ADEA CASE SERIES GLP1 Receptor Agonists Merlin Thomas






















- Slides: 54

ADEA CASE SERIES GLP-1 Receptor Agonists Merlin Thomas Gary Kilov Nicole Frayne Giuliana Murfet Facilitator: Rachel Mc. Keown, Professional Services Manager, ADEA (NSW)

The right target. . . Motivated/adherent Good self-care Short duration Low hypo risk Long life expectancy No co-morbidity Good resources <7 Non-compliant Poor self-care Longstanding High hypo risk Short life expectancy Co-morbidity Limited resources COMPROMISE TARGET Adapted from Inzucchi et al Diabetes Care 2012

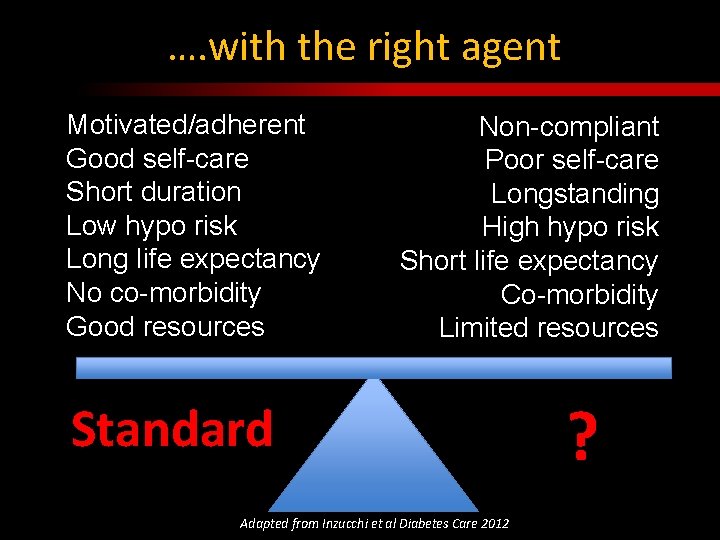
…. with the right agent Motivated/adherent Good self-care Short duration Low hypo risk Long life expectancy No co-morbidity Good resources Non-compliant Poor self-care Longstanding High hypo risk Short life expectancy Co-morbidity Limited resources Standard Adapted from Inzucchi et al Diabetes Care 2012 ?

1. BACKGROUND How does GLP-1 lower glucose levels in diabetes?

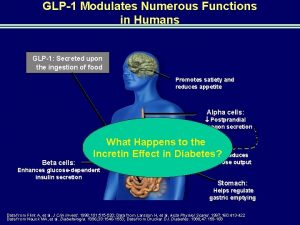
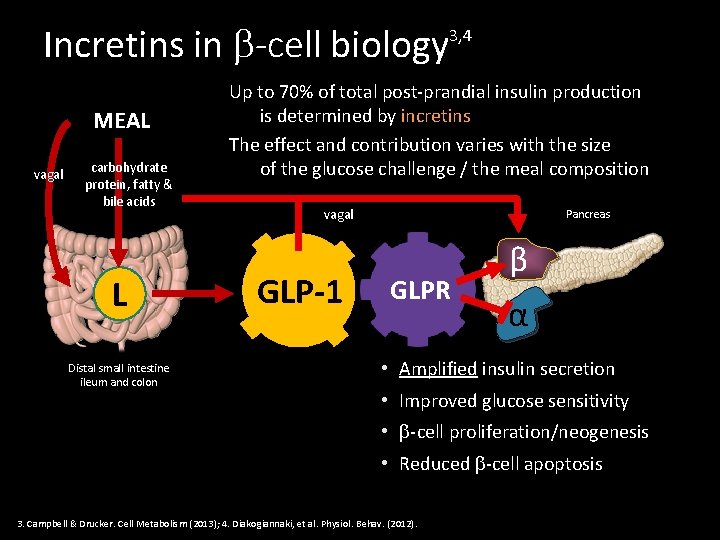
Incretins in -cell biology MEAL vagal carbohydrate protein, fatty & bile acids L Distal small intestine ileum and colon 3, 4 Up to 70% of total post-prandial insulin production is determined by incretins The effect and contribution varies with the size of the glucose challenge / the meal composition vagal GLP-1 Pancreas GLPR β α • Amplified insulin secretion • Improved glucose sensitivity • -cell proliferation/neogenesis • Reduced -cell apoptosis 3. Campbell & Drucker. Cell Metabolism (2013); 4. Diakogiannaki, et al. Physiol. Behav. (2012).

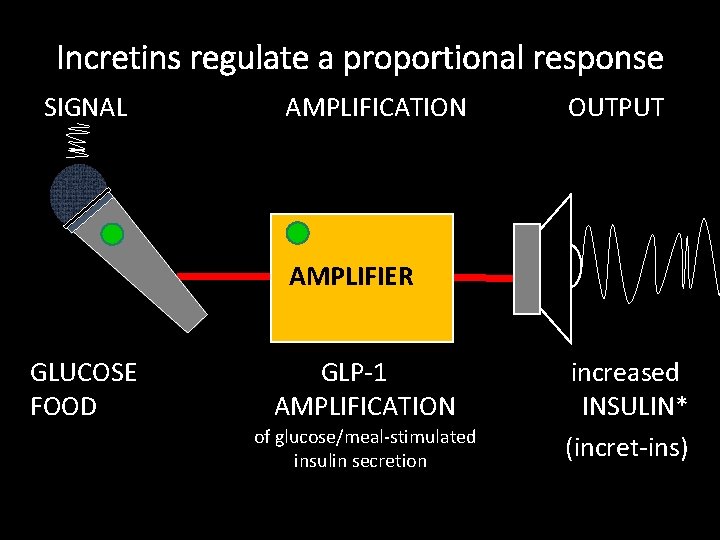
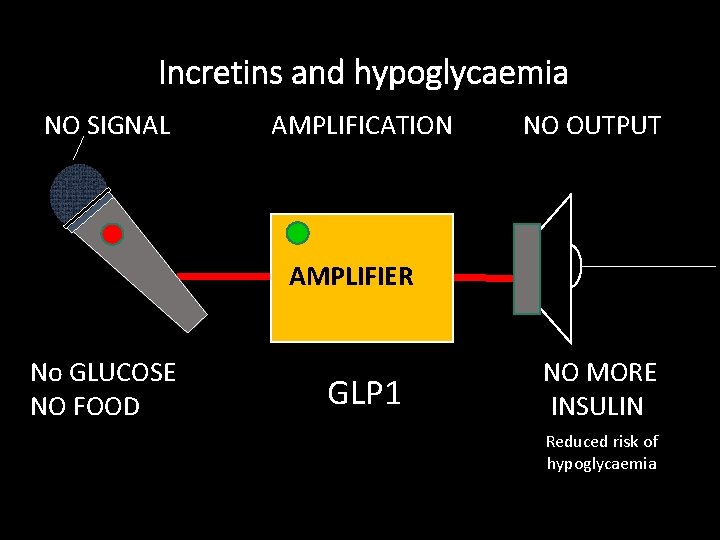
Incretins regulate a proportional response SIGNAL AMPLIFICATION OUTPUT AMPLIFIER GLUCOSE FOOD GLP-1 AMPLIFICATION of glucose/meal-stimulated insulin secretion increased INSULIN* (incret-ins)

What happens to the incretin effect in diabetes? As a consequence of hyperglycaemia and/or other metabolic manifestations of diabetes itself incretin effect is reduced by ~50% in diabetes 8 ý Normal secretion of GLP-1, but. . þ Down-regulation of GLP-1 receptor 8 þ Less substrate to act on (β-cells, δ-cells, etc) þ Gastroparesis 8 þ Resistance to GLP-1 (which needs pharmacological doses to overcome it)8, 10 8. Nauck et al. Diabetologia (2013); 9. De Frionzo Diabetes (2009) 10. Vilsbøll T, et al. Diabetologia. (2002)

Incretins and hypoglycaemia NO SIGNAL AMPLIFICATION NO OUTPUT AMPLIFIER No GLUCOSE NO FOOD GLP 1 NO MORE INSULIN Reduced risk of hypoglycaemia

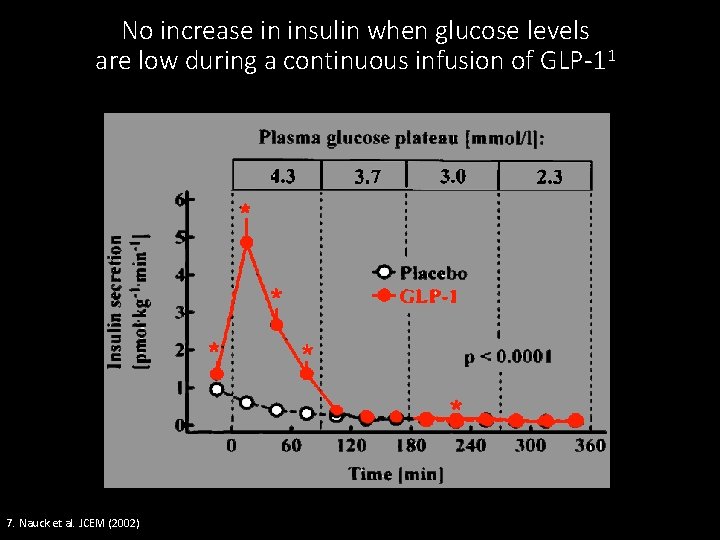
No increase in insulin when glucose levels are low during a continuous infusion of GLP-11 7. Nauck et al. JCEM (2002)

What happens to -cells in diabetes? Diabetes is associated with the blunting of hyperglycaemia or postprandial suppression of glucagon secretion Reduced incretin effect Hyperplasia of -cells Increased sensitivity to glucagon Contributes to the inappropriately increased rate of hepatic glucose output characteristic of T 2 DM =ALPHA CELL ANARCHY 8. Defronzo. Diabetes (2009)

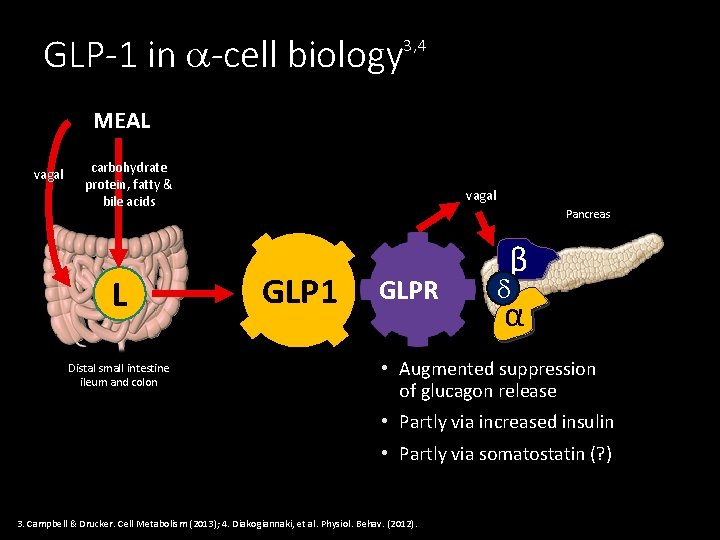
GLP-1 in -cell biology 3, 4 MEAL vagal carbohydrate protein, fatty & bile acids L Distal small intestine ileum and colon vagal Pancreas GLP 1 GLPR β α • Augmented suppression of glucagon release • Partly via increased insulin • Partly via somatostatin (? ) 3. Campbell & Drucker. Cell Metabolism (2013); 4. Diakogiannaki, et al. Physiol. Behav. (2012).

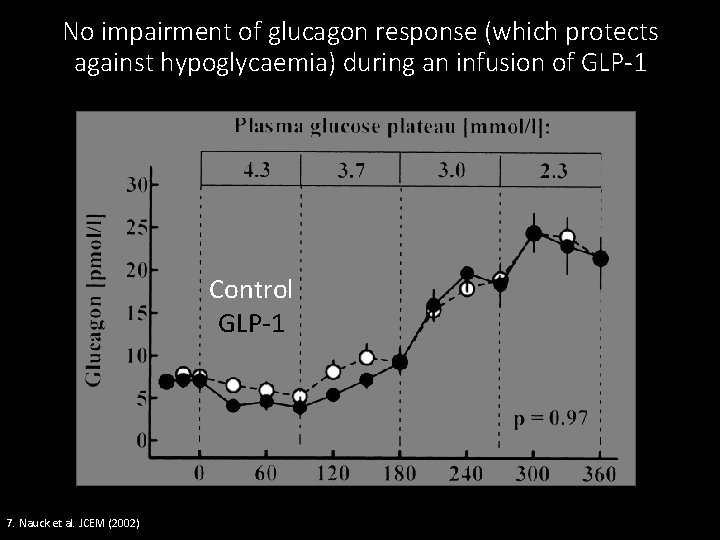
No impairment of glucagon response (which protects against hypoglycaemia) during an infusion of GLP-1 Control GLP-1 7. Nauck et al. JCEM (2002)

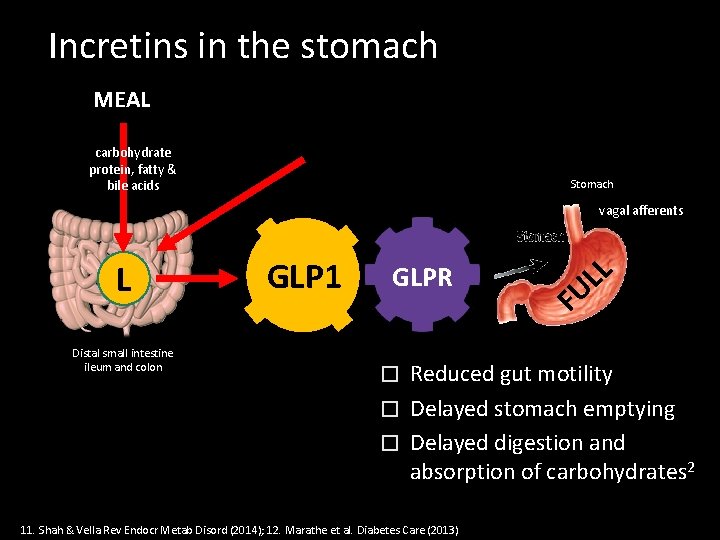
Incretins in the stomach MEAL carbohydrate protein, fatty & bile acids Stomach vagal afferents L Distal small intestine ileum and colon GLP 1 GLPR L L U F Reduced gut motility � Delayed stomach emptying � Delayed digestion and absorption of carbohydrates 2 � 11. Shah & Vella Rev Endocr Metab Disord (2014); 12. Marathe et al. Diabetes Care (2013)

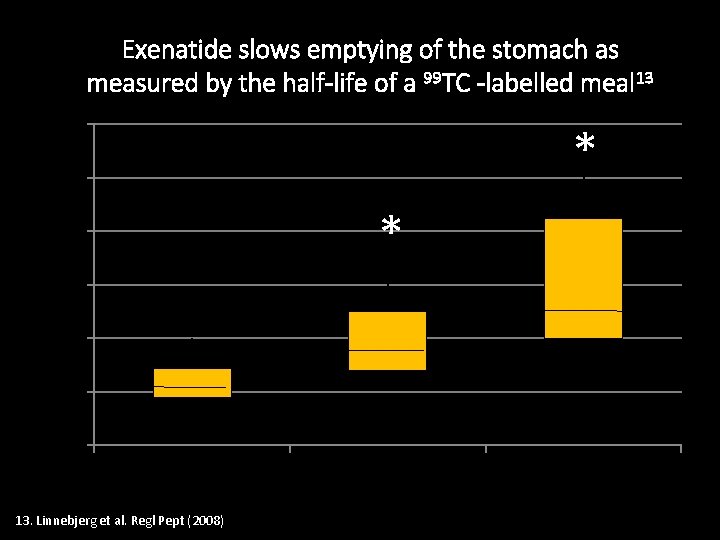
Exenatide slows emptying of the stomach as measured by the half-life of a 99 TC -labelled meal 13 T(50) h of labelled meal 6 * 5 * 4 3 2 1 0 Placebo 13. Linnebjerg et al. Regl Pept (2008) Exenatide (5µg) Exenatide (10µg)

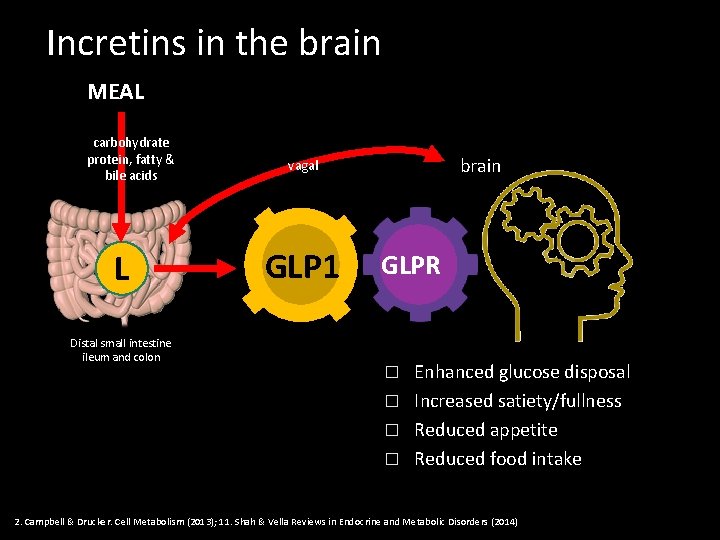
Incretins in the brain MEAL carbohydrate protein, fatty & bile acids L Distal small intestine ileum and colon brain vagal GLP 1 GLPR Enhanced glucose disposal � Increased satiety/fullness � Reduced appetite � Reduced food intake � 2. Campbell & Drucker. Cell Metabolism (2013); 11. Shah & Vella Reviews in Endocrine and Metabolic Disorders (2014)

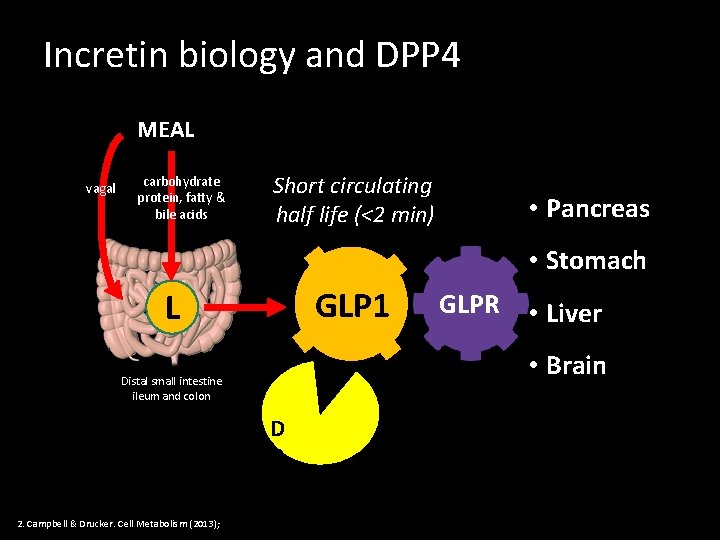
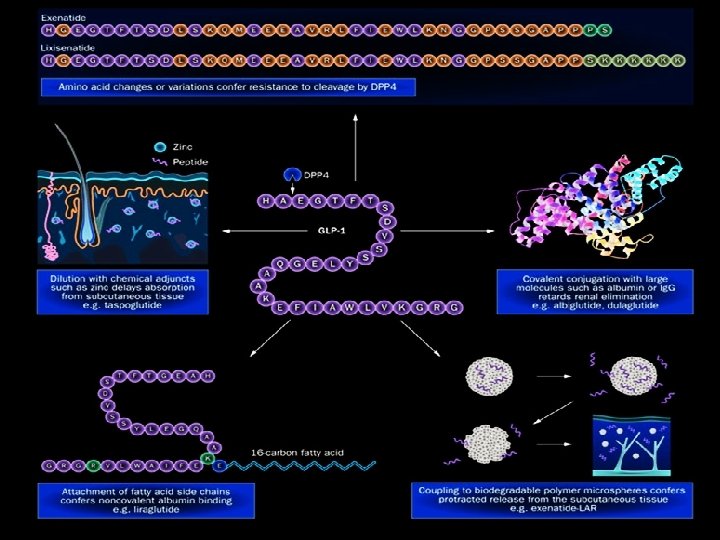
Incretin biology and DPP 4 MEAL vagal carbohydrate protein, fatty & bile acids Short circulating half life (<2 min) • Pancreas • Stomach GLP 1 L • Liver • Brain Distal small intestine ileum and colon 2. Campbell & Drucker. Cell Metabolism (2013); GLPR D P P 4

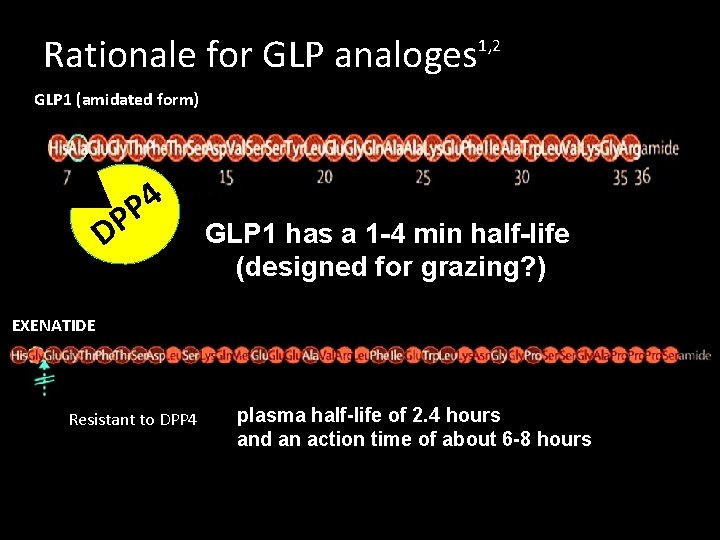
Rationale for GLP analoges 1, 2 GLP 1 (amidated form) 4 P DP GLP 1 has a 1 -4 min half-life (designed for grazing? ) EXENATIDE Resistant to DPP 4 plasma half-life of 2. 4 hours and an action time of about 6 -8 hours

The Gila monster

From Lizard Saliva to Diabetes Drug “I want some of that (adaptation) for my patients”


All lower the Hb. A 1 c by approximately the same amount when added-on metformin 40 RCT (n=17795): 6 -12 months trials, added-on after MFM failure Mc. Intosh B et al. Open Med 2011; 5: E 35 -E 48

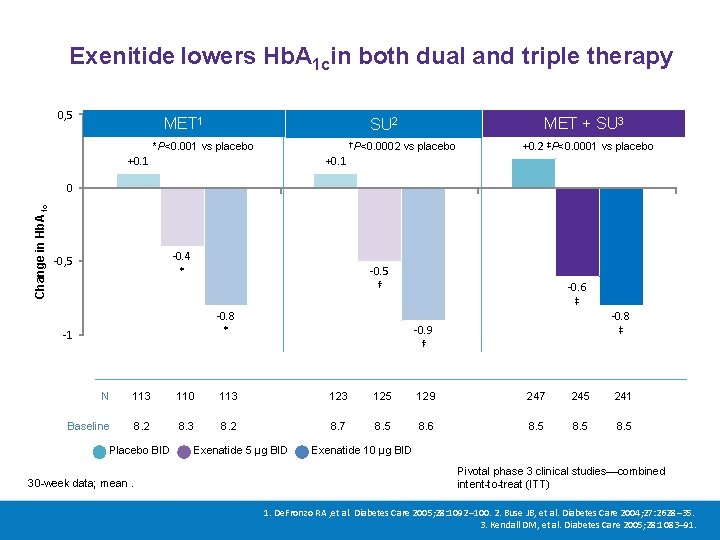
Exenitide lowers Hb. A 1 cin both dual and triple therapy 0, 5 MET 1 MET + SU 3 SU 2 †P<0. 0002 vs placebo *P<0. 001 vs placebo +0. 1 +0. 2 ‡P<0. 0001 vs placebo +0. 1 Change in Hb. A 1 c 0 -0. 4 * -0, 5 -0. 5 † -0. 8 * -1 -0. 6 ‡ -0. 8 ‡ -0. 9 † N 113 110 113 125 129 247 245 241 Baseline 8. 2 8. 3 8. 2 8. 7 8. 5 8. 6 8. 5 Placebo BID 30 -week data; mean. Exenatide 5 μg BID Exenatide 10 μg BID Pivotal phase 3 clinical studies—combined intent-to-treat (ITT) 1. De. Fronzo RA , et al. Diabetes Care 2005; 28: 1092– 100. 2. Buse JB, et al. Diabetes Care 2004; 27: 2628– 35. 3. Kendall DM, et al. Diabetes Care 2005; 28: 1083– 91.

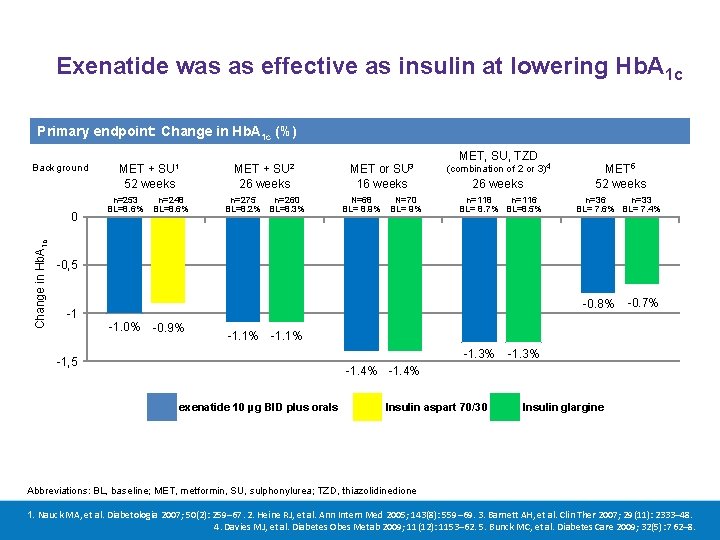
Exenatide was as effective as insulin at lowering Hb. A 1 c Primary endpoint: Change in Hb. A 1 c (%) Background Change in Hb. A 1 c 0 SU 1 SU 2 MET + 52 weeks MET + 26 weeks n=253 n=248 BL=8. 6% n=275 n=260 BL=8. 2% BL=8. 3% SU 3 MET or 16 weeks N=68 N=70 BL= 8. 9% BL= 9% MET, SU, TZD (combination of 2 or 3)4 26 weeks n=118 n=116 BL= 8. 7% BL=8. 5% MET 5 52 weeks n=36 n=33 BL= 7. 6% BL= 7. 4% -0, 5 -0. 8% -1 -1. 0% -0. 9% -1. 1% -0. 7% -1. 1% -1. 3% -1, 5 -1. 3% -1. 4% exenatide 10 µg BID plus orals Insulin aspart 70/30 Insulin glargine Abbreviations: BL, baseline; MET, metformin, SU, sulphonylurea; TZD, thiazolidinedione 1. Nauck MA, et al. Diabetologia 2007; 50(2): 259– 67. 2. Heine RJ, et al. Ann Intern Med 2005; 143(8): 559– 69. 3. Barnett AH, et al. Clin Ther 2007; 29(11): 2333– 48. 4. Davies MJ, et al. Diabetes Obes Metab 2009; 11(12): 1153– 62. 5. Bunck MC, et al. Diabetes Care 2009; 32(5): 762– 8.

CASE#1 Starting GLP-1 R agonists

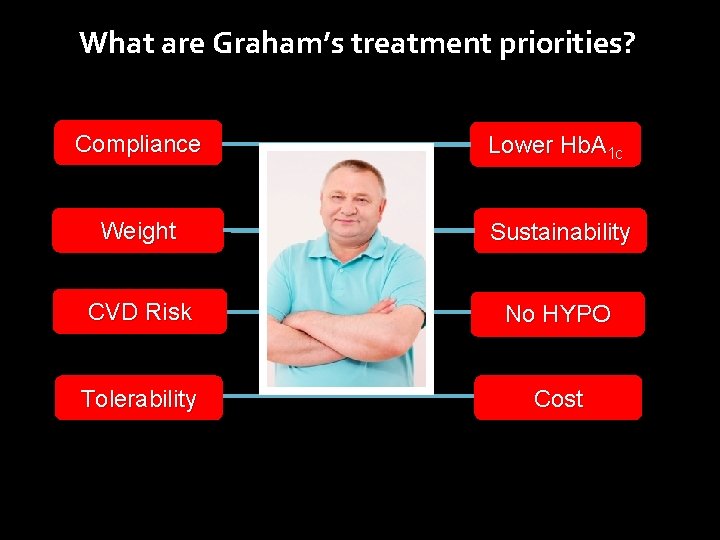
Graham presents to his GP Patient history • • Age 50 years Married, 3 children Works as a taxi driver BP 132/85 mm. Hg BMI 30 kg/m 2 Non-smoker Diet and exercise not optimal Graham, aged 50 Medical history • Diabetes diagnosed 18 months ago • Dyslipidaemia and hypertension diagnosed 3 years ago Laboratory parameters Hb. A 1 c 7. 9% � Total cholesterol: 3. 8 mmol/L � Normal albuminuria and e. GFR � Current Medications • Metformin 1500 mg/day • Rosuvastatin 20 mg/day • Telmisartan/hydrochlorothiazide 80/12. 5 mg fixed dose combination

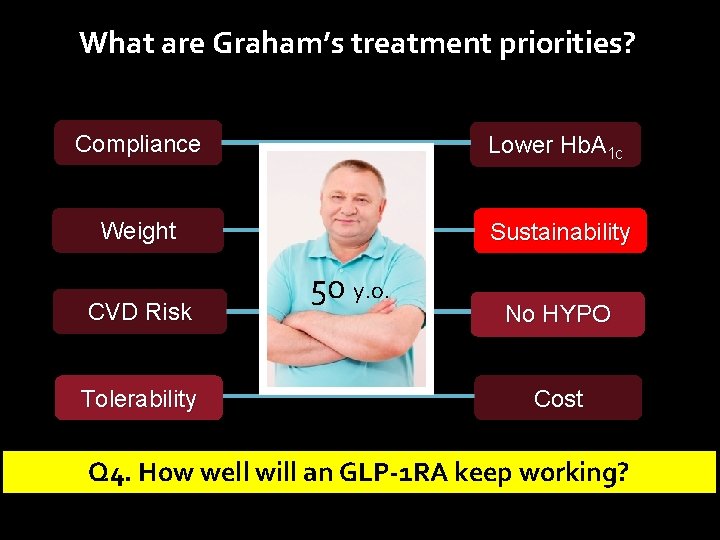
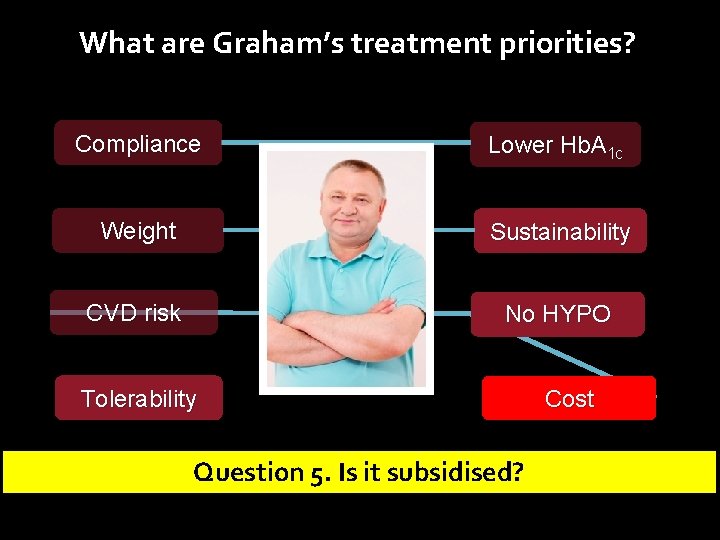
What are Graham’s treatment priorities? Compliance Lower Hb. A 1 c Weight Sustainability CVD Risk No HYPO Tolerability Cost

What are Graham’s treatment priorities? Compliance Lower Hb. A 1 c Weight Sustainability CVD Risk No HYPO Tolerability Cost Question 1. How well will he tolerate an GLP-1 R agonist?

Common side effects Nausea (20 -30%) Vomiting (~10%) Diarrhoea (~10%) Injection site reactions (10 -20%)

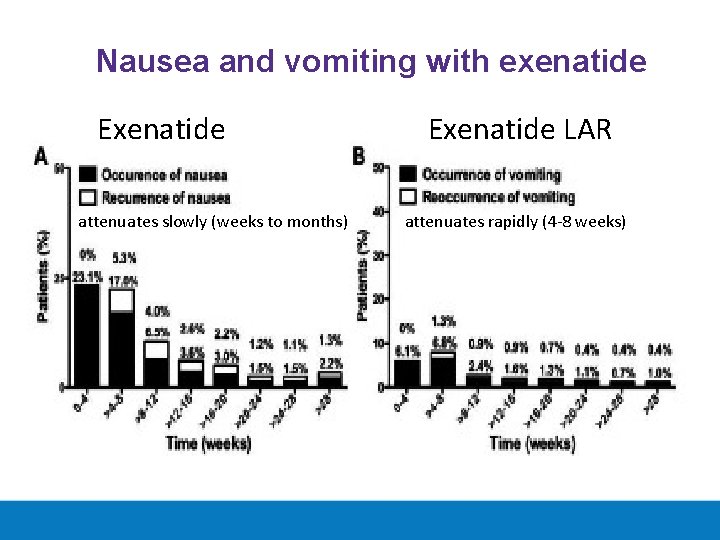
Nausea and vomiting with exenatide Exenatide attenuates slowly (weeks to months) Exenatide LAR attenuates rapidly (4 -8 weeks)

Tricks for tolerability • Cautious dose escalation 5µg bd to 10µg bd • Reducing the size as well as the fat content of meals can sometimes help get patients through. • Although exenatide can be injected at any time within 60 minutes of meal, starting off at ~15 minutes prior meal and slowly extending this depending on tolerability can also help. Question 1 a. What other tricks do you have? 30

What are Graham’s treatment priorities? Compliance Lower Hb. A 1 c Weight Sustainability CVD Risk No HYPO Tolerability Cost Question 2. What about driving on a GLP-1 RA?

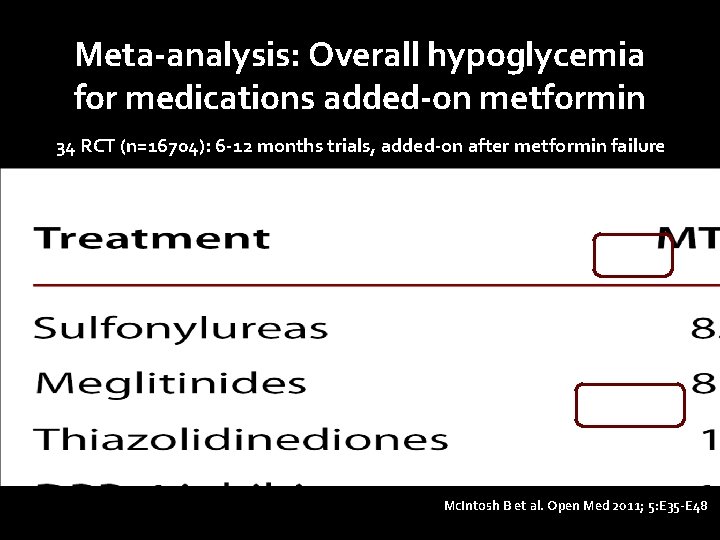
Meta-analysis: Overall hypoglycemia for medications added-on metformin 34 RCT (n=16704): 6 -12 months trials, added-on after metformin failure Mc. Intosh B et al. Open Med 2011; 5: E 35 -E 48

GLP 1 doesn’t cause hypoglycemia GLP-1 actions on the β cell are normally tightly coupled to the level of ambient glucose. The insulinotropic actions of GLP-1 are rapidly terminated once the plasma glucose falls into the normal range 7 6 5 4 3 2 1 33

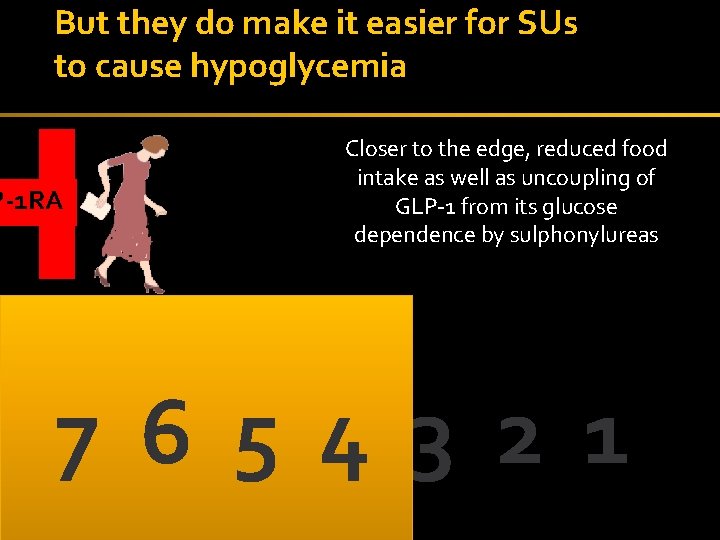
But they do make it easier for SUs to cause hypoglycemia P-1 RA Closer to the edge, reduced food intake as well as uncoupling of GLP-1 from its glucose dependence by sulphonylureas 7 6 5 4 3 2 1 34

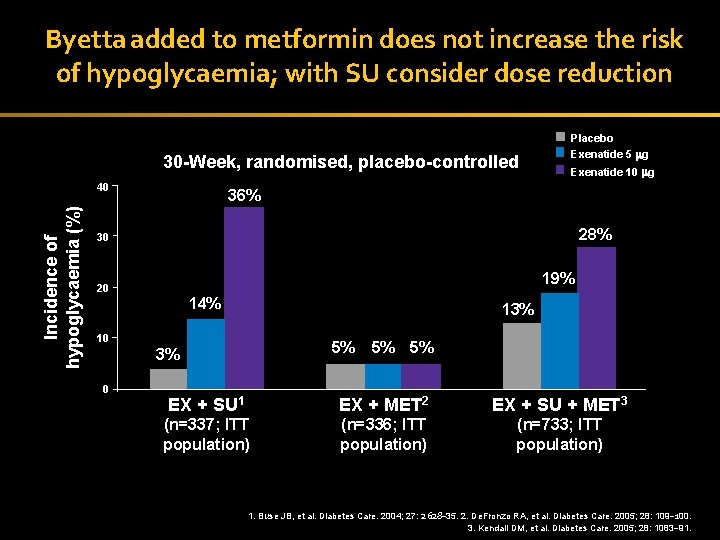
Byetta added to metformin does not increase the risk of hypoglycaemia; with SU consider dose reduction Placebo 30 -Week, randomised, placebo-controlled Incidence of hypoglycaemia (%) 40 Exenatide 5 g Exenatide 10 g 36% 28% 30 19% 20 14% 13% 10 5% 3% 0 5% 5% EX + SU 1 EX + MET 2 EX + SU + MET 3 (n=337; ITT population) (n=336; ITT population) (n=733; ITT population) 35 1. Buse JB, et al. Diabetes Care. 2004; 27: 2628– 35. 2. De. Fronzo RA, et al. Diabetes Care. 2005; 28: 109– 100. 3. Kendall DM, et al. Diabetes Care. 2005; 28: 1083– 91.

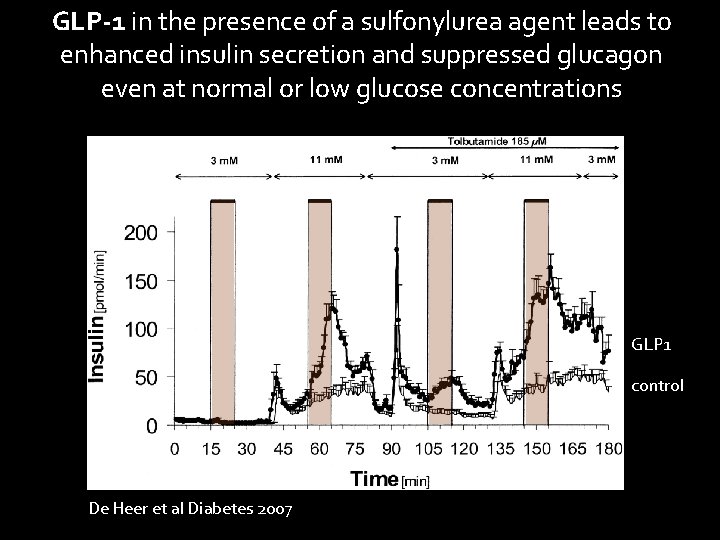
GLP-1 in the presence of a sulfonylurea agent leads to enhanced insulin secretion and suppressed glucagon even at normal or low glucose concentrations GLP 1 control De Heer et al Diabetes 2007

What are Graham’s treatment priorities? Compliance Lower Hb. A 1 c Weight Loss Sustainability CVD Risk No HYPO Tolerability Cost Question 3. How will GLP-1 R agonist affect his weight?

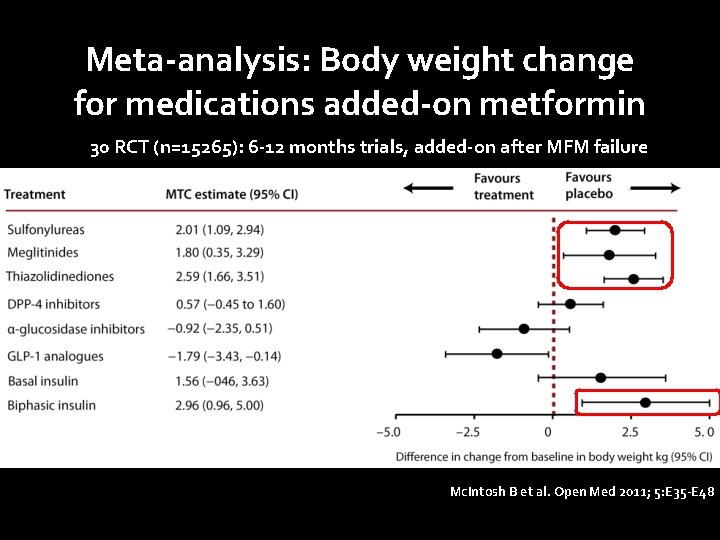
Meta-analysis: Body weight change for medications added-on metformin 30 RCT (n=15265): 6 -12 months trials, added-on after MFM failure Mc. Intosh B et al. Open Med 2011; 5: E 35 -E 48

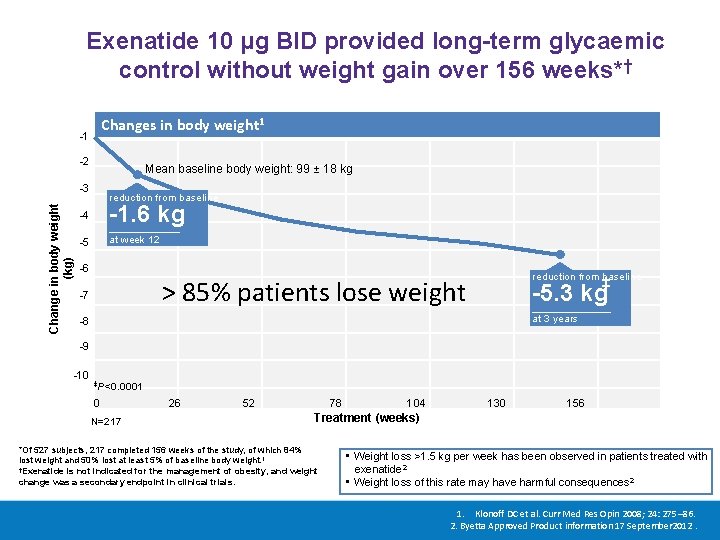
Exenatide 10 μg BID provided long-term glycaemic control without weight gain over 156 weeks*† Changes in body weight 1 -1 -2 Mean baseline body weight: 99 ± 18 kg Change in body weight (kg) -3 reduction from baseline -4 -1. 6 kg -5 at week 12 -6 reduction from baseline > 85% patients lose weight -7 ‡ -5. 3 kg at 3 years -8 -9 -10 ‡P<0. 0001 0 N=217 26 52 78 104 130 156 Treatment (weeks) *Of 527 subjects, 217 completed 156 weeks of the study, of which 84% lost weight and 50% lost at least 5% of baseline body weight. 1 †Exenatide is not indicated for the management of obesity, and weight change was a secondary endpoint in clinical trials. • Weight loss >1. 5 kg per week has been observed in patients treated with exenatide 2 • Weight loss of this rate may have harmful consequences 2 1. Klonoff DC et al. Curr Med Res Opin 2008; 24: 275– 86. 2. Byetta Approved Product information 17 September 2012.

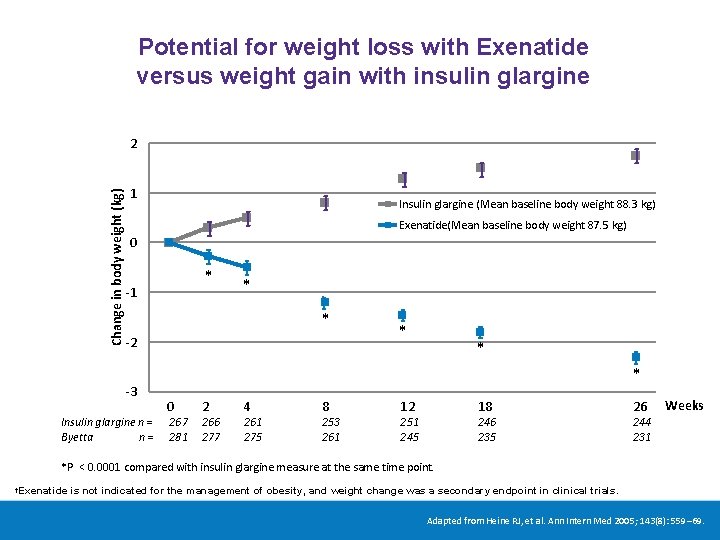
Potential for weight loss with Exenatide versus weight gain with insulin glargine Change in body weight (kg) 2 1 Insulin glargine (Mean baseline body weight 88. 3 kg) Exenatide(Mean baseline body weight 87. 5 kg) 0 * -1 * * -2 -3 Insulin glargine n = Byetta n= * * * 0 267 281 2 266 277 4 261 275 8 253 261 12 18 251 245 246 235 26 Weeks 244 231 *P < 0. 0001 compared with insulin glargine measure at the same time point. Exenatide is not indicated for the management of obesity, and weight change was a secondary endpoint in clinical trials. † Adapted from Heine RJ, et al. Ann Intern Med 2005; 143(8): 559– 69.

What are Graham’s treatment priorities? Compliance Lower Hb. A 1 c Weight Sustainability CVD Risk Tolerability 50 y. o. No HYPO Cost Q 4. How well will an GLP-1 RA keep working?

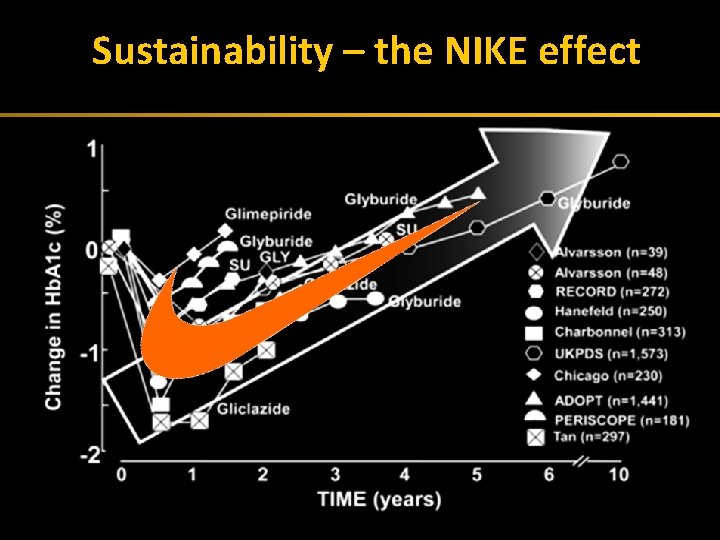
Sustainability –(nike the NIKE effect Exhaustion effect) 42

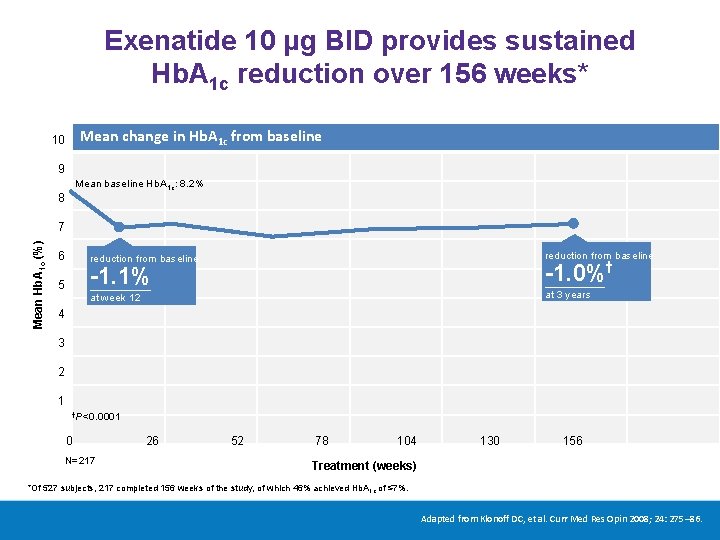
Exenatide 10 μg BID provides sustained Hb. A 1 c reduction over 156 weeks* Mean change in Hb. A 1 c from baseline 10 9 Mean baseline Hb. A 1 c: 8. 2% 8 Mean Hb. A 1 c (%) 7 6 reduction from baseline -1. 0%† -1. 1% 5 at 3 years at week 12 4 3 2 1 †P<0. 0001 0 N=217 26 52 78 104 130 156 Treatment (weeks) *Of 527 subjects, 217 completed 156 weeks of the study, of which 46% achieved Hb. A 1 c of ≤ 7%. Adapted from Klonoff DC, et al. Curr Med Res Opin 2008; 24: 275– 86.

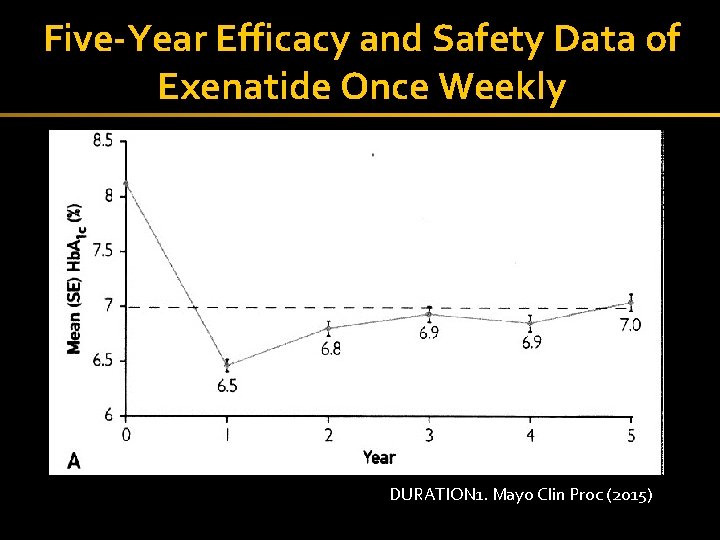
Five-Year Efficacy and Safety Data of Exenatide Once Weekly 44 DURATION 1. Mayo Clin Proc (2015)

What are Graham’s treatment priorities? Compliance Lower Hb. A 1 c Weight Sustainability CVD Risk No HYPO Tolerability Cost Question 5. Will a GLP 1 RA protect his heart?

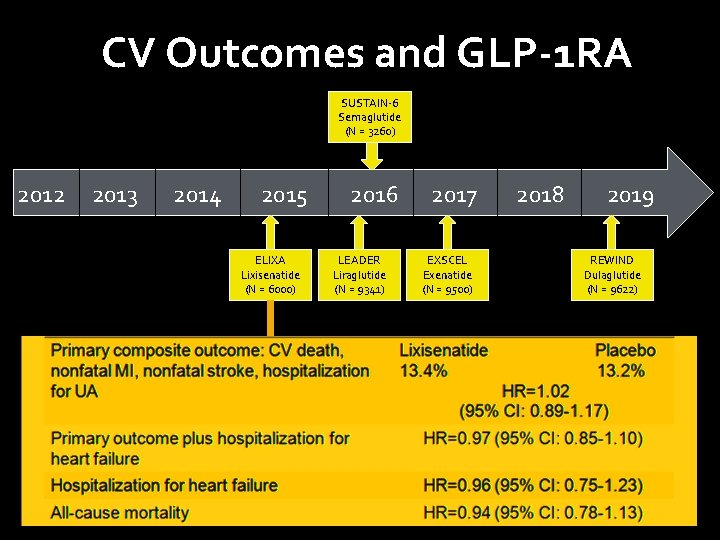
CV Outcomes and GLP-1 RA SUSTAIN-6 Semaglutide (N = 3260) 2012 2013 2014 2015 ELIXA Lixisenatide (N = 6000) 2016 LEADER Liraglutide (N = 9341) 2017 EXSCEL Exenatide (N = 9500) 2018 2019 REWIND Dulaglutide (N = 9622)

What are Graham’s treatment priorities? Compliance Lower Hb. A 1 c Weight Sustainability CVD risk No HYPO Tolerability Question 5. Is it subsidised? Cost

CASE#2 GLP-1 RA in addition to insulin

Kevin presents to his GP Patient history • • • Age 50 years BP 142/75 mm. Hg BMI 37 kg/m 2 Non-smoker Diet and exercise not optimal Kevin aged 50 Medical history • Diabetes diagnosed 5 years ago • Failed oral therapy Now on insulin injections • But control is still suboptimal Laboratory parameters Hb. A 1 c 8. 2% � Total cholesterol: 5. 8 mmol/L � e. GFR 125 ml/min/1. 73 m 2 � Current Medications • • Metformin 2 g/day Insulin 100 U/day Rosuvastatin 20 mg/day Telmisartan/hydrochlorothiazide 80/12. 5 mg fixed dose combination

What are Kevin’s treatment priorities? Compliance Weight Lower Hb. A 1 c Sustainability CVD Risk No HYPO Tolerability Cost Q 6. How well will an GLP 1 RA work for Kevin (on top of his insulin regimen)?

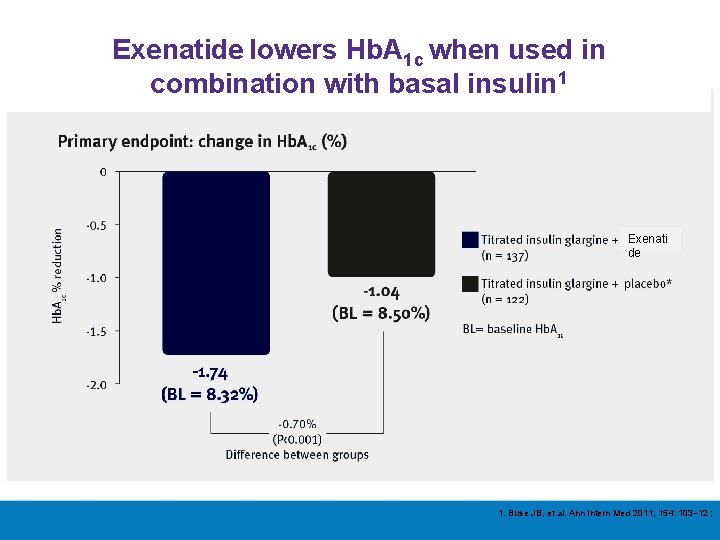
Over the past 3 years, however, the effectiveness of combining GLP-1 receptor agonists (both shorter -acting and newer weekly formulations) with basal insulin has been demonstrated, with most studies showing equal or slightly superior efficacy to the addition of prandial insulin, and with weight loss and less hypoglycemia. The available data now suggest that either a GLP-1 receptor agonist or prandial insulin could be used in this setting, with the former arguably safer, at least for short-term outcomes Inzucchi Diabetes (2015)

Exenatide lowers Hb. A 1 c when used in combination with basal insulin 1 Exenati de 1. Buse JB, et al. Ann Intern Med 2011; 154: 103– 12 ;

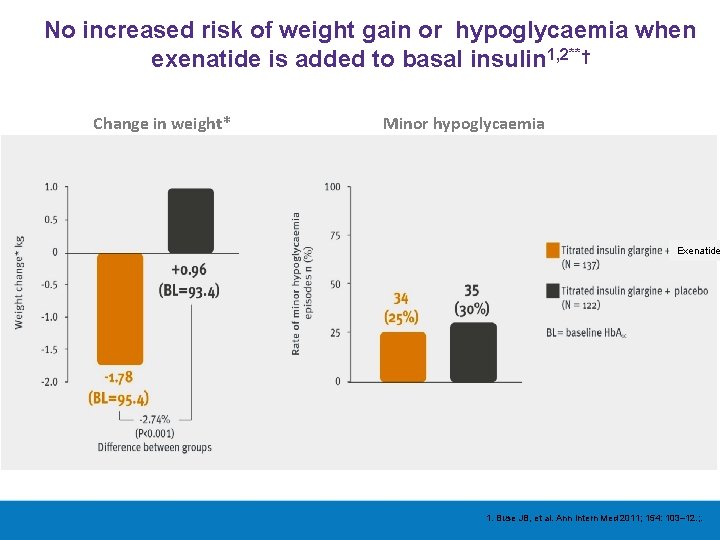
No increased risk of weight gain or hypoglycaemia when exenatide is added to basal insulin 1, 2**† Change in weight* Minor hypoglycaemia Exenatide 1. Buse JB, et al. Ann Intern Med 2011; 154: 103– 12. ; .

ADEA CASE SERIES THANK YOU FOR YOUR PARTICIPATION Merlin Thomas Gary Kilov Nicole Frayne
 Dpp4 vs glp1
Dpp4 vs glp1 Adea candito
Adea candito Adea book
Adea book Ambenomium
Ambenomium Dopamine agonists
Dopamine agonists Indirect acting cholinergic agonists
Indirect acting cholinergic agonists Best case worst case average case
Best case worst case average case Forensic pathologist vs forensic anthropologist
Forensic pathologist vs forensic anthropologist Puude tuvastamine
Puude tuvastamine Power of merlin entertainments suppliers
Power of merlin entertainments suppliers Unin merlin
Unin merlin Merlin pelicula disney
Merlin pelicula disney Pocket merlin figurative language
Pocket merlin figurative language Merlin timi 36
Merlin timi 36 Fornecedor leroy merlin
Fornecedor leroy merlin Estoire de merlin
Estoire de merlin Quantum merlin arthur
Quantum merlin arthur Merlin sepp
Merlin sepp Merlin communications
Merlin communications Merlin entertainments organisational structure
Merlin entertainments organisational structure Merlin tg
Merlin tg Drager merlin entry control board
Drager merlin entry control board Seven songs of merlin
Seven songs of merlin Merlin kirbits
Merlin kirbits Merlin kirbits
Merlin kirbits Merlin kirbits
Merlin kirbits Kevadine linnulaul
Kevadine linnulaul Merlin bergmann
Merlin bergmann Via pontano 43 milano
Via pontano 43 milano Merlin's beard figurative language
Merlin's beard figurative language Merlin tatrik
Merlin tatrik Merlin linde
Merlin linde Uther pendragon and igraine
Uther pendragon and igraine King arthur and guinevere merlin
King arthur and guinevere merlin Merlin gem
Merlin gem Merlin linde
Merlin linde Fea
Fea Eesti
Eesti Leroy jacobs
Leroy jacobs Andres lokk
Andres lokk Google drive system design
Google drive system design Merlin tatrik
Merlin tatrik Piq
Piq Merlin tatrik
Merlin tatrik Dr merlin willcox
Dr merlin willcox Maclaurin series vs taylor series
Maclaurin series vs taylor series Heisenberg 1925 paper
Heisenberg 1925 paper Maclaurin expansion
Maclaurin expansion Deret maclaurin
Deret maclaurin Ibm p series
Ibm p series Current series feedback topology mixing is
Current series feedback topology mixing is Series aiding and series opposing
Series aiding and series opposing Arithmetic sequence sum formula
Arithmetic sequence sum formula Retrospective cohort vs case series
Retrospective cohort vs case series Penelitian case series adalah
Penelitian case series adalah