Advanced Therapy Medicinal Products April 18 2012 Eliane













- Slides: 30

Advanced Therapy Medicinal Products April 18, 2012 Eliane. schutte@signifix. com 1

Agenda • ATMP definition/ classification? • What is my human cell based product? • Exemptions and different routes? 2

Definitions 2001/83/EC/ ; 2004/24/EC; 93/42/EC 3

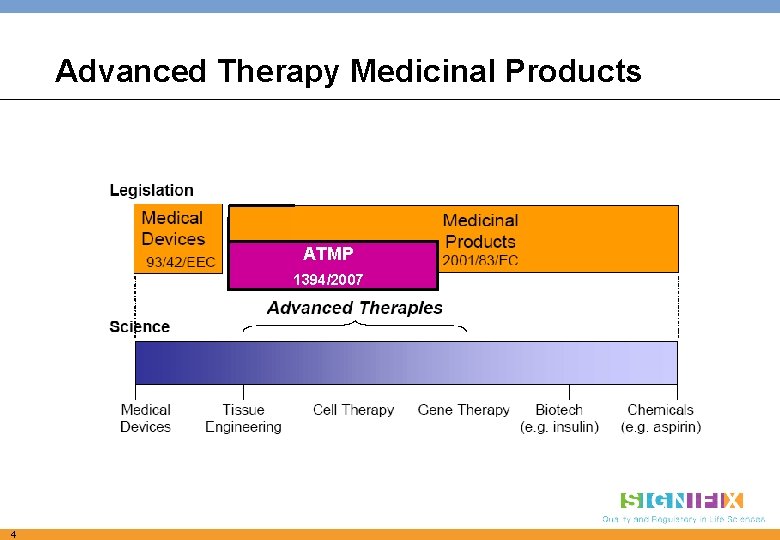
Advanced Therapy Medicinal Products ATMP 1394/2007 4

Status EU ATMP’s • 1 registered ATMP in EU : Chondroselect (Tigenex) • Only six applications have been submitted for MA (four ATMPs) • In 2009 -2011 • • • a product was intended for treatment of squamous cell carcinoma (head and neck) was withdrawn the CAT adopted a negative opinion on the MA application of a gene therapy product intended for treatment of cerebral cancer (high grade glioma); the manufacturer withdrew its application in March 2010. A negative CAT opinion was adopted for the MA application a gene therapy product intended for use in severe lipid metabolic disease. On re-examination of Glyberain October 2011, the CAT adopted a positive opinion but this was not endorsed by the CHMP. • 50 Summaries of scientific recommendations on classification of advancedtherapy medicinal products (4 not classified a ATMP) 5

ATMP or Medical Device or Human tissue Or Blood product or Combo? • Where does my product fit? • Where do I want it to fit? Carefull consideration required to define best strategy Ask for classification is that wise? • CAT/ CBG/CA human tissues/ Notified Body? • Where to go best? 6

1) Advanced Therapy Medicinal Products • ATMPs are medical products for human use and include: q Gene therapy products q Somatic cell therapy products q Tissue engineering products • ATMPs are regulated under consolidated framework for advanced therapies (Regulation 1394/2007) as pharmaceutical products • ATMP Regulation, Article 3 Where an ATMP contains human cells or tissues, the donation, procurement and testing of those tissues or cells shall be made in accordance with Directive 2004/23/EC 7

Cells or tissues shall be considered ‘engineered’ if they fulfill at least one of the following conditions: • Industrial process • the cells or tissues have been “engineered”; subject to substantial manipulation, • are not intended to be used for the same essential function or functions in the recipient as in the donor. 8

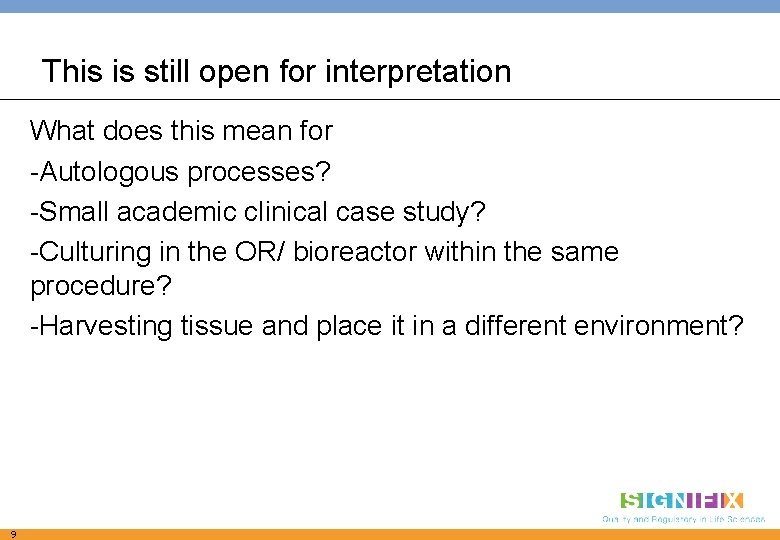
This is still open for interpretation What does this mean for -Autologous processes? -Small academic clinical case study? -Culturing in the OR/ bioreactor within the same procedure? -Harvesting tissue and place it in a different environment? 9

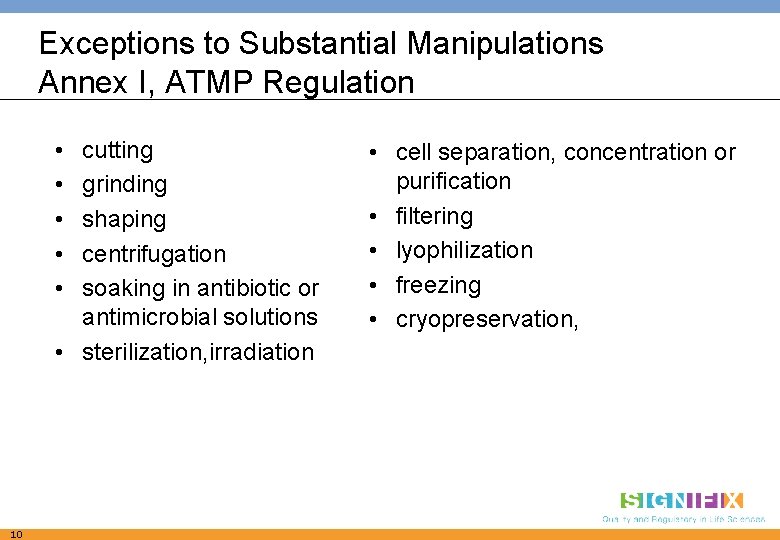
Exceptions to Substantial Manipulations Annex I, ATMP Regulation • • • cutting grinding shaping centrifugation soaking in antibiotic or antimicrobial solutions • sterilization, irradiation 10 • cell separation, concentration or purification • filtering • lyophilization • freezing • cryopreservation,

What if my product is an exception or gray zone? What are the other options? 11

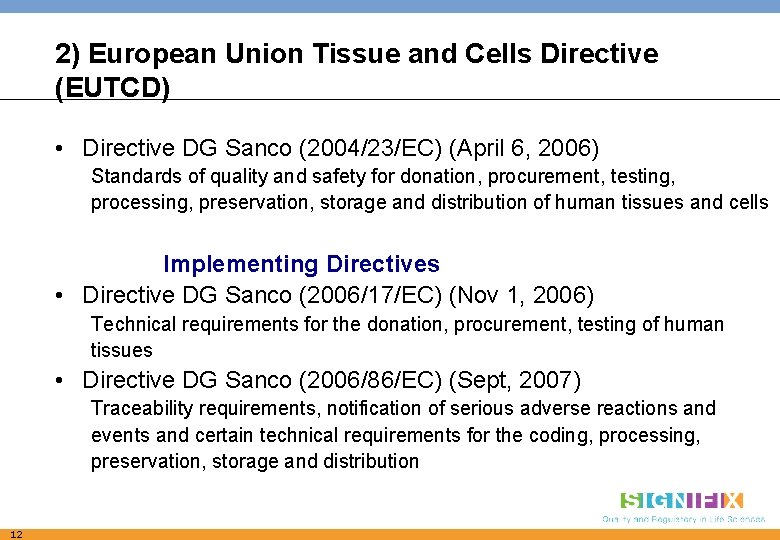
2) European Union Tissue and Cells Directive (EUTCD) • Directive DG Sanco (2004/23/EC) (April 6, 2006) Standards of quality and safety for donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells Implementing Directives • Directive DG Sanco (2006/17/EC) (Nov 1, 2006) Technical requirements for the donation, procurement, testing of human tissues • Directive DG Sanco (2006/86/EC) (Sept, 2007) Traceability requirements, notification of serious adverse reactions and events and certain technical requirements for the coding, processing, preservation, storage and distribution 12

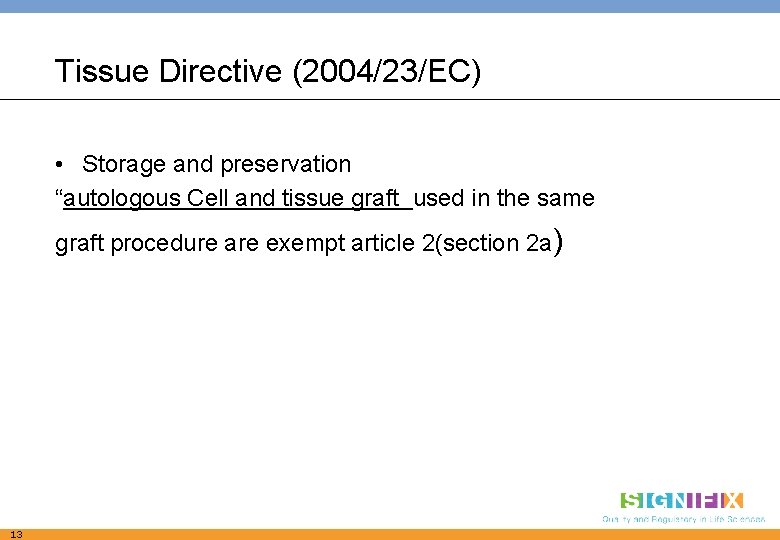
Tissue Directive (2004/23/EC) • Storage and preservation “autologous Cell and tissue graft used in the same graft procedure are exempt article 2(section 2 a) 13

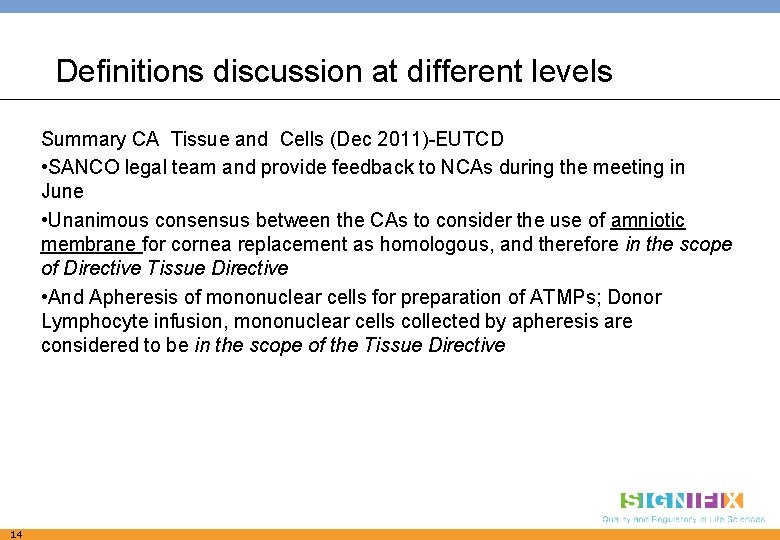
Definitions discussion at different levels Summary CA Tissue and Cells (Dec 2011)-EUTCD • SANCO legal team and provide feedback to NCAs during the meeting in June • Unanimous consensus between the CAs to consider the use of amniotic membrane for cornea replacement as homologous, and therefore in the scope of Directive Tissue Directive • And Apheresis of mononuclear cells for preparation of ATMPs; Donor Lymphocyte infusion, mononuclear cells collected by apheresis are considered to be in the scope of the Tissue Directive 14

Medical Device Directive (93/42/EC) • Article 1. 5(f) This Directive does not apply to: (a) in vitro diagnostic devices; (b) active implantable devices covered by Directive 90/385/EEC; (c) medicinal products covered by Directive 65/65/EEC; (d) cosmetic products covered by Directive 76/768/EEC (18); (e) human blood, human blood products, human plasma or blood cells of human origin or to devices which incorporate at the time of placing on the market such blood products, plasma or cells; (f) transplants or tissues or cells of human origin nor to products incorporating or derived from tissues or cells of human origin; (g) transplants or tissues or cells of animal origin, unless a device is manufactured utilizing animal tissue which is rendered non-viable or non-viable products derived from animal tissue. 15

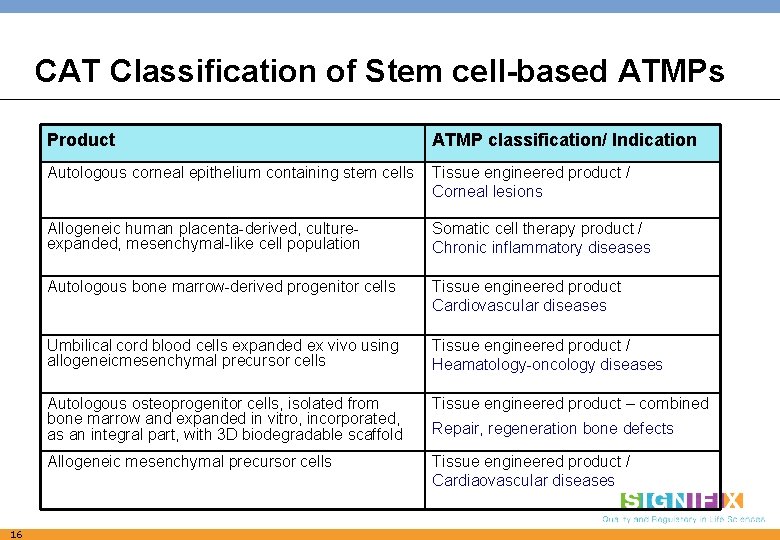
CAT Classification of Stem cell-based ATMPs Product ATMP classification/ Indication Autologous corneal epithelium containing stem cells Tissue engineered product / Corneal lesions 16 Allogeneic human placenta-derived, cultureexpanded, mesenchymal-like cell population Somatic cell therapy product / Chronic inflammatory diseases Autologous bone marrow-derived progenitor cells Tissue engineered product Cardiovascular diseases Umbilical cord blood cells expanded ex vivo using allogeneicmesenchymal precursor cells Tissue engineered product / Heamatology-oncology diseases Autologous osteoprogenitor cells, isolated from bone marrow and expanded in vitro, incorporated, as an integral part, with 3 D biodegradable scaffold Tissue engineered product – combined Allogeneic mesenchymal precursor cells Tissue engineered product / Cardiaovascular diseases Repair, regeneration bone defects

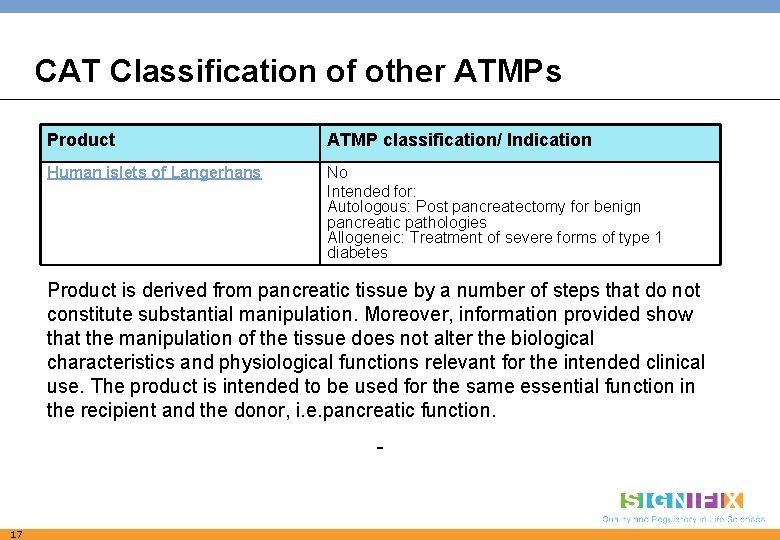
CAT Classification of other ATMPs Product ATMP classification/ Indication Human islets of Langerhans No Intended for: Autologous: Post pancreatectomy for benign pancreatic pathologies Allogeneic: Treatment of severe forms of type 1 diabetes Product is derived from pancreatic tissue by a number of steps that do not constitute substantial manipulation. Moreover, information provided show that the manipulation of the tissue does not alter the biological characteristics and physiological functions relevant for the intended clinical use. The product is intended to be used for the same essential function in the recipient and the donor, i. e. pancreatic function. - 17

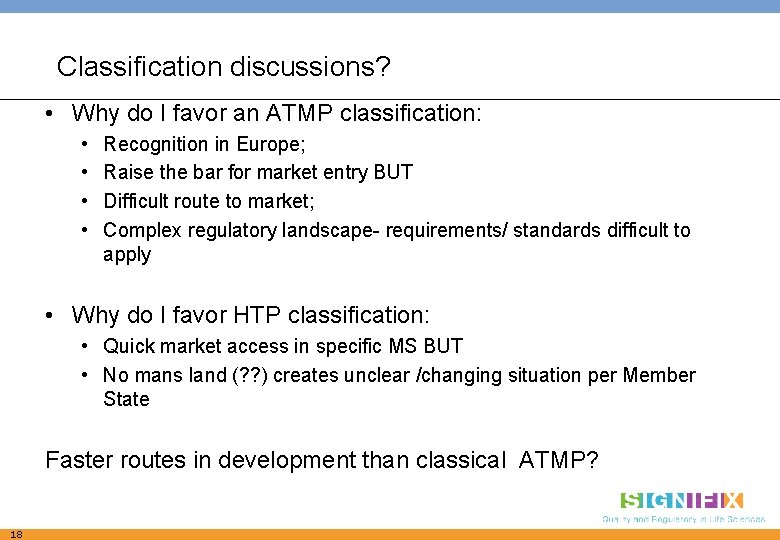
Classification discussions? • Why do I favor an ATMP classification: • • Recognition in Europe; Raise the bar for market entry BUT Difficult route to market; Complex regulatory landscape- requirements/ standards difficult to apply • Why do I favor HTP classification: • Quick market access in specific MS BUT • No mans land (? ? ) creates unclear /changing situation per Member State Faster routes in development than classical ATMP? 18

1) Advanced Therapy Medicinal Products • Key aspects of the ATMP Regulation: • • 19 Centralized Marketing Authorization procedure (EMA) Committee for Advanced Therapies (CAT) Hospital Exemption ruling Specific GMP regulations Incentives for small and medium sized enterprises (SME) Risk management and long term traceability Post authorization pharmacovigilance systems

3 options : treatment of patients using ATMPs 1. Treatment as registered Medicinal Product (via EMA, see www. ema. europa. eu. ) 2. Products, which were legally on the Community market when the Regulation became applicable, should comply to the Regulation by December 30, 2012. 3. Treatment as registered Medicinal Product in a clinical study ( www. ccmo-online. nl for information). 4. Treatment as a hospital exemption • De IGZ has to authorize the hospital exemption including manufacturing (GMP) ) 20

CAT: Committee for Advanced Therapies • Products covered subject to centralized authorization procedure involving a single scientific evaluation of the quality, safety and efficacy of the product by the EMEA • CAT (Committee for Advanced Therapies) responsible for q Draft opinions on the Quality, Safety and Efficacy of each ATMP for final approval by CHMP (Committee for medicinal Products for Human Use) q Advice on whether the product falls within the definition ATMP q Contribute to scientific advice procedures • Comprised of multidisciplinary scientific experts representing all EU member states, as well as and patient and medical associations 21

Hospital Exemption (Article 28) • ATMPs exempted from the centralized marketing authorization procedure • Hospital Exemption requirements: q Non-routine basis q Manufactured and used in same member state q Used in a hospital under exclusive professional responsibility of a medical practitioner q Specific quality standards q Individual medical prescription q Custom-made product for an individual patient • Same requirements apply as for ATMPs for which a centralized procedure is mandatory (GMP, QP release, pharmacovigilance) • Not intended for clinical trials but rather case studies 22

Hospital exemption • Member states are responsible for the implementation of the Hospital Exemption • Lack of clear guidance in most Member States • Differences in interpretation of the Hospital Exemption are likely to occur • Many member states are reluctant to grant hospital exemption for clinical Phase 1 trials • Variation per country ( ie Netherlands difficult vs Spain easier) 23

Hospital exemption procedure • A request for hospital exemption needs to be adressed to IGZ, digitally via a form and has to be sent to atmp@igz. nl. • IGZ reviews requests monthly, the request should be sent at least 2 weeks before the date IGZ has an ATMP review meeting. • IGZ sents the decision 2 weeks after the review meeting • Hospital Exemptions are enclined for one year only and maximum 10 patients. • Hospital exemptions from companies are most likely deferred 24

• Good Manufacturing Practices 25

GMP Manufacturing of ATMPs • ATMPs need to be manufactured in accordance with the GMP guidelines for human medicinal products for human use (Directive 2003/94/EC) • Control of consistency, reproducibility and uniformity are key aspects • Annex 2 of the EU Guidelines for GMP for medicinal products for human and veterinary use (Eudralex Vol 4) has been updated to include GMP specific to ATMPs • The Annex recognizes the inherent variability and increased risks for microbial contamination and transfer of pathogens associated with biological culturing processes and materials 26

GMP Human tissues • Only as defined in the Directives (light GMP system) 27

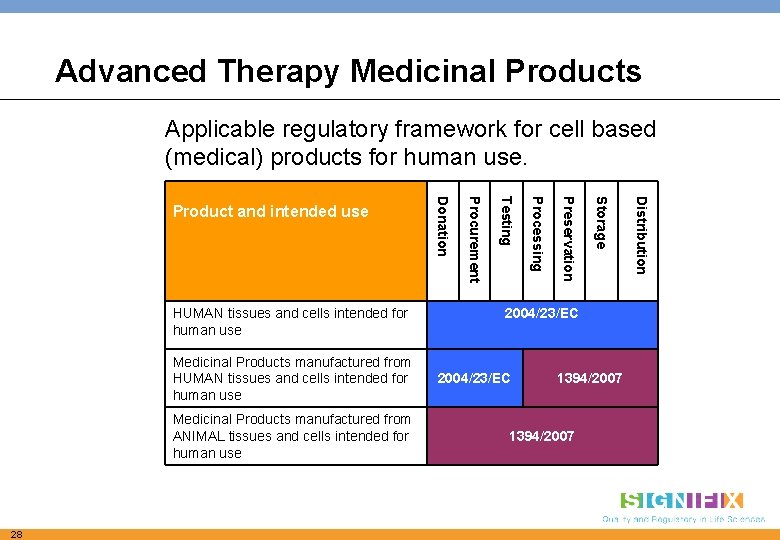
Advanced Therapy Medicinal Products Applicable regulatory framework for cell based (medical) products for human use. 2004/23/EC 1394/2007 Distribution Storage 28 Preservation Medicinal Products manufactured from ANIMAL tissues and cells intended for human use Processing Medicinal Products manufactured from HUMAN tissues and cells intended for human use Testing HUMAN tissues and cells intended for human use Procurement Product and intended use Donation

ATMP and Stem Cells • EMA closely monitors developments in the area of stem cell therapy • Depending on the indication and process, stem-cell based medicinal products can be classified as ATMPs • Stem cells preparations that are not substantially manipulated or intended to be used for the same essential function in the recipient as in the donor are outside the scope of the ATMP Regulation • To date, no stem-cell product has received marketing authorization within the EU 29

ATMP and Stem Cells • EMA expressed concerns about ‘stem-cell tourism’: use of unauthorized stem-cell based treatment in the absence of rigorous scientific and ethical requirements (Lancet 2010; Vol 376: 514) • CAT has classified various medicinal products containing stem cells as ATMPs • Committee of Orhpan Medicinal Products (COMP) has granted orphan designation to a number of medicines containing stemcells for the treatment of rare diseases 30
 Olseltamivir
Olseltamivir Advanced medicinal chemistry
Advanced medicinal chemistry Bioness bits cost
Bioness bits cost What are the major humanistic therapies
What are the major humanistic therapies Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress Pepsi vs coke marketing strategy
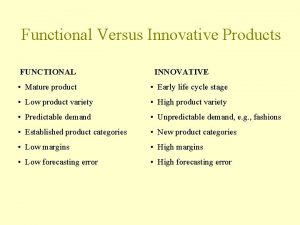
Pepsi vs coke marketing strategy Functional products vs innovative products
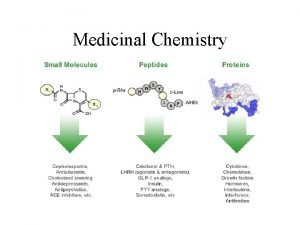
Functional products vs innovative products Medicinal chemistry definition
Medicinal chemistry definition Medicinal plants definition
Medicinal plants definition Gas medicinal
Gas medicinal Cosechadora de stevia
Cosechadora de stevia Medicinal algae
Medicinal algae Herbal medicine approved by doh
Herbal medicine approved by doh Medicinal algae
Medicinal algae Drug receptor interaction medicinal chemistry
Drug receptor interaction medicinal chemistry Veterinary medicinal product dossier
Veterinary medicinal product dossier Jewel weed medicinal
Jewel weed medicinal Burmm
Burmm Vanilla planifolia medicinal uses
Vanilla planifolia medicinal uses Quality control methods for medicinal plant materials
Quality control methods for medicinal plant materials Electrophilic substitution reaction of quinoline
Electrophilic substitution reaction of quinoline Farmacocinetica
Farmacocinetica Objective of medicinal plants
Objective of medicinal plants Bachelorkontrakt
Bachelorkontrakt Quimica medicinal
Quimica medicinal Patrick: an introduction to medicinal chemistry 3e
Patrick: an introduction to medicinal chemistry 3e Toilet preparation act 1955
Toilet preparation act 1955 Pyrrole medicinal uses
Pyrrole medicinal uses Drug-receptor interaction
Drug-receptor interaction Mixtures of organic substances and a medicinal agent are:
Mixtures of organic substances and a medicinal agent are: April ford incentives
April ford incentives