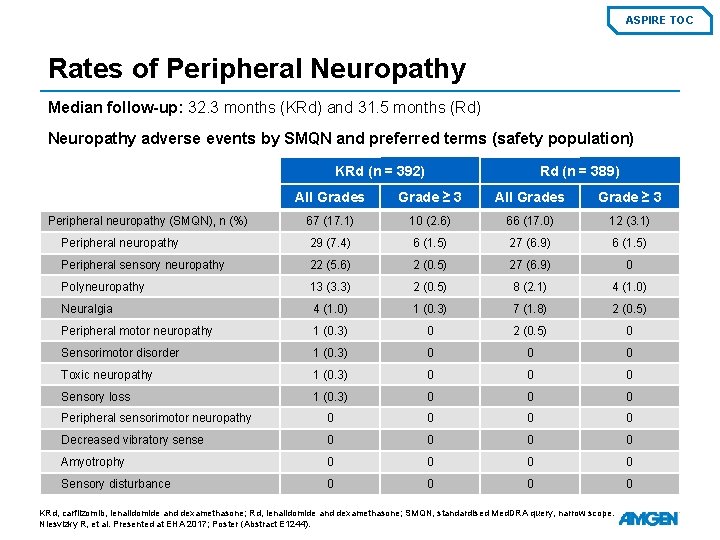
ASPIRE TOC Rates of Peripheral Neuropathy Median follow-up: 32. 3 months (KRd) and 31. 5 months (Rd) Neuropathy adverse events by SMQN and preferred terms (safety population) KRd (n = 392) Rd (n = 389) All Grades Grade ≥ 3 67 (17. 1) 10 (2. 6) 66 (17. 0) 12 (3. 1) Peripheral neuropathy 29 (7. 4) 6 (1. 5) 27 (6. 9) 6 (1. 5) Peripheral sensory neuropathy 22 (5. 6) 2 (0. 5) 27 (6. 9) 0 Polyneuropathy 13 (3. 3) 2 (0. 5) 8 (2. 1) 4 (1. 0) Neuralgia 4 (1. 0) 1 (0. 3) 7 (1. 8) 2 (0. 5) Peripheral motor neuropathy 1 (0. 3) 0 2 (0. 5) 0 Sensorimotor disorder 1 (0. 3) 0 0 0 Toxic neuropathy 1 (0. 3) 0 0 0 Sensory loss 1 (0. 3) 0 0 0 Peripheral sensorimotor neuropathy 0 0 Decreased vibratory sense 0 0 Amyotrophy 0 0 Sensory disturbance 0 0 Peripheral neuropathy (SMQN), n (%) KRd, carfilzomib, lenalidomide and dexamethasone; Rd, lenalidomide and dexamethasone; SMQN, standardised Med. DRA query, narrow scope. Niesvizky R, et al. Presented at EHA 2017; Poster (Abstract E 1244).

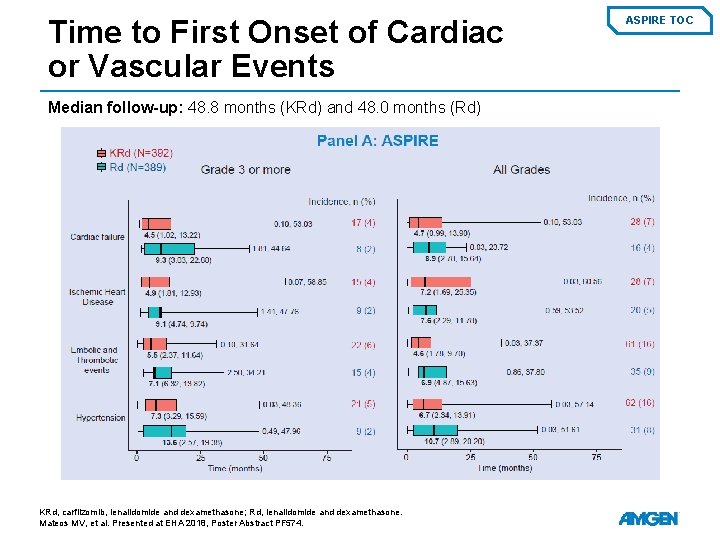
Time to First Onset of Cardiac or Vascular Events Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) KRd, carfilzomib, lenalidomide and dexamethasone; Rd, lenalidomide and dexamethasone. Mateos MV, et al. Presented at EHA 2018, Poster Abstract PF 574. ASPIRE TOC

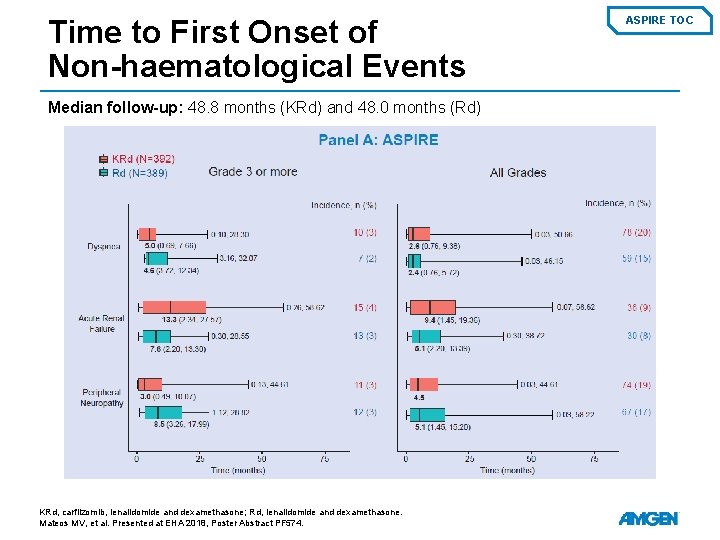
Time to First Onset of Non-haematological Events Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) KRd, carfilzomib, lenalidomide and dexamethasone; Rd, lenalidomide and dexamethasone. Mateos MV, et al. Presented at EHA 2018, Poster Abstract PF 574. ASPIRE TOC

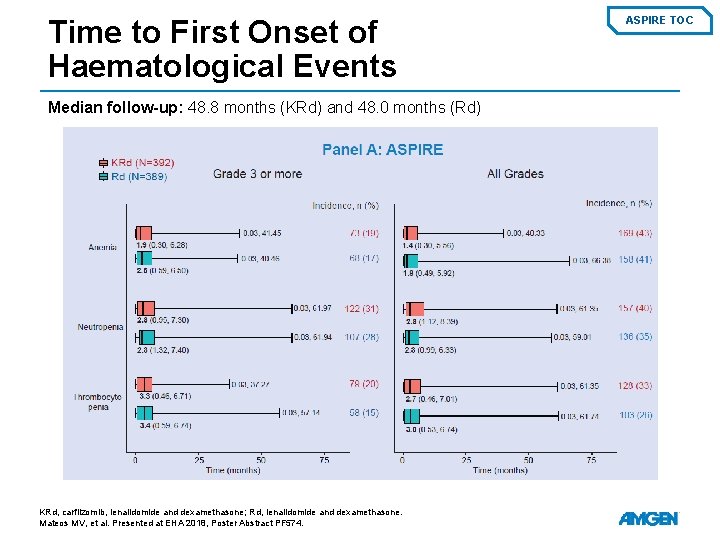
Time to First Onset of Haematological Events Median follow-up: 48. 8 months (KRd) and 48. 0 months (Rd) KRd, carfilzomib, lenalidomide and dexamethasone; Rd, lenalidomide and dexamethasone. Mateos MV, et al. Presented at EHA 2018, Poster Abstract PF 574. ASPIRE TOC

ASPIRE TOC Summary: Adverse Events • AEs led to fewer discontinuations in the KRd group and patients remained on study treatment longer • Grade ≥ 3 AEs were reported at similar rates between KRd and Rd groups in patients with: • • ≥ 2 prior lines of therapy in the standard-risk group • Grade ≥ 3 AEs were reported more frequently with KRd vs Rd in the high-risk group and 1 prior line of therapy group • Among patients aged < 70 years, hypophosphatemia was the only grade ≥ 3 AE to occur ≥ 5. 0% more frequently with KRd vs Rd; among patients aged ≥ 70 years, grade ≥ 3 AEs reported ≥ 5. 0% more frequently with KRd vs Rd include neutropaenia, thrombocytopaenia, hypokalemia, and cardiac failure (grouped term) • For patients treated with second-line KRd, safety results for the subgroups with and without prior bortezomib exposure were consistent with those reported previously • Safety profiles for early and late relapse patients were comparable with previous reports of safety • Safety findings in the final analysis were consistent with previously reported profile AE, adverse event; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone.

Diese Präsentation ist urheberechtlich geschützt durch Amgen Gmb. H stellt dieses Präsentationsmaterial für Angehörige des medizinischen Fachkreises mit Zugang zur Amgen Schweiz Webseite zur Verfügung. Es dient ausschliesslich zur eigenen Verwendung und darf nicht an Dritte weitergeleitet werden. Es dürfen keine inhaltlichen Änderungen vorgenommen wird. en