Adrenal and retropetionium Disorders of the Adrenal Cortex















- Slides: 25

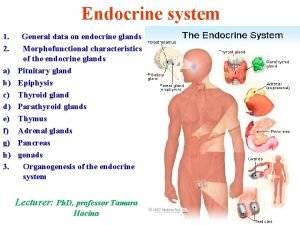
Adrenal and retropetionium

• Disorders of the Adrenal Cortex • Hyperaldosteronism: • Hyperaldosteronism may be secondary to stimulation of the renin-angiotensin system from renal artery stenosis and to low-flow states such as congestive heart failure and cirrhosis. • Primary hyperaldosteronism results from autonomous aldosterone secretion, leads to suppression of renin secretion. Hypokalemia. Most cases result from a solitary functioning adrenal adenoma (∼ 70%) and idiopathic bilateral hyperplasia (30%). Adrenocortical carcinoma and glucocorticoid-suppressible hyperaldosteronism are rare. • Symptoms and Signs: hypertension, muscle weakness, polydipsia, polyuria, nocturia, headaches, and fatigue. Weakness and fatigue are related to the presence of hypokalemia. • Diagnostic Studies • Laboratory Studies Hypokalemia. plasma aldosterone concentration–to–plasma renin activity ratio of 1: 25 to 30 is strongly suggestive of the diagnosis. • Radiologic Studies. CT scans with 0. 5 -cm cuts in the adrenal area can localize aldosteronomas with a sensitivity of 90%. MRI scans are less sensitive but more specific. Selective venous catheterization and adrenal vein sampling for aldosterone. Scintigraphy with 131 I-6β-iodomethyl noriodocholesterol (NP-59) also may be used for the same purpose. • Treatment: Unilateral tumors producing aldosterone are best managed by adrenalectomy. For the other patients, medical therapy with spironolactone, amiloride, or triamterene is the mainstay of management.

• Cushing’s Syndrome. • Cushing described patients with a peculiar fat deposition, amenorrhea, impotence (in men), hirsutism, purple striae, hypertension, diabetes, and other features that constitute the syndrome. • Today, the term Cushing’s syndrome refers to a complex of symptoms and signs resulting from hypersecretion of cortisol regardless of etiology. • In contrast, Cushing’s disease refers to a pituitary tumor, usually an adenoma, which leads to bilateral adrenal hyperplasia and hypercortisolism. • Although most individuals have sporadic disease. Cushing’s syndrome may be found in MEN 1 families.

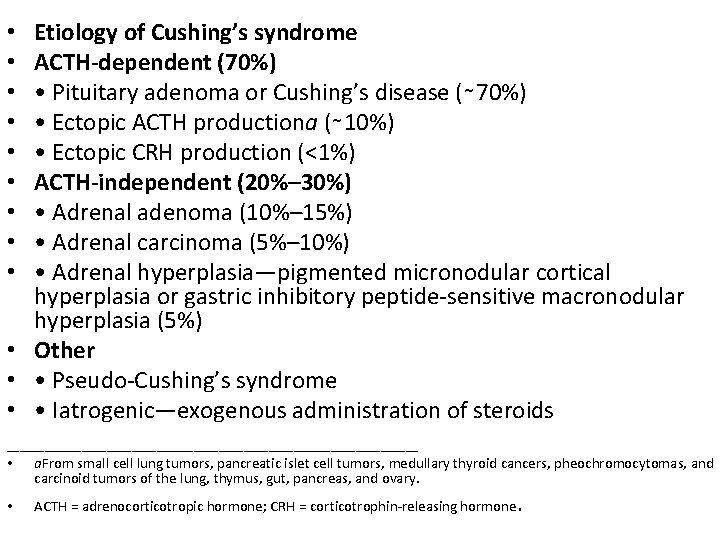
Etiology of Cushing’s syndrome ACTH-dependent (70%) • Pituitary adenoma or Cushing’s disease (∼ 70%) • Ectopic ACTH productiona (∼ 10%) • Ectopic CRH production (<1%) ACTH-independent (20%– 30%) • Adrenal adenoma (10%– 15%) • Adrenal carcinoma (5%– 10%) • Adrenal hyperplasia—pigmented micronodular cortical hyperplasia or gastric inhibitory peptide-sensitive macronodular hyperplasia (5%) • Other • • Pseudo-Cushing’s syndrome • • Iatrogenic—exogenous administration of steroids _________________ • • • a. From small cell lung tumors, pancreatic islet cell tumors, medullary thyroid cancers, pheochromocytomas, and carcinoid tumors of the lung, thymus, gut, pancreas, and ovary. • ACTH = adrenocorticotropic hormone; CRH = corticotrophin-releasing hormone .

• Patients with major depression, alcoholism, pregnancy, chronic renal failure, or stress also may have elevated cortisol levels and symptoms o hypercortisolism. However, these manifestations resolve with treatment of the underlying disorder, and these patients are deemed to have pseudo. Cushing’s syndrome.

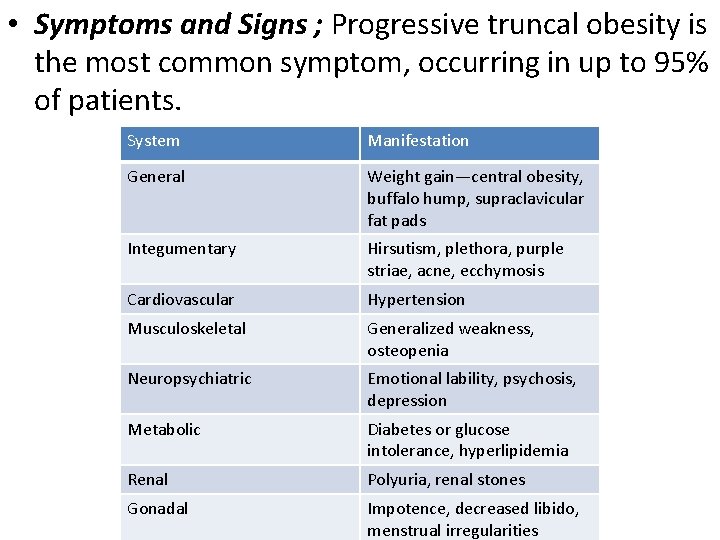
• Symptoms and Signs ; Progressive truncal obesity is the most common symptom, occurring in up to 95% of patients. System Manifestation General Weight gain—central obesity, buffalo hump, supraclavicular fat pads Integumentary Hirsutism, plethora, purple striae, acne, ecchymosis Cardiovascular Hypertension Musculoskeletal Generalized weakness, osteopenia Neuropsychiatric Emotional lability, psychosis, depression Metabolic Diabetes or glucose intolerance, hyperlipidemia Renal Polyuria, renal stones Gonadal Impotence, decreased libido, menstrual irregularities

• Diagnostic Tests: confirm the presence of Cushing’s syndrome and to determine its etiology. • characterized by elevated glucocorticoid levels that are not suppressible by exogenous hormone administration and loss of diurnal variation. • Radiologic Studies: CT and MRI scans. Radioscintigraphic imaging. • Treatment: Laparoscopic adrenalectomy is the treatment of choice for patients with adrenal adenomas. Open adrenalectomy is reserved for large tumors (≥ 6 cm) or those suspected to be adrenocortical cancers. Bilateral adrenalectomy is curative for primary adrenal hyperplasia.

• The treatment of choice in Cushing’s disease is transsphenoidal excision of the pituitary adenoma. • Pituitary irradiation has been used for patients with persistent or recurrent disease after surgery. • Patients with ectopic ACTH production are best managed by treating the primary tumor.

• Adrenocortical Cancer. • Adrenal carcinomas are rare neoplasms with a worldwide incidence of two per 1 million. • The majority are sporadic, but adrenocortical carcinomas also occur in (Li-Fraumeni syndrome) and MENIN (multiple endocrine neoplasia type 1). • Symptoms and Signs Approximately 50% of adrenocortical cancers are nonfunctioning. The remaining secrete cortisol (30%), androgens (20%), estrogens (10%), aldosterone (2%), or multiple hormones (35%). • Diagnostic Tests: measurement of serum electrolyte levels to rule out hypokalemia, urinary catecholamines to rule out pheochromocytomas, an overnight 1 -mg dexamethasone suppression test, and a 24 -hour urine collection for cortisol and 17 -ketosteroids to rule out Cushing’s syndrome. • CT and MRI scans. FDGPET or PET-CT scans may have some utility in distinguishing benign from malignant lesions.

• Treatment: The most important predictor of survival in patients with adrenal cancer is the adequacy of resection.

• Sex Steroid Excess: • Adrenal adenomas or carcinomas that secrete adrenal androgens lead to virilizing syndromes. Although women with virilizing tumors develop hirsutism, amenorrhea, infertility, and other signs of masculinization, suchas increased muscle mass, deepened voice, and temporal balding, men with these tumors are more difficult to diagnose and, hence, usually present with disease in advanced stages. • Children with virilizing tumors have accelerated growth, premature development of facial and pubic hair, acne, genital enlargement, and deepening of their voice. • Feminizing adrenal tumors are less common and occur in men in the third to fifth decades of life. These tumors lead to gynecomastia, impotence, and testicular atrophy. • Women with these tumors develop irregular menses or dysfunctional uterine bleeding. Vaginal bleeding may occur I postmenopausal women. Girls with these tumors experience precocious puberty with breast enlargement and early menarche. • Diagnostic Tests: lab teste. Eg androgen precursor, plasma or urine as 17 -ketosteroids, etc.

• Treatment Virilizing and feminizing tumors are treated by adrenalectomy. Malignancy is difficult to diagnose histologically but is suggested by the presence of local invasion, recurrence, or distal metastases. Adrenolytic drugs such as mitotane, aminoglutethimide, and ketoconazole may be useful in controlling symptoms in patients with metastatic disease.

• Disorders of the Adrenal Medulla • Pheochromocytomas: • Pheochromocytomas are rare tumors with prevalence rates ranging from 0. 3% to 0. 95% in autopsy series and approximately 1. 9% in series using biochemical screening. They can occur at any age, with a peak incidence in the fourth and fifth decades of life, and have no gender predilection. • Extra-adrenal tumors, also called functional paragangliomas. • Pheochromocytomas often are called the 10 percent tumor because 10% are bilateral, 10% are malignant, 10% occur in pediatricpatients, 10% are extra-adrenal, and 10% are familial. • Pheochromocytomas occur in families with MEN 2 A and MEN 2 B in approximately 50% of patients. MEN 1 syndrome. • Symptoms and Signs: Headache, palpitations, and diaphoresis constitute the “classic triad” of pheochromocytomas. • Cardiovascular complications, . • The most common clinical sign is hypertension. • may be paroxysmal • with intervening normotension, sustained with paroxysms • or sustained hypertension alone. • Sudden death may occur in patients with undiagnosed tumors who undergo other surgeries or biopsy.

• Diagnostic Tests • Biochemical Studies: 24 -hour urine samples for catecholamines and their metabolitesas well as by determining plasma metanephrine levels. • VMA measurements are slightly less sensitive and specific. • Extraadrenal tumors secrete norepinephrine, whereas epinephrine is the main hormone secreted from adrenal pheochromocytomas. • Radiologic Studies: o localize tumors and to assess the extent of spread once th diagnosis has been made with biochemical tests. • CT scans, MRI scans, • Metaiodobenzylguanidine (MIBG) is taken up and concentrated by vesicles in the adrenal medullar cells because its structure is similar to norepinephrine. 131 Iradiolabeled MIBG especially those in ectopic positions.

• Treatment: • The medical management of pheochromocytomas is aimed chiefly at blood pressure control and volume repletion. • long-acting α-blockers such as phenoxybenzamine are started 1 to 3 weeks before surgery • β-Blockers such as propranolol often need to be added preoperatively in patients who have persistent tachycardia and arrhythmias • β-Blockers should only be instituted after adequate αblockade and hydration to avoid the effects of unopposed α stimulation (i. e. , hypertensive crisis and congestive heart failure). • Adrenalectomy is the treatment of choice for patients with pheochromocytoma. to resect the tumor completely with minimal tumor manipulation or rupture of the tumor capsule.

• Hereditary Pheochromocytomas • Malignant Pheochromocytomas: Malignancy usually is diagnosed when there is evidence of invasion into surrounding structures or distant metastases. • The Adrenal Incidentaloma Adrenal lesions discovered during imaging performed for unrelate reasons are referred to as incidentalomas. • Adrenal Insufficiency: may be primary, resulting from adrenal disease, or secondary, due to a deficiency of ACTH. Treatment must be initiated based on clinical suspicion alone, even before test results are obtained, or the patient is unlikely to survive.

• multiple endocrine neoplasia type I (MEN I), which consists of parathyroid, pituitary, and pancreatic (or duodenal) tumors. • MEN 2 A: MTC, pheochromocytoma, primary hyperparathyroidism. • MEN 2 A is associated with medullary thyroid carcinoma and pheochromocytoma or parathyroid adenoma • MEN 2 B: MTC, pheochromocytoma, Marfanoid habitus, mucocutaneous ganglioneuromatosis • MEN 2 B is associated with medullary thyroid carcinoma, marfanoid habitus, mucosal neuromas, and ganglioneuromatosis.

• RETROPERITONEUM • Surgical Anatomy: The retroperitoneum is defined as the space between the posterior envelopment of the peritoneum and the posterior body wall. • The retroperitoneal space is bounded superiorly bythe diaphragm, posteriorly by the spinal column and iliopsoas muscles, and inferiorly by the levator ani muscles. • Retroperitoneal Infections: • Retrocecal appendicitis, contained perforation of duodenal ulcers, iatrogenic perforation with esophagogastroduodenoscopy or endoscopic retrograde cholangiopancreatography, and complicated pancreatitis may all lead to retroperitoneal infection with or without abscess formation.

• The site of pain may be variable and can include the back, pelvis, or thighs. Erythema may be observed around the umbilicus or flank. • The diagnosis is best established by CT. • Management includes identification and treatment of the underlying condition, intravenous antibiotics, and drainage of all well-defined collections.

• Retroperitoneal Fibrosis: Retroperitoneal fibrosis is a class of disorders characterized by hyperproliferation of fibrous tissue in the retroperitoneum. • may be a primary disorder as in idiopathic retroperitoneal fibrosis, also known as Ormond’s disease, or a secondary reaction to an inciting inflammatory process, malignancy, or medication. • Presenting symptoms depend on the structure or structures affected by the fibrotic process. Initially, patients complain of the insidious onset of dull, poorly localized abdominal pain. Sudden-onset or severe abdominal pain may signify acute mesenteric ischemia. Other symptoms of retroperitoneal fibrosis may include unilateral leg swelling, intermittent claudication, oliguria, hematuria, or dysuria.

• findings on physical examination vary with the retroperitoneal structure involved. Consequently, findings may include hypertension, the palpation of an abdominal or flank mass, lower-extremity edema (unilateral or bilateral), or diminished lower-extremity pulses (unilateral or bilateral). • Laboratory evaluation and Many imaging modalities have been used. • Once malignancy, drug-induced, and infectious etiologies are ruled out, treatment of the retroperitoneal fibrotic process is instituted. Corticosteroids, with or without surgery, are the mainstay of medical therapy. Surgical treatment consists primarily of ureterolysis or ureteral stenting and is required in patients who present with significant hydronephrosis. Endovascular interventions for iliocaval occlusion • Cyclosporin, tamoxifen, and azathioprine have also been used to treat patients who respond poorly to corticosteroids.


• The outermost layer, the zona glomerulosa is the main site for production of aldosterone. • Situated between the glomerulosa and reticularis the zona fasciculata is responsible for prdoucing glucocorticoids. Such as 11 deoxycorticosterone, and cortisol • The inner most cortical layer, the zona reticularis produces androgens, mainly dehydroepiandrosterone (DHEA), DHEA sulfate (DHEA-S), androstenedione (the precursor to testosterone) in humans.

• Adrenal Physiology • Cholesterol, derived from the plasma or synthesized in the adrenal, is the common precursor of all steroid hormones derived from the adrenal cortex. • Mineralocorticoids: aldosterone, 11 deoxycorticosterone (DOC), and cortisol. • Aldosterone secretion is regulated primarily by the renin-angiotensin system. • Decreased renal blood flow, decreased plasma sodium, and increased sympathetic tone all stimulate the release of renin from juxtaglomerular cells. • Glucocorticoids: cortisol, the major adrenal glucocorticoid, is regulated by ACTH secreted by the anterior pituitary, which, in turn, is under the control of corticotrophinreleasing hormone (CRH) secreted by the hypothalamus

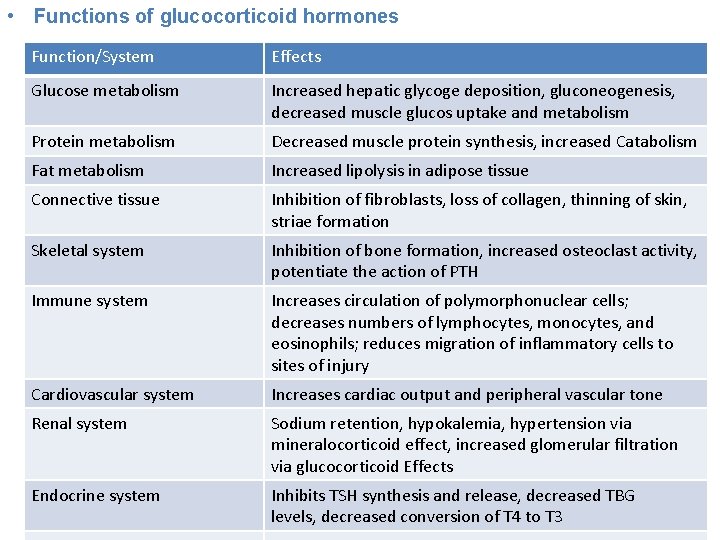
• Functions of glucocorticoid hormones Function/System Effects Glucose metabolism Increased hepatic glycoge deposition, gluconeogenesis, decreased muscle glucos uptake and metabolism Protein metabolism Decreased muscle protein synthesis, increased Catabolism Fat metabolism Increased lipolysis in adipose tissue Connective tissue Inhibition of fibroblasts, loss of collagen, thinning of skin, striae formation Skeletal system Inhibition of bone formation, increased osteoclast activity, potentiate the action of PTH Immune system Increases circulation of polymorphonuclear cells; decreases numbers of lymphocytes, monocytes, and eosinophils; reduces migration of inflammatory cells to sites of injury Cardiovascular system Increases cardiac output and peripheral vascular tone Renal system Sodium retention, hypokalemia, hypertension via mineralocorticoid effect, increased glomerular filtration via glucocorticoid Effects Endocrine system Inhibits TSH synthesis and release, decreased TBG levels, decreased conversion of T 4 to T 3
 Zona
Zona Glucocorticoids
Glucocorticoids Adrenal cortex develops from
Adrenal cortex develops from Adrenal medulla cortex
Adrenal medulla cortex Motor cortex sensory cortex
Motor cortex sensory cortex Prefrontal cortex and amygdala
Prefrontal cortex and amygdala Prefrontal cortex and amygdala
Prefrontal cortex and amygdala Outermost layer of cerebrum
Outermost layer of cerebrum Prefrontal and frontal cortex
Prefrontal and frontal cortex Medulla kidney
Medulla kidney Adrenal gland sympathetic nervous system
Adrenal gland sympathetic nervous system Adrenal bez histolojisi
Adrenal bez histolojisi Arritimias
Arritimias Dr dawn lim
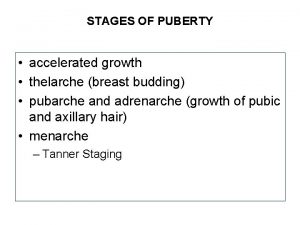
Dr dawn lim Estadiamento de tanner
Estadiamento de tanner Adrenogenital sendrom
Adrenogenital sendrom Adrenal gland relations
Adrenal gland relations Medulla
Medulla Adrenal yetmezlik acilci
Adrenal yetmezlik acilci Congenital adrenal hyperplasia characteristics
Congenital adrenal hyperplasia characteristics Menopause and mania
Menopause and mania Summary of adrenal gland
Summary of adrenal gland Gonads glands
Gonads glands Ectocrine
Ectocrine 21 hydroxylase deficiency
21 hydroxylase deficiency Feminization tubes
Feminization tubes