African and American trypanosomiasis Jarmila Kliescikova MD 1





















- Slides: 74

African and American trypanosomiasis Jarmila Kliescikova, MD, 1 st Faculty of Medicine, Charles University in Prague

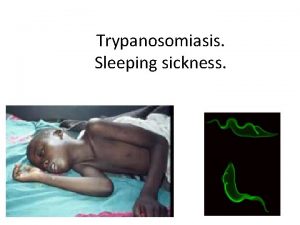
Sleeping sickness

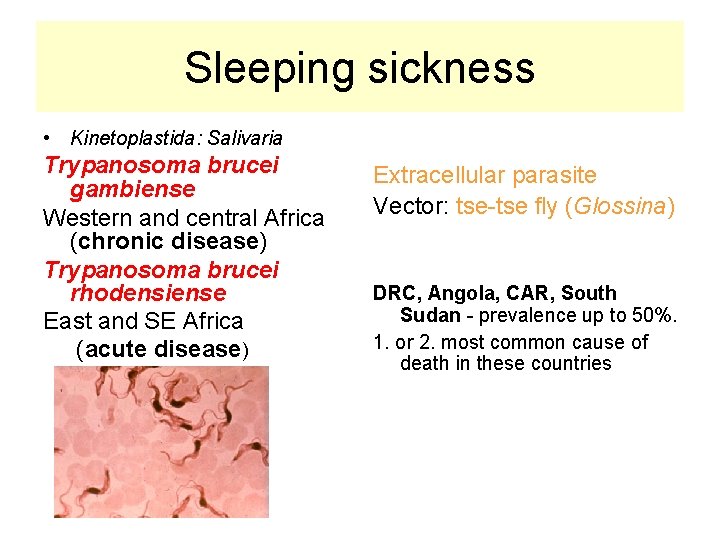
Sleeping sickness • Kinetoplastida: Salivaria Trypanosoma brucei gambiense Western and central Africa (chronic disease) Trypanosoma brucei rhodensiense East and SE Africa (acute disease) Extracellular parasite Vector: tse-tse fly (Glossina) DRC, Angola, CAR, South Sudan - prevalence up to 50%. 1. or 2. most common cause of death in these countries

In Africa, patients with sleeping sickness are poor live in remote / poor / unstable / neglected areas Patient prognosis is dependent on accurate and early diagnosis and staging The incidence of sleeping sickness has decreased in the most affected countries since 2000 ( elimination ? ) The maintenance of vertical programmes are more difficult to justify and fund integration into existing health structures is the trend practical and cheap diagnostic tools must be used

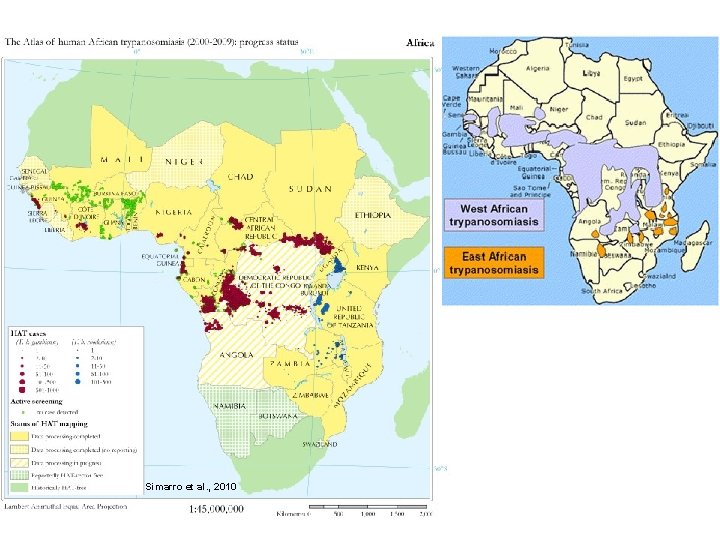
Epidemiology Distribution: tropical Africa (Chad, Congo, Cote de Ivoire, Guinea, Malawi, Uganda, Tanzania, CAR) Botswana, Swaziland Namibia – trasmission seems interruped Connected to the vector distribution Prevalence approximately - 50 mil. 20 – 50 thousand new cases per year Approximately 55 thousand deaths/year Belongs to so called neglected diseases East African form rarely imported to Europe – infection usually during safari

Simarro et al. , 2010

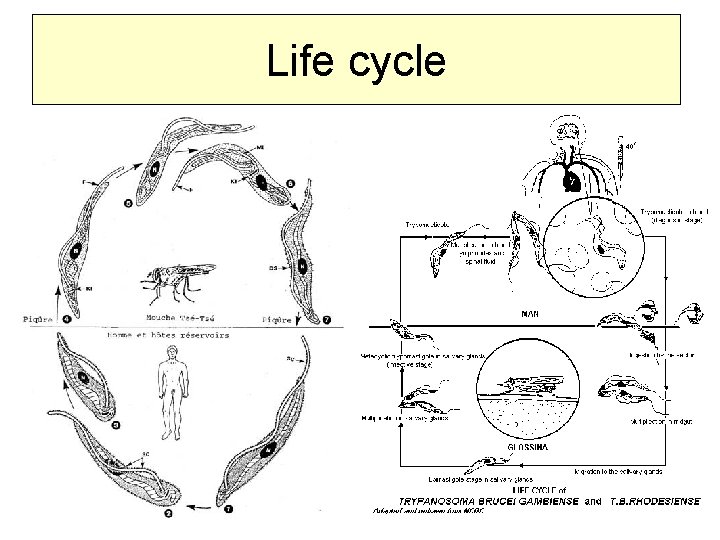
The vector = Glossina spp. – both genders able to transmit the disease T. b. gambiensae Gl. palpalis/tachinoides – River glossina The maximum is the end of dry season Antroponoosis – human is the main reservoir, rarely dog, swine, sheep, cattle, . . T. b. rhodesiensae Gl. morsitans/fuscipes Savannah glossina Zoonosis – reservoir antelope, lions, cattle, sheeps, dogs


Life cycle

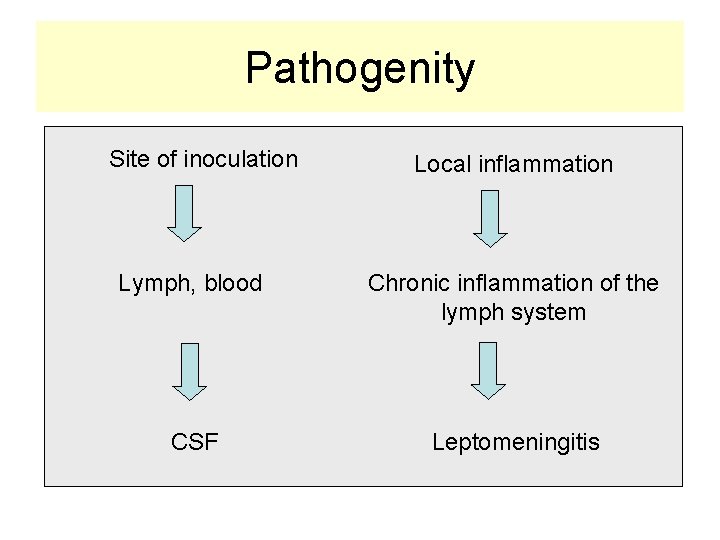
Pathogenity Site of inoculation Local inflammation Lymph, blood Chronic inflammation of the lymph system CSF Leptomeningitis

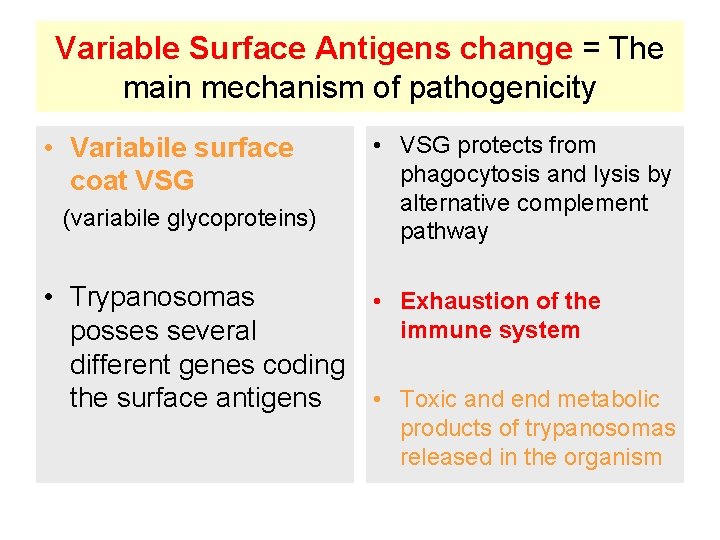
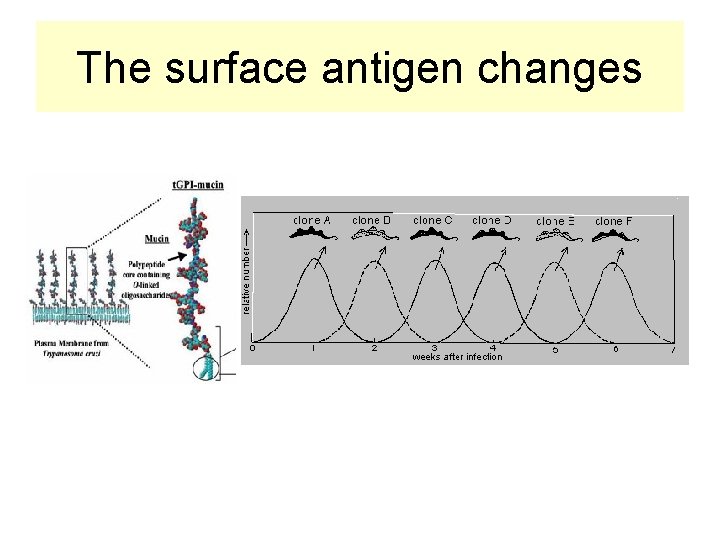
Variable Surface Antigens change = The main mechanism of pathogenicity • Variabile surface coat VSG (variabile glycoproteins) • VSG protects from phagocytosis and lysis by alternative complement pathway • Trypanosomas • Exhaustion of the immune system posses several different genes coding the surface antigens • Toxic and end metabolic products of trypanosomas released in the organism

The surface antigen changes

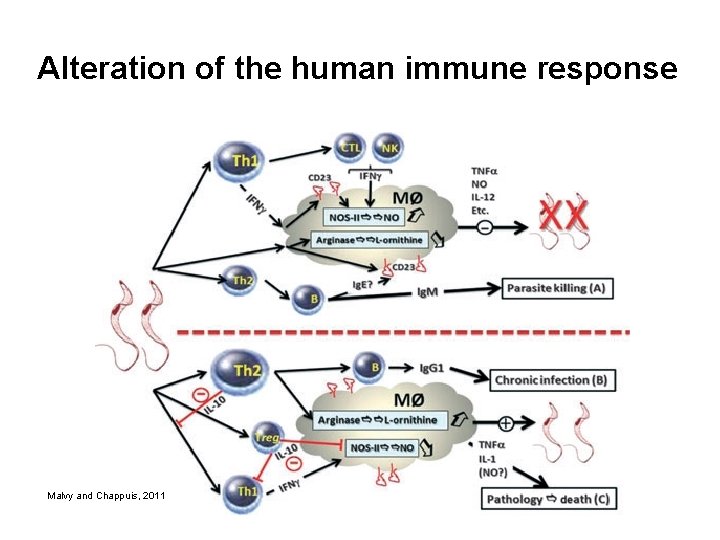
Alteration of the human immune response Malvy and Chappuis, 2011

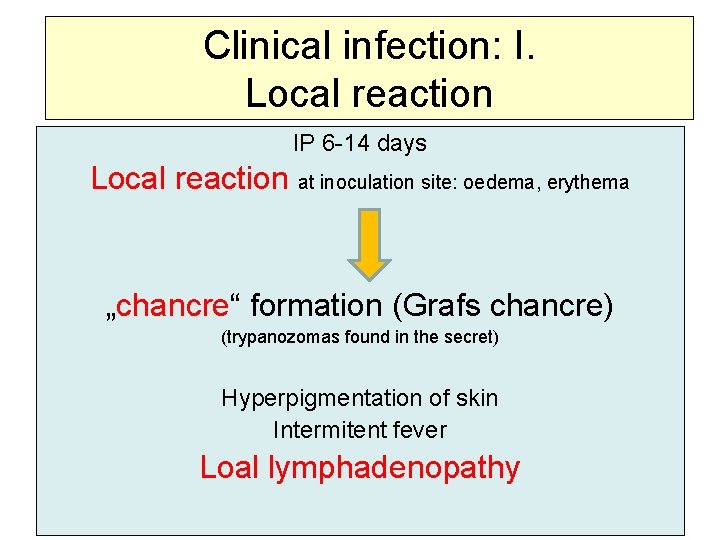
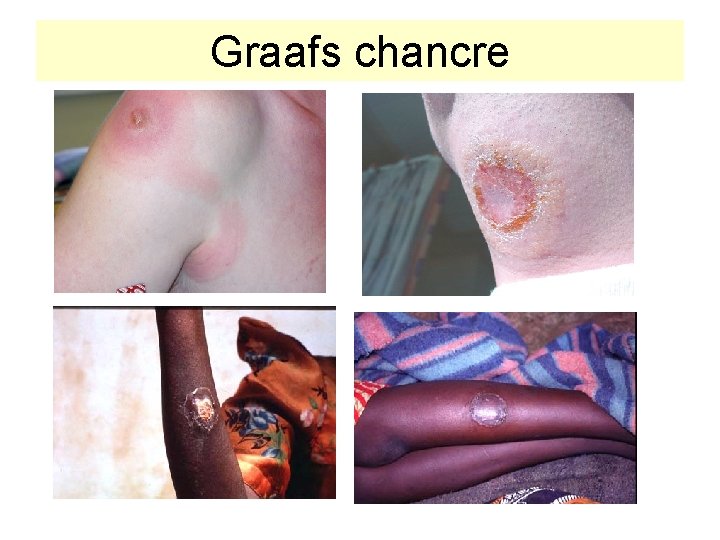
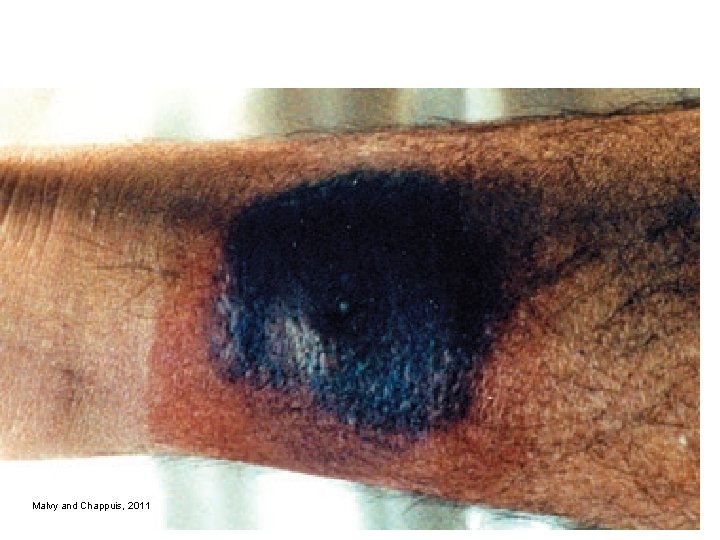
Clinical infection: I. Local reaction IP 6 -14 days Local reaction at inoculation site: oedema, erythema „chancre“ formation (Grafs chancre) (trypanozomas found in the secret) Hyperpigmentation of skin Intermitent fever Loal lymphadenopathy

Graafs chancre

Malvy and Chappuis, 2011

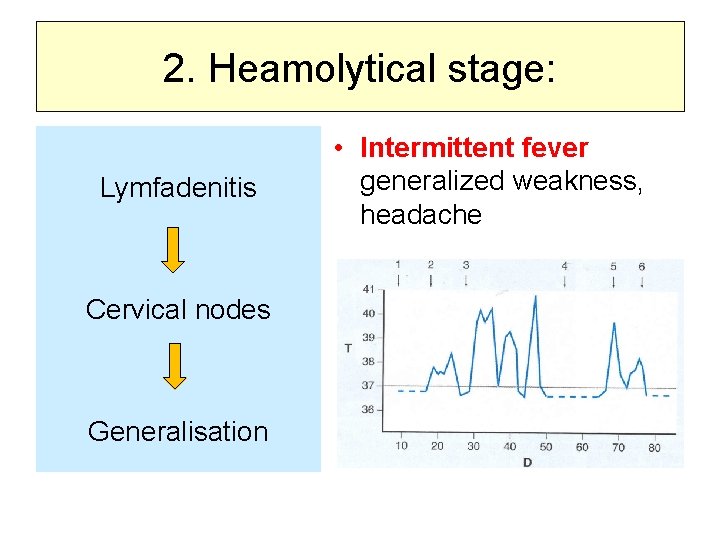
2. Heamolytical stage: Lymfadenitis Cervical nodes Generalisation • Intermittent fever generalized weakness, headache

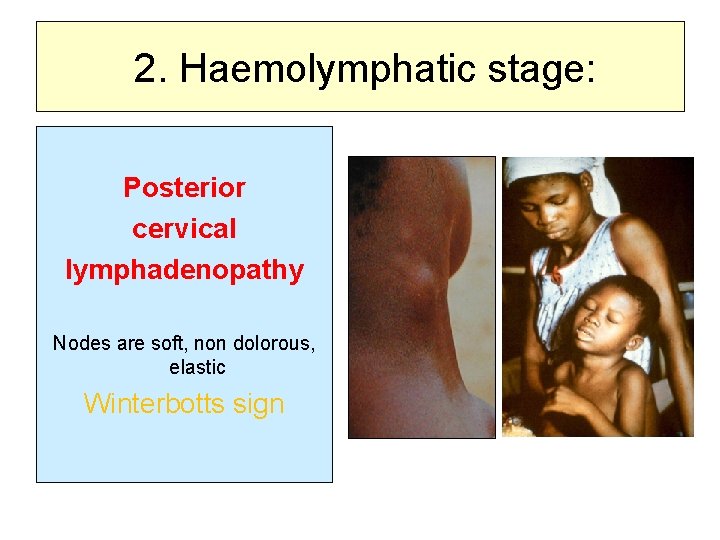
2. Haemolymphatic stage: Posterior cervical lymphadenopathy Nodes are soft, non dolorous, elastic Winterbotts sign

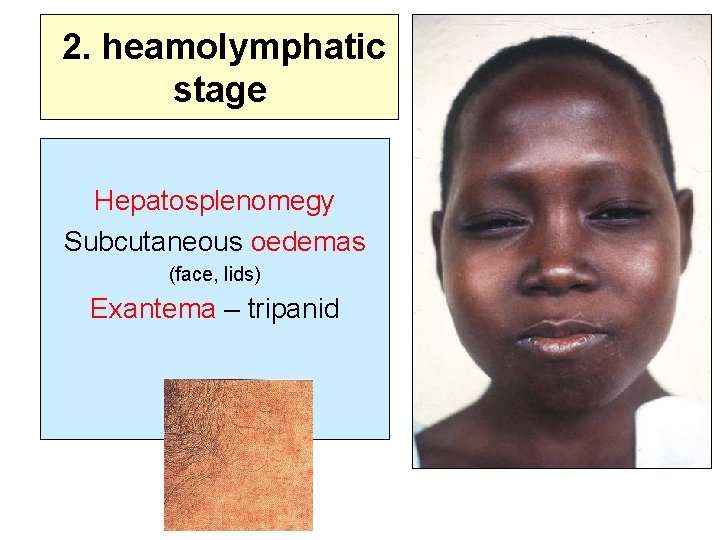
2. heamolymphatic stage Hepatosplenomegy Subcutaneous oedemas (face, lids) Exantema – tripanid

2. Heamolymphatic stage: Myocarditis tachycardia (100 -140/min); heart failure Anaemia Polyneuropathy sensitive, motoric Weakness kachexia

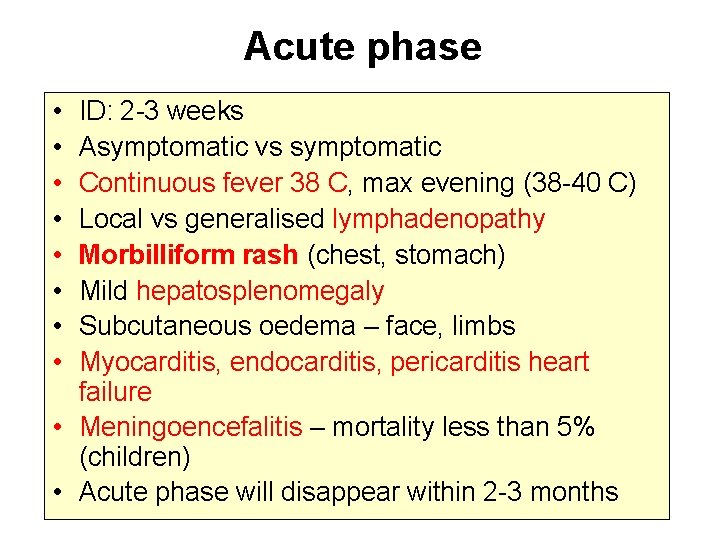
3. Meningoencefalic stage Periferal polyneuropathy (late hypersteasia after pressure on limbs and muscles, pruritus) Headache Inverse sleep Personality, character changes Chorea, atetosis, dyskinesis, tremor, ataxia, tonic-clonic seizures Sexual behaviour dysfunctions, endocrinne dysregulation Wasting syndrome


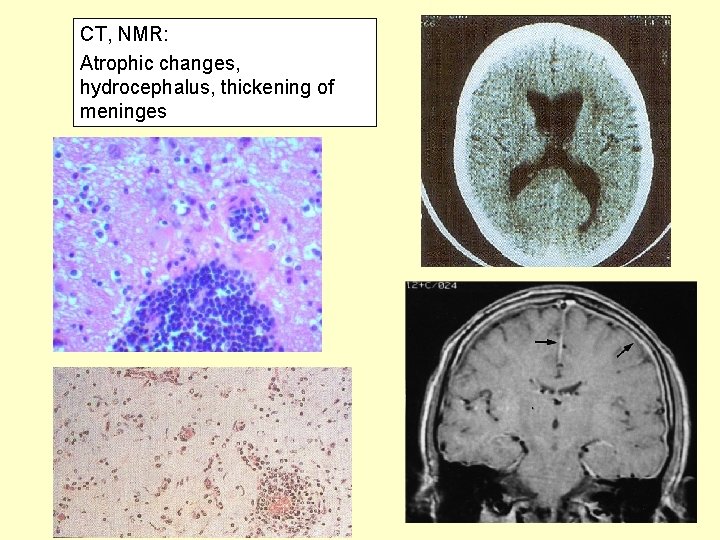
CT, NMR: Atrophic changes, hydrocephalus, thickening of meninges

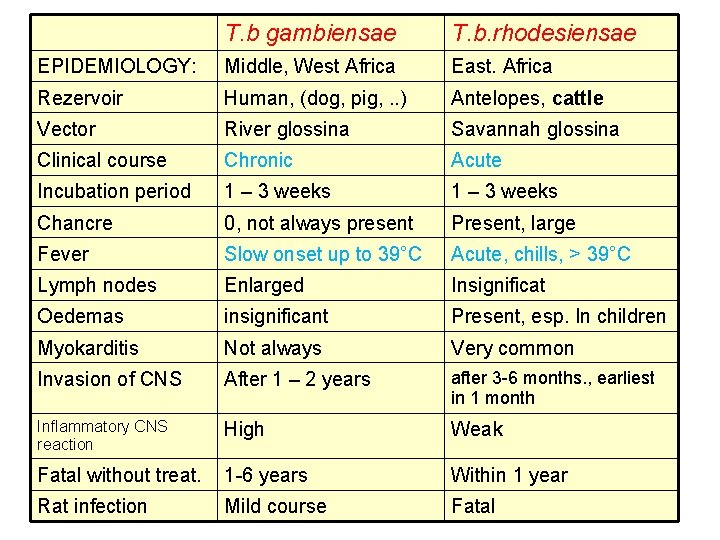
T. b gambiensae T. b. rhodesiensae EPIDEMIOLOGY: Middle, West Africa East. Africa Rezervoir Human, (dog, pig, . . ) Antelopes, cattle Vector River glossina Savannah glossina Clinical course Chronic Acute Incubation period 1 – 3 weeks Chancre 0, not always present Present, large Fever Slow onset up to 39°C Acute, chills, > 39°C Lymph nodes Enlarged Insignificat Oedemas insignificant Present, esp. In children Myokarditis Not always Very common Invasion of CNS After 1 – 2 years after 3 -6 months. , earliest in 1 month Inflammatory CNS reaction High Weak Fatal without treat. 1 -6 years Within 1 year Rat infection Mild course Fatal

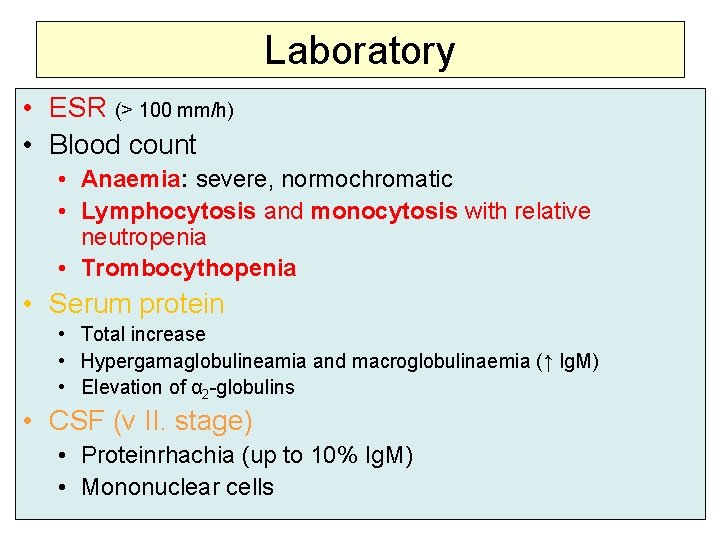
Laboratory • ESR (> 100 mm/h) • Blood count • Anaemia: severe, normochromatic • Lymphocytosis and monocytosis with relative neutropenia • Trombocythopenia • Serum protein • Total increase • Hypergamaglobulineamia and macroglobulinaemia (↑ Ig. M) • Elevation of α 2 -globulins • CSF (v II. stage) • Proteinrhachia (up to 10% Ig. M) • Mononuclear cells

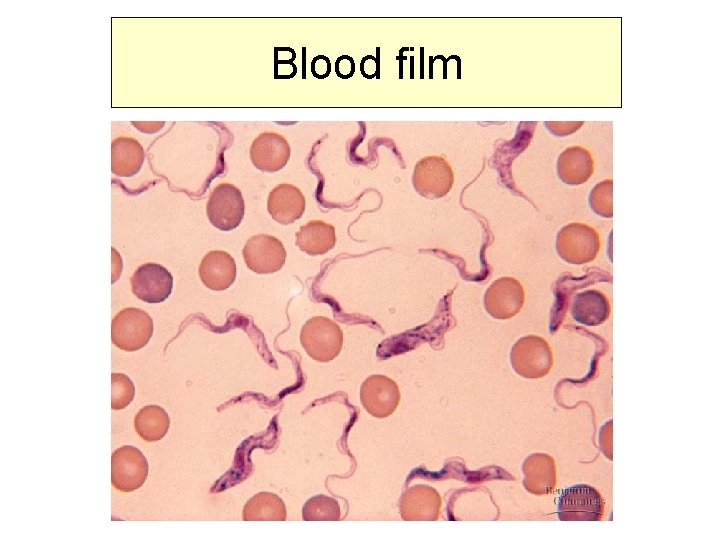
Diagnostics – direct methods • Biopsy of ulcus, local lymph nodes • Blood film, thick film • Concentration techniques • CSF examination

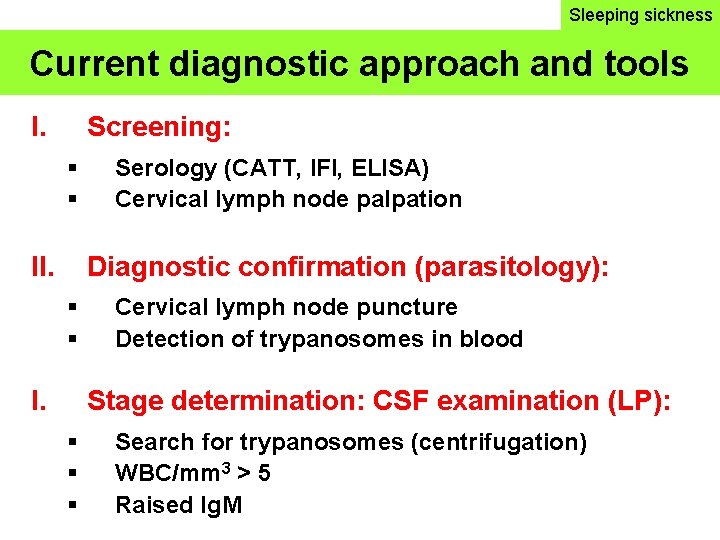
Sleeping sickness Current diagnostic approach and tools I. Screening: II. Serology (CATT, IFI, ELISA) Cervical lymph node palpation Diagnostic confirmation (parasitology): I. Cervical lymph node puncture Detection of trypanosomes in blood Stage determination: CSF examination (LP): Search for trypanosomes (centrifugation) WBC/mm 3 > 5 Raised Ig. M

Blood film

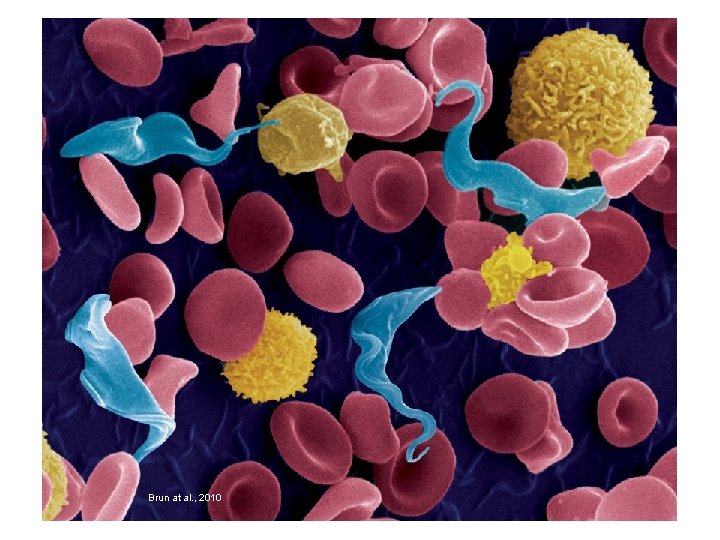
Brun at al. , 2010

Serology Agglutination tests: Paper stripes Only for T. b. gambiense

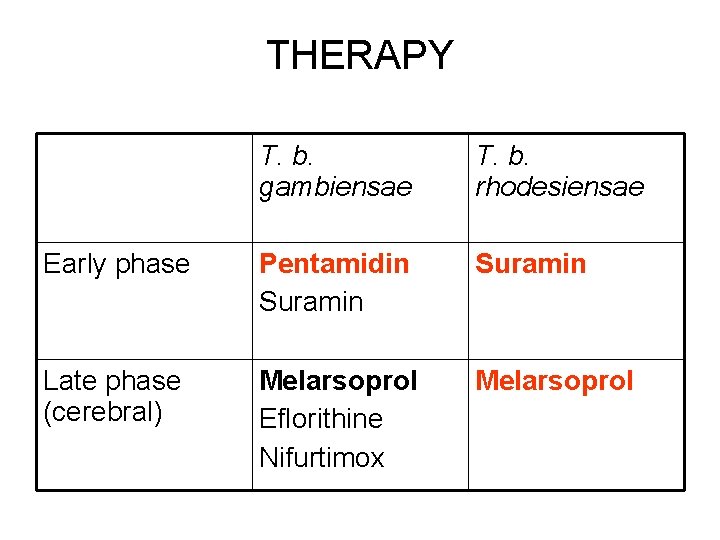
THERAPY T. b. gambiensae T. b. rhodesiensae Early phase Pentamidin Suramin Late phase (cerebral) Melarsoprol Eflorithine Nifurtimox Melarsoprol

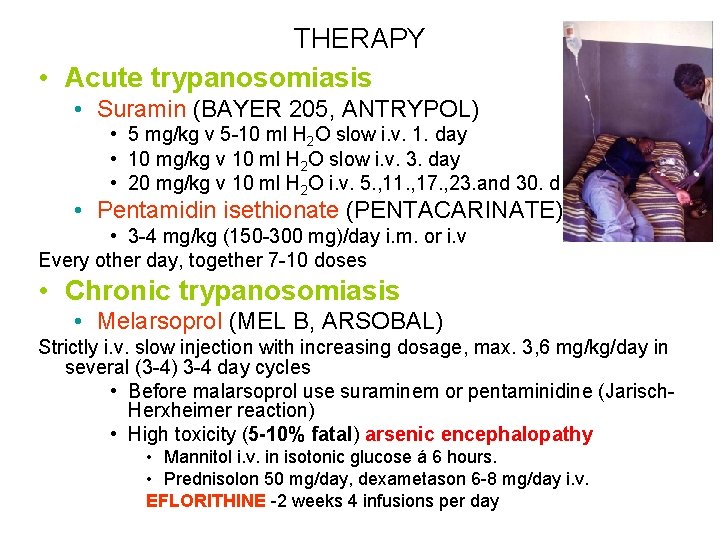
THERAPY • Acute trypanosomiasis • Suramin (BAYER 205, ANTRYPOL) • 5 mg/kg v 5 -10 ml H 2 O slow i. v. 1. day • 10 mg/kg v 10 ml H 2 O slow i. v. 3. day • 20 mg/kg v 10 ml H 2 O i. v. 5. , 11. , 17. , 23. and 30. d • Pentamidin isethionate (PENTACARINATE) • 3 -4 mg/kg (150 -300 mg)/day i. m. or i. v Every other day, together 7 -10 doses • Chronic trypanosomiasis • Melarsoprol (MEL B, ARSOBAL) Strictly i. v. slow injection with increasing dosage, max. 3, 6 mg/kg/day in several (3 -4) 3 -4 day cycles • Before malarsoprol use suraminem or pentaminidine (Jarisch. Herxheimer reaction) • High toxicity (5 -10% fatal) arsenic encephalopathy • Mannitol i. v. in isotonic glucose á 6 hours. • Prednisolon 50 mg/day, dexametason 6 -8 mg/day i. v. EFLORITHINE -2 weeks 4 infusions per day

Vector control http: //influentialpoints. com/Gallery/Tsetse-flies_Louse-flies_and_Lice. htm

American trypanosomiasis Trypanosoma cruzi – Kinetoplastida: Stercoraria • Vector: Triatoma; Rhodnius • Chagas disease • Rezervoir: human, live stock • Transmission: by vector transfusion/transplantation transplacentary

Where are we now: 2012 • Transmission by Triatoma infestans halted in 1999 in Uruguay, 1999 in Chile, 2006 in Brazil and 2009 in Guatemala • Triatoma eliminated also from some parts of Argentina and Paraguay • Disease now „common“ in non-endemic areas: Europe and USA • WHO launched an initiative for controlling of disease in non-endemic areas • USA Food and Drug Administration approved the first serological screening for blood donors

• Emergence of secondary domestic and peridomestic vectors • 8 -11 mil people infected predominantly in Mexico, Central and South America • Incidence has dropped from 700 000 new cases per year to 40 000 • The number of deaths has dropped from approximately 45 thousand to 12 500 (chronic kardiomyopathy)

Europe and Chagas disease 3 periods: Description of Chagas disease and in 1980 first case description in Europe Description of non-endemic transmission via transfusion or congenital transmission (southern Europe, Spain) Chagas disease recognized as global problem – transmission reported in 28 countries worldwide

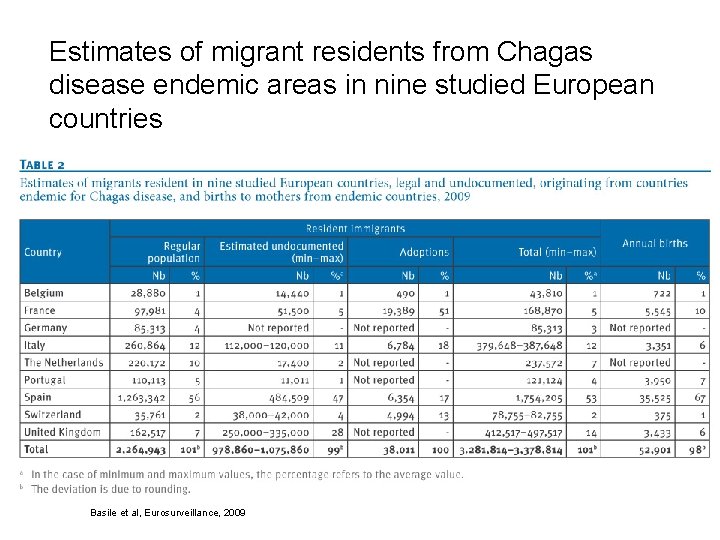
Estimates of migrant residents from Chagas disease endemic areas in nine studied European countries Basile et al, Eurosurveillance, 2009

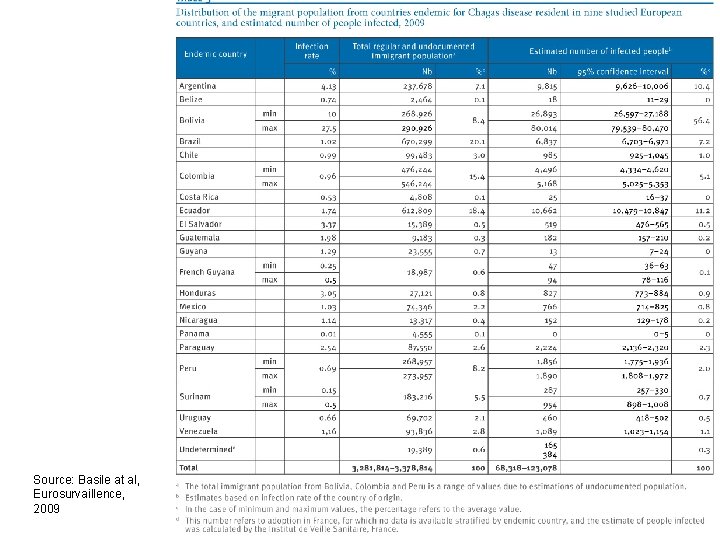
Source: Basile at al, Eurosurvaillence, 2009

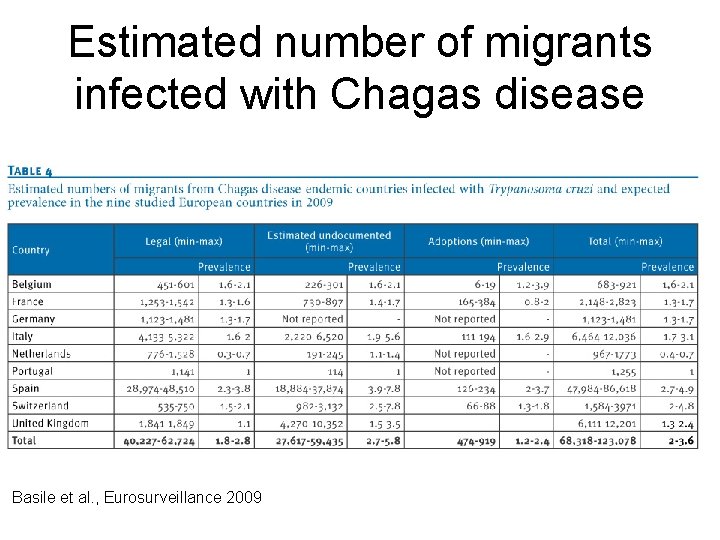
Estimated number of migrants infected with Chagas disease Basile et al. , Eurosurveillance 2009

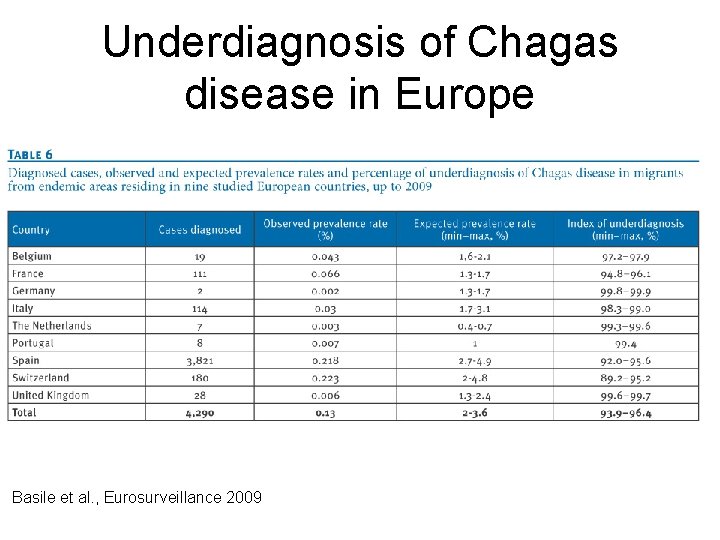
Underdiagnosis of Chagas disease in Europe Basile et al. , Eurosurveillance 2009

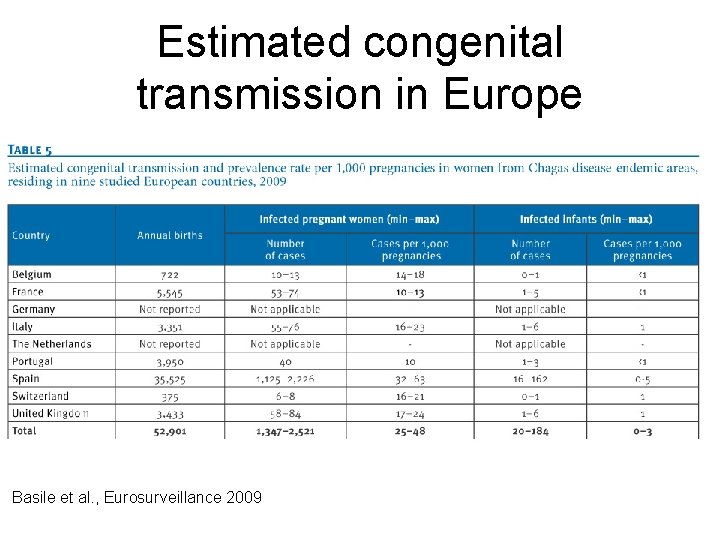
Estimated congenital transmission in Europe Basile et al. , Eurosurveillance 2009

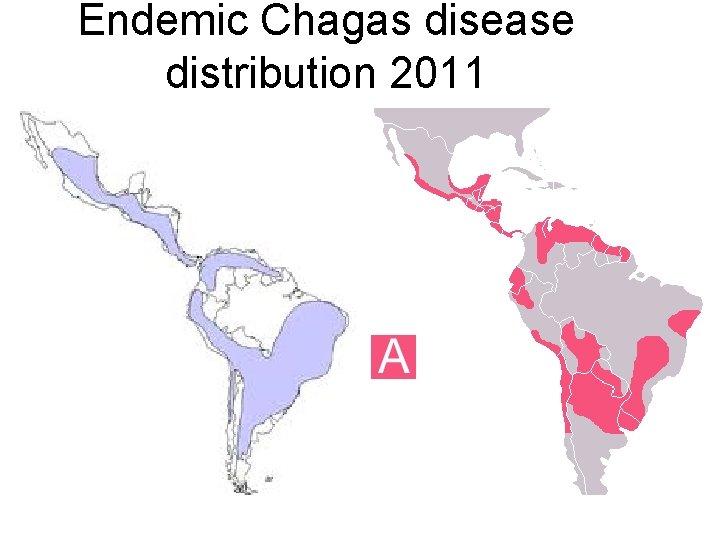
Endemic Chagas disease distribution 2011

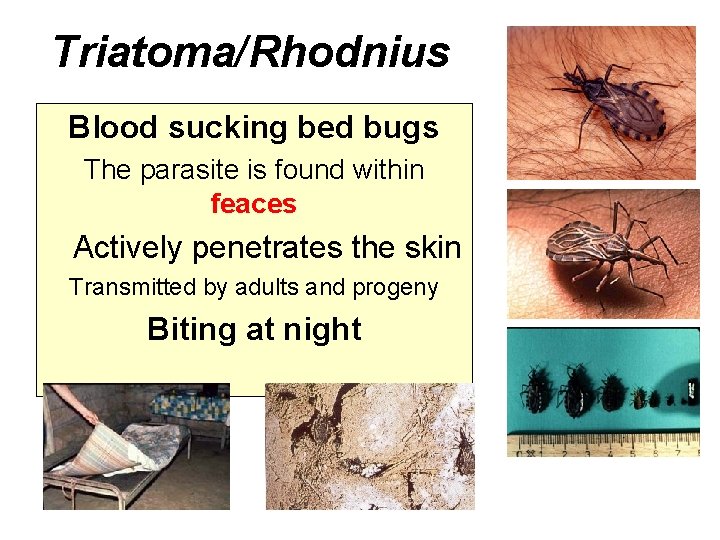
Triatoma/Rhodnius Blood sucking bed bugs The parasite is found within feaces Actively penetrates the skin Transmitted by adults and progeny Biting at night

Typical sites of vector multiplication

Typical sites of vector multiplication The vector can live in the crevices that are common in the mud and wood used to build walls and floor

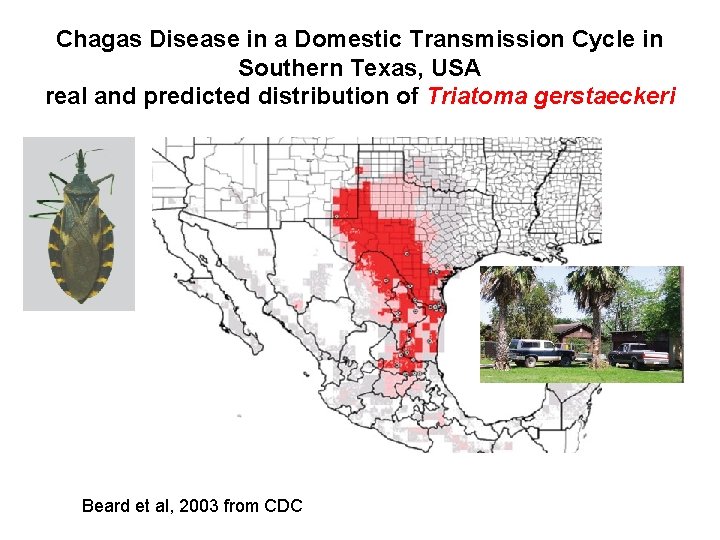
Chagas Disease in a Domestic Transmission Cycle in Southern Texas, USA real and predicted distribution of Triatoma gerstaeckeri Beard et al, 2003 from CDC

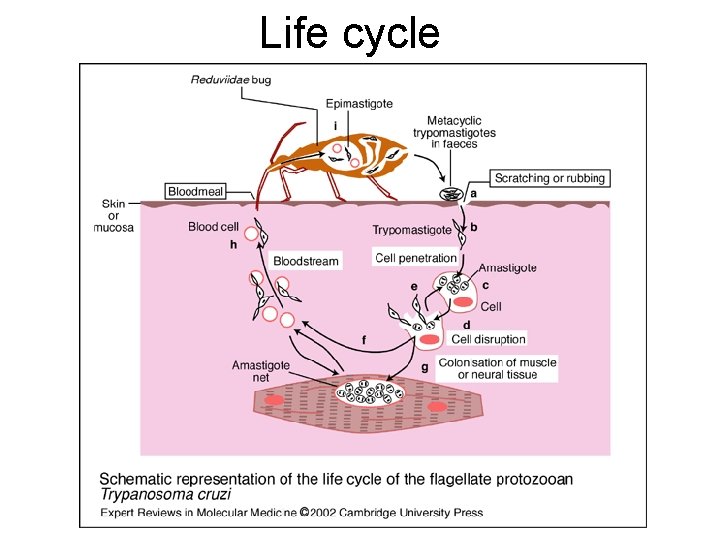
Life cycle

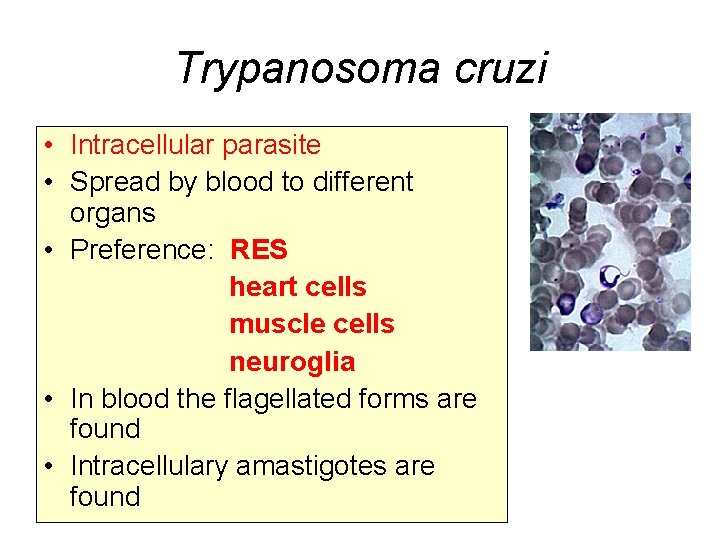
Trypanosoma cruzi • Intracellular parasite • Spread by blood to different organs • Preference: RES heart cells muscle cells neuroglia • In blood the flagellated forms are found • Intracellulary amastigotes are found

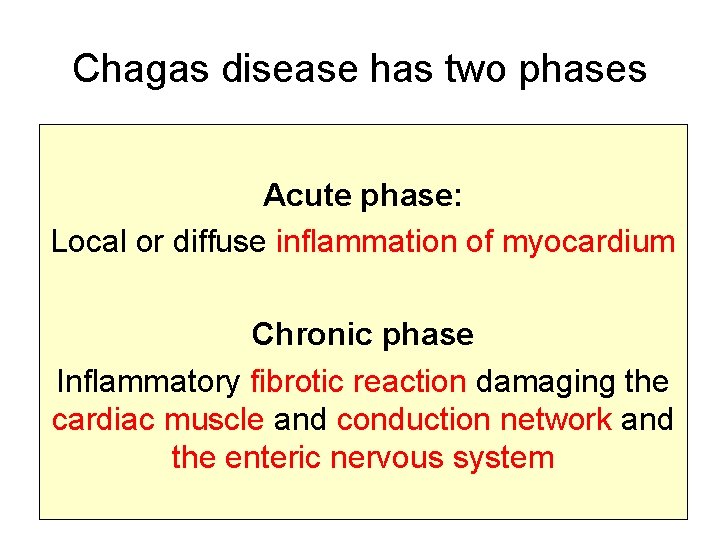
Chagas disease has two phases Acute phase: Local or diffuse inflammation of myocardium Chronic phase Inflammatory fibrotic reaction damaging the cardiac muscle and conduction network and the enteric nervous system

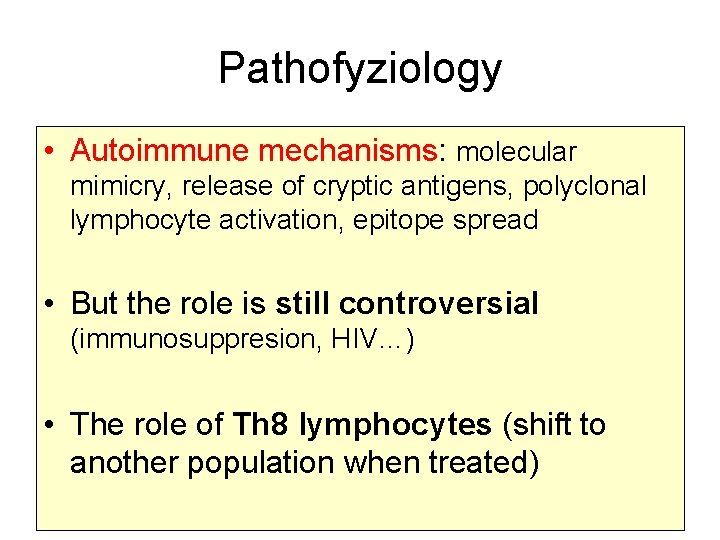
Pathofyziology • Autoimmune mechanisms: molecular mimicry, release of cryptic antigens, polyclonal lymphocyte activation, epitope spread • But the role is still controversial (immunosuppresion, HIV…) • The role of Th 8 lymphocytes (shift to another population when treated)

Pathophysiology • Host response can cause tissue damage (Th 8 lymphocytes producing granzymes and other cytokines) • Progression to symptomatic disease involves imbalance between T-helper 1 and 2 responses • Heart: conduct system, parasympatic nerve • Hypertrophy, fibrosis, thinning of the left ventricular wall, aneurysma, thrombes formation

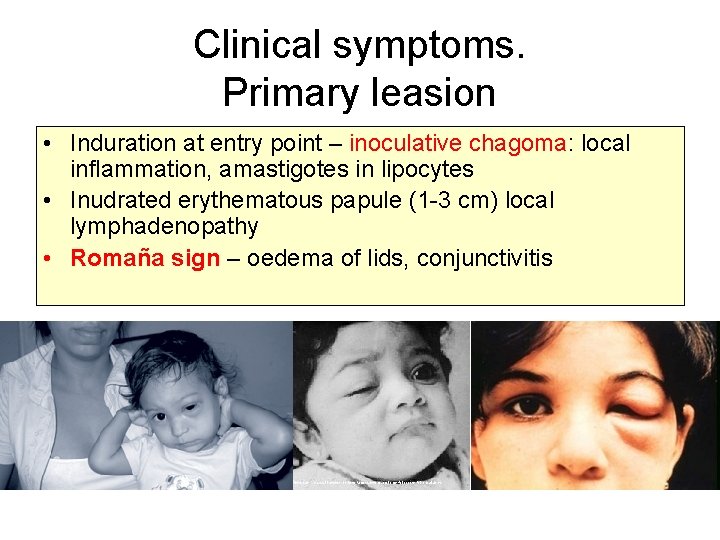
Clinical symptoms. Primary leasion • Induration at entry point – inoculative chagoma: local inflammation, amastigotes in lipocytes • Inudrated erythematous papule (1 -3 cm) local lymphadenopathy • Romaña sign – oedema of lids, conjunctivitis

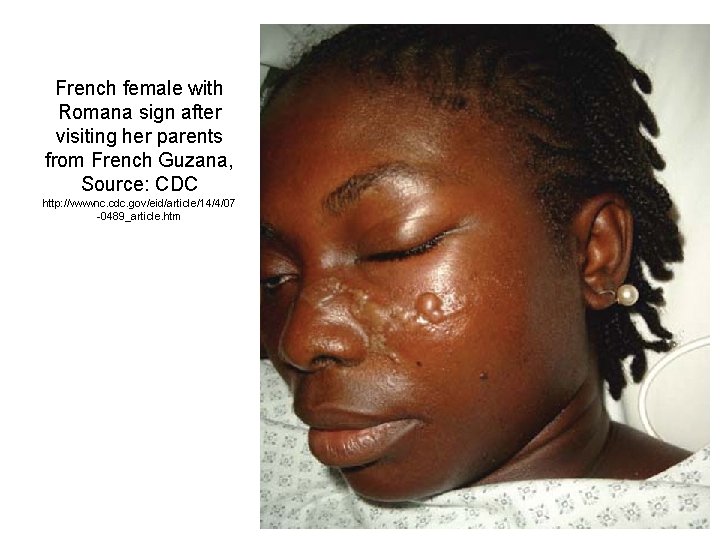
French female with Romana sign after visiting her parents from French Guzana, Source: CDC http: //wwwnc. cdc. gov/eid/article/14/4/07 -0489_article. htm

Indeterminate Chagas disease • Seropositivity for Ch. disease • Normal chest radiograph and EKG • Abscence of clinical signs and symptoms • One third of patients progresses to symptomatic disease Some patients: abnormal contractility on Echo, Areas of cardiac fibrosis…

Acute phase • • ID: 2 -3 weeks Asymptomatic vs symptomatic Continuous fever 38 C, max evening (38 -40 C) Local vs generalised lymphadenopathy Morbilliform rash (chest, stomach) Mild hepatosplenomegaly Subcutaneous oedema – face, limbs Myocarditis, endocarditis, pericarditis heart failure • Meningoencefalitis – mortality less than 5% (children) • Acute phase will disappear within 2 -3 months

Silva N et al. J Acquir Immune Defic Syndr Hum Retrovirol, 1999.

Two thirds of patients – cardiac form, one third GIT form Progression 10 -30 years after infection

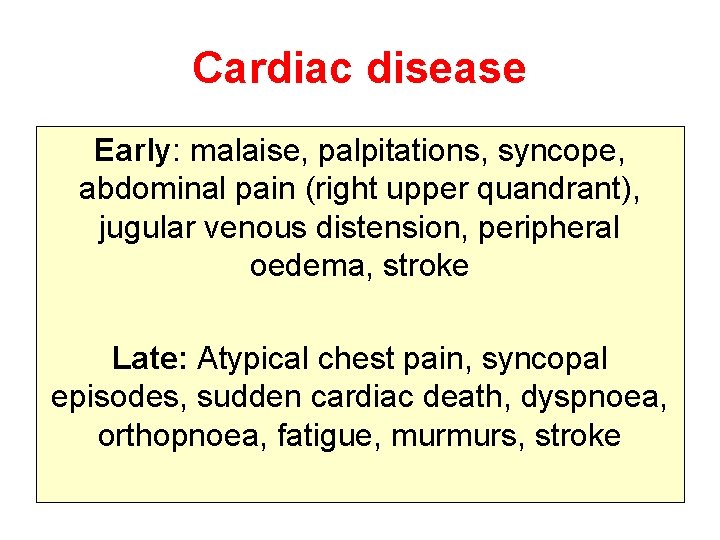
Cardiac disease Early: malaise, palpitations, syncope, abdominal pain (right upper quandrant), jugular venous distension, peripheral oedema, stroke Late: Atypical chest pain, syncopal episodes, sudden cardiac death, dyspnoea, orthopnoea, fatigue, murmurs, stroke




GIT disease • Megaoesophagus: dysphagia, regurgitation, odynophagia, oesophagitis, aspiratory pneumonia, hiccups • Megacolon: chronic constipation, meteorism, chronic abdominal pain, bacterial overgrowth syndrome, malabsorbtion, ileus – toxické megacolon • Megaureteres



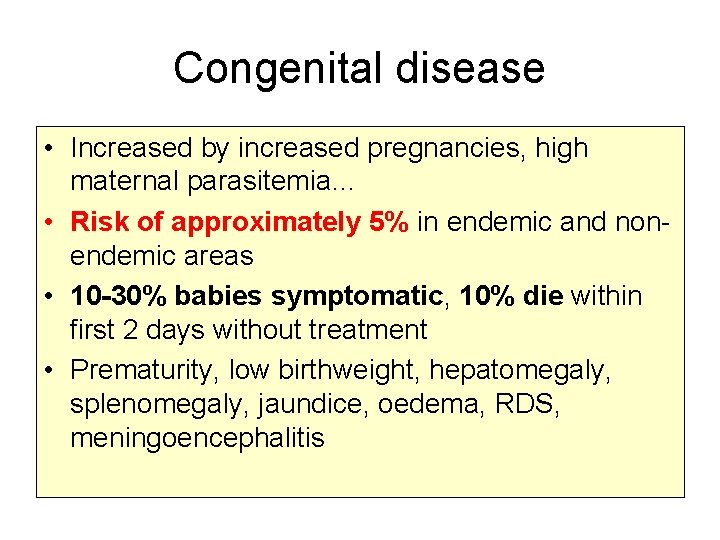
Congenital disease • Increased by increased pregnancies, high maternal parasitemia… • Risk of approximately 5% in endemic and nonendemic areas • 10 -30% babies symptomatic, 10% die within first 2 days without treatment • Prematurity, low birthweight, hepatomegaly, splenomegaly, jaundice, oedema, RDS, meningoencephalitis

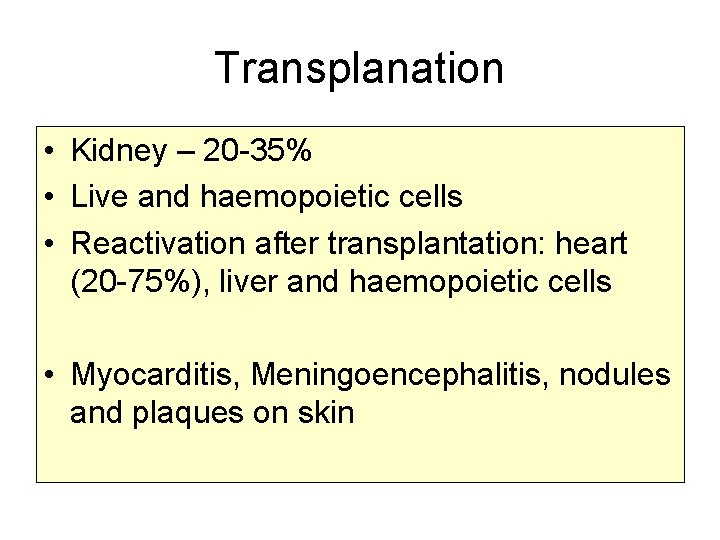
Transplanation • Kidney – 20 -35% • Live and haemopoietic cells • Reactivation after transplantation: heart (20 -75%), liver and haemopoietic cells • Myocarditis, Meningoencephalitis, nodules and plaques on skin

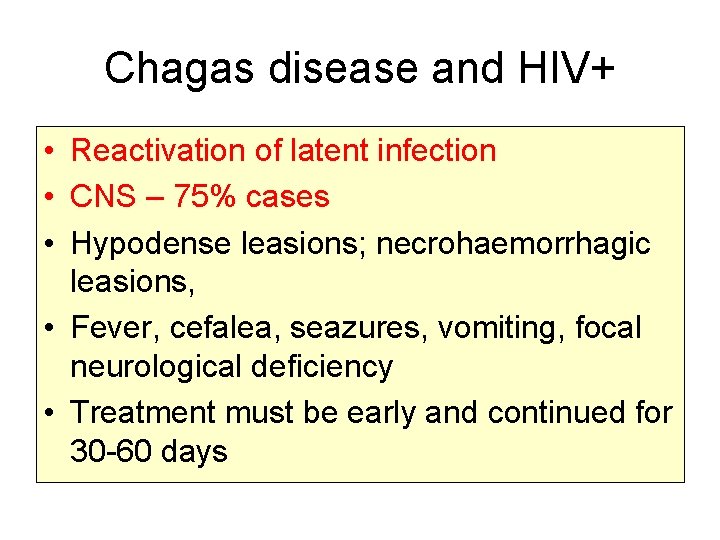
Chagas disease and HIV+ • Reactivation of latent infection • CNS – 75% cases • Hypodense leasions; necrohaemorrhagic leasions, • Fever, cefalea, seazures, vomiting, focal neurological deficiency • Treatment must be early and continued for 30 -60 days

Prognosis Score for the progression of cardiac involvement • Age older than 50 years; 2 points • Systolic diameter more than 40 mm; 3 points • Intraventricular conduction disorders; 2 points • Sustained ventricular tachycardia; 3 points • Benznidazole treatment; – 2 points Risk of progression is • 3・ 6% for a score of 0 • 6・ 9% for a score between 1 and 3 • 16% for a score between 4 and 6 • 52・ 5% for a score above 7 Prognostic score for mortality from Chagas disease • New York Heart Association III–IV; 5 points • Cardiomegaly; 5 points • Wall motion disorders; 3 points • Non-sustained ventricular tachycardia; 3 points • Broadened QRS complex; 2 points • Male sex; 2 points Risk of death is • 2% at 5 years and 10% at 10 years for a score between 0 and 6 points • 18% at 5 years and 44% at 10 years for a score between 7 and 11 points • 63% at 5 years and 84% at 10 years for a score between 12 and 20 points

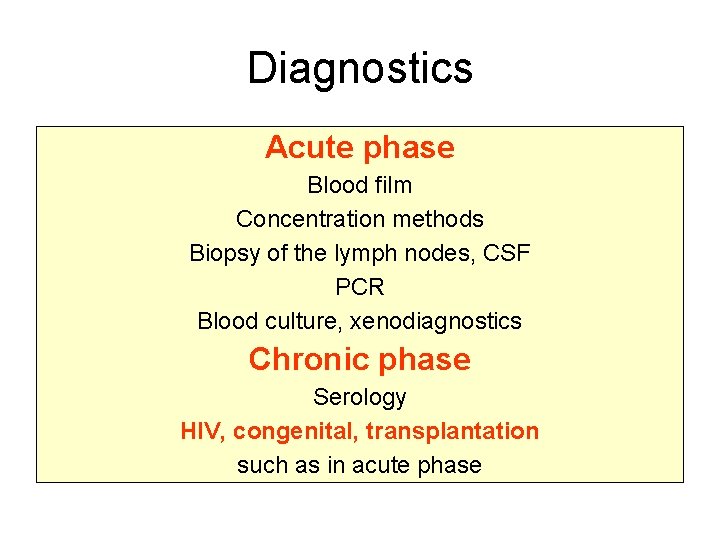
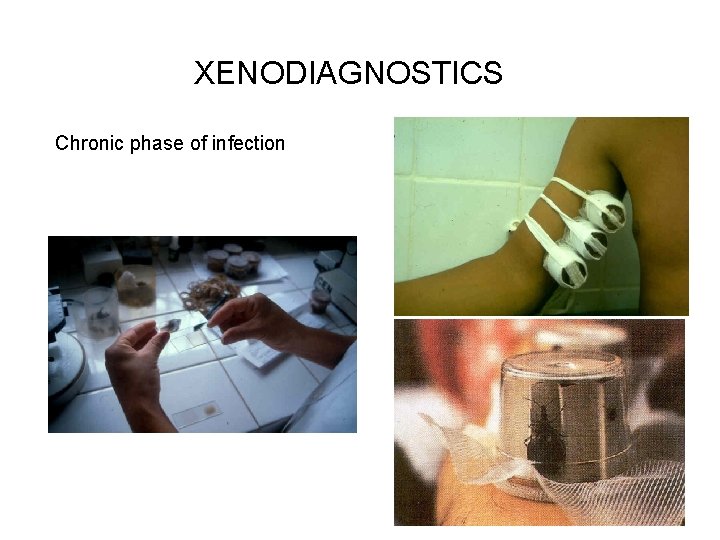
Diagnostics Acute phase Blood film Concentration methods Biopsy of the lymph nodes, CSF PCR Blood culture, xenodiagnostics Chronic phase Serology HIV, congenital, transplantation such as in acute phase

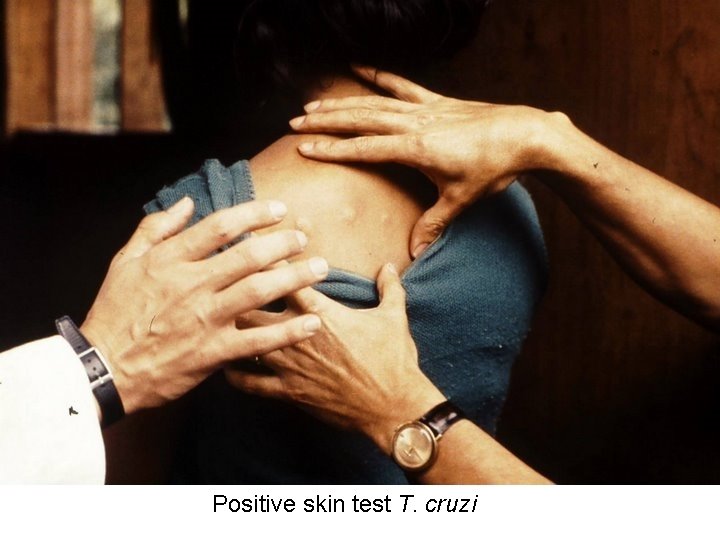
Positive skin test T. cruzi

XENODIAGNOSTICS Chronic phase of infection

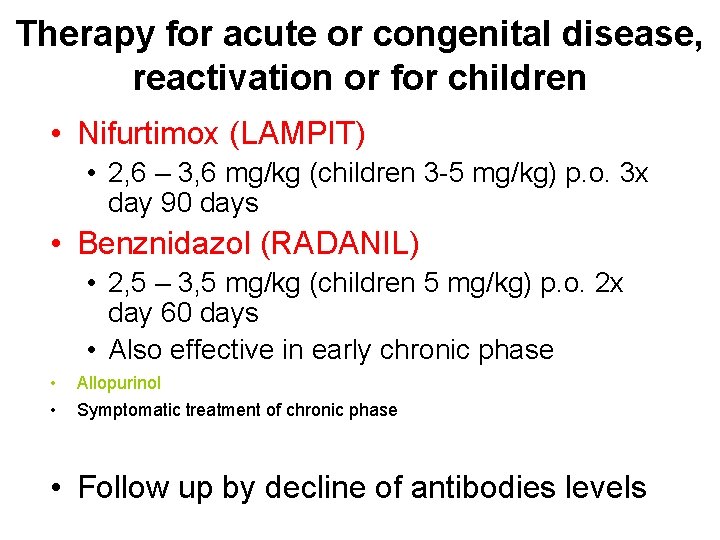
Therapy for acute or congenital disease, reactivation or for children • Nifurtimox (LAMPIT) • 2, 6 – 3, 6 mg/kg (children 3 -5 mg/kg) p. o. 3 x day 90 days • Benznidazol (RADANIL) • 2, 5 – 3, 5 mg/kg (children 5 mg/kg) p. o. 2 x day 60 days • Also effective in early chronic phase • • Allopurinol Symptomatic treatment of chronic phase • Follow up by decline of antibodies levels

 African trypanosomiasis
African trypanosomiasis African trypanosomiasis life cycle
African trypanosomiasis life cycle Leishmania
Leishmania Chagoma
Chagoma Jarmila gábová
Jarmila gábová Jarmila gábová
Jarmila gábová Sleeping sickness disease
Sleeping sickness disease Kristen walter
Kristen walter Trypanosomiasis
Trypanosomiasis Lesson 5 african american culture and politics
Lesson 5 african american culture and politics African american fighter pilots
African american fighter pilots African american vernacular english
African american vernacular english African american vernacular english
African american vernacular english Prentice hall african american history
Prentice hall african american history First african american poet
First african american poet Oxford african american studies center
Oxford african american studies center Realism naturalism modernism in african american literature
Realism naturalism modernism in african american literature African american cinema history
African american cinema history Chapter 5 african american in the new nation
Chapter 5 african american in the new nation African american beauty academy
African american beauty academy Urban street books
Urban street books African american english teacher
African american english teacher African american doctors in kansas city
African american doctors in kansas city African american vernacular english example
African american vernacular english example Holt african american history
Holt african american history Pan-african and independence comprehension check
Pan-african and independence comprehension check Center for african peace and conflict resolution
Center for african peace and conflict resolution African thunderstorm
African thunderstorm African mythology gods and goddesses
African mythology gods and goddesses North and central african societies
North and central african societies Name the elements of a folktale
Name the elements of a folktale African mask project
African mask project Person and community in african traditional thought
Person and community in african traditional thought How are elephants a keystone species
How are elephants a keystone species African charter on democracy, elections and governance
African charter on democracy, elections and governance West african society and culture section 3
West african society and culture section 3 African institute for economic development and planning
African institute for economic development and planning North and central african societies
North and central african societies Mansa musa temple
Mansa musa temple African rainstick
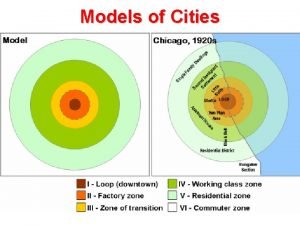
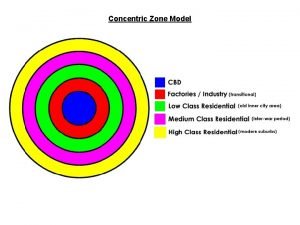
African rainstick City model
City model Urban realms model
Urban realms model Southeast asian urban model
Southeast asian urban model African religion
African religion Hamilton surgeon
Hamilton surgeon Pan african federalist movement
Pan african federalist movement Saaqis
Saaqis Northwest african countries
Northwest african countries Homer hoyt model
Homer hoyt model Ministry of east african community affairs uganda
Ministry of east african community affairs uganda French speaking african countries
French speaking african countries Closest african country to europe
Closest african country to europe Furniture industry south africa
Furniture industry south africa Elephant.it
Elephant.it African independence movements
African independence movements Latin city model
Latin city model Nigeria xxxvideo
Nigeria xxxvideo Food chain with lion
Food chain with lion Negative effects of african imperialism
Negative effects of african imperialism Languages in africa
Languages in africa African cubism
African cubism Sector zone model
Sector zone model Proclaimed as the “father of african literature,”
Proclaimed as the “father of african literature,” African transition zone
African transition zone Bumba god
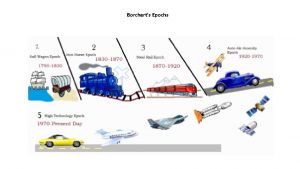
Bumba god Borchert's epochs model
Borchert's epochs model African country that speaks spanish
African country that speaks spanish African thunderstorm
African thunderstorm Great african rift valley
Great african rift valley Macro cosmos psychology
Macro cosmos psychology African monsoon
African monsoon African farmer game
African farmer game African creation stories
African creation stories Are west african bantu
Are west african bantu Bantu culture
Bantu culture