ACTG 5273 Randomized Trial of Secondline ART Supports


- Slides: 19

ACTG 5273 Randomized Trial of Second-line ART Supports WHO Guidance Alberto La Rosa 1, Linda Harrison 2, Babafemi Taiwo 3, Carole L Wallis 4, Lu Zheng 2, Peter Kim 5, Nagalingeswaran Kumarasamy 6, Mina Hosseinipour 7, John W. Mellors 8, Ann C. Collier 9. 1 Asociacion Civil Impacta Salud y Educacion, Lima, Peru; 2 Harvard TH Chan School of Public Health, Boston, Massachusetts, USA; 3 Northwestern University, Chicago, USA; 4 BARC-SA and Lancet Laboratories, Johannesburg, South Africa; 5 Division of AIDS, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA; 6 YRG CARE, Chennai, India; 7 University of North Carolina, Chapel Hill, North Carolina, USA; 8 University of Pittsburgh, Pennsylvania, USA; 9 University of Washington, Seattle, Washington, USA. 1

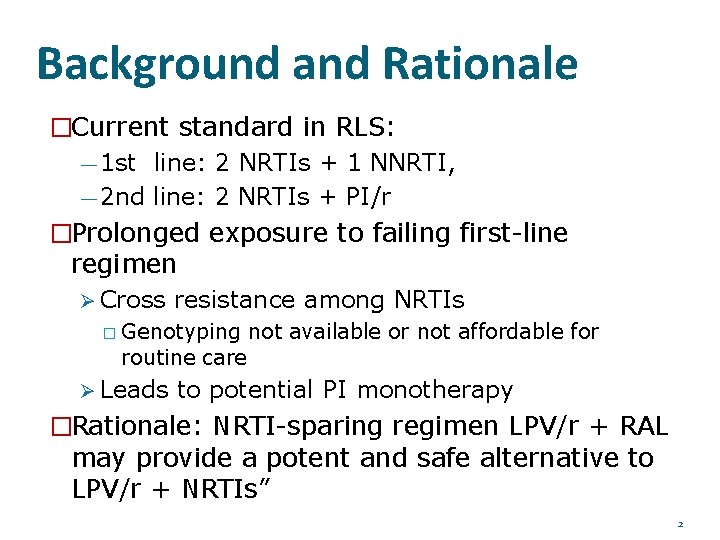
Background and Rationale �Current standard in RLS: — 1 st line: 2 NRTIs + 1 NNRTI, — 2 nd line: 2 NRTIs + PI/r �Prolonged exposure to failing first-line regimen Cross resistance among NRTIs � Genotyping routine care not available or not affordable for Leads to potential PI monotherapy �Rationale: NRTI-sparing regimen LPV/r + RAL may provide a potent and safe alternative to LPV/r + NRTIs” 2

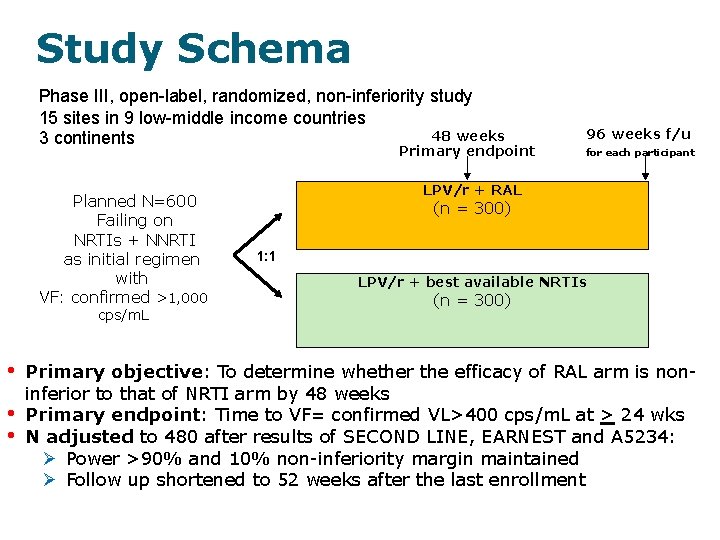
Study Schema Phase III, open-label, randomized, non-inferiority study 15 sites in 9 low-middle income countries 48 weeks 3 continents Primary endpoint Planned N=600 Failing on NRTIs + NNRTI as initial regimen with VF: confirmed >1, 000 cps/m. L • • • 96 weeks f/u for each participant LPV/r + RAL (n = 300) 1: 1 LPV/r + best available NRTIs (n = 300) Primary objective: To determine whether the efficacy of RAL arm is noninferior to that of NRTI arm by 48 weeks Primary endpoint: Time to VF= confirmed VL>400 cps/m. L at > 24 wks N adjusted to 480 after results of SECOND LINE, EARNEST and A 5234: Power >90% and 10% non-inferiority margin maintained Follow up shortened to 52 weeks after the last enrollment

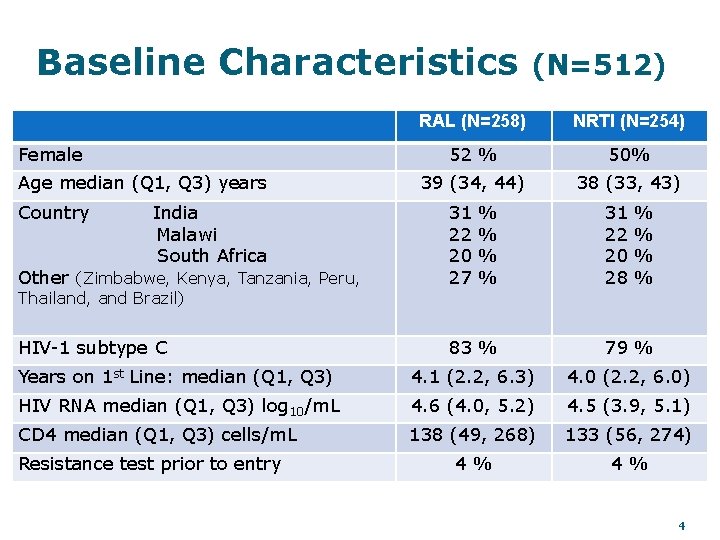
Baseline Characteristics Female Age median (Q 1, Q 3) years Country India Malawi South Africa (N=512) RAL (N=258) NRTI (N=254) 52 % 50% 39 (34, 44) 38 (33, 43) Other (Zimbabwe, Kenya, Tanzania, Peru, 31 22 20 27 HIV-1 subtype C 83 % 79 % Years on 1 st Line: median (Q 1, Q 3) 4. 1 (2. 2, 6. 3) 4. 0 (2. 2, 6. 0) HIV RNA median (Q 1, Q 3) log 10/m. L 4. 6 (4. 0, 5. 2) 4. 5 (3. 9, 5. 1) CD 4 median (Q 1, Q 3) cells/m. L 138 (49, 268) 133 (56, 274) 4% 4% Thailand, and Brazil) Resistance test prior to entry % % 31 22 20 28 % % 4

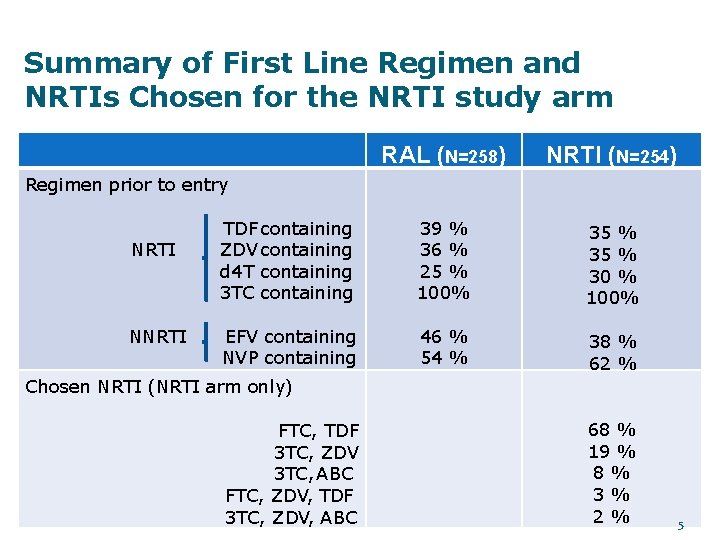
Summary of First Line Regimen and NRTIs Chosen for the NRTI study arm RAL (N=258) NRTI (N=254) TDF containing ZDV containing d 4 T containing 3 TC containing 39 % 36 % 25 % 100% 35 % 30 % 100% EFV containing NVP containing 46 % 54 % 38 % 62 % Regimen prior to entry NRTI NNRTI Chosen NRTI (NRTI arm only) FTC, TDF 3 TC, ZDV 3 TC, ABC FTC, ZDV, TDF 3 TC, ZDV, ABC 68 % 19 % 8% 3% 2% 5

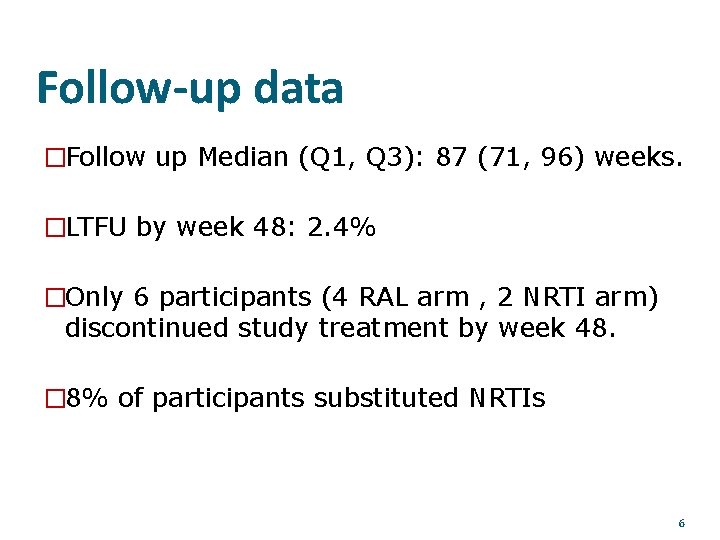
Follow-up data �Follow up Median (Q 1, Q 3): 87 (71, 96) weeks. �LTFU by week 48: 2. 4% �Only 6 participants (4 RAL arm , 2 NRTI arm) discontinued study treatment by week 48. � 8% of participants substituted NRTIs 6

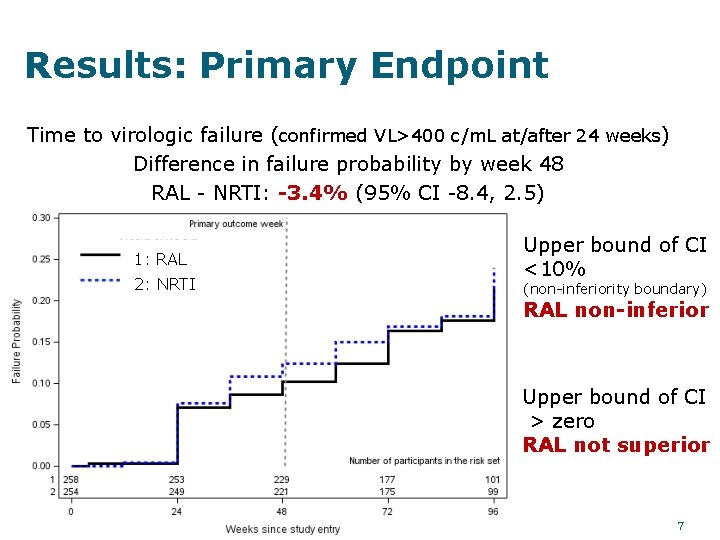
Results: Primary Endpoint Time to virologic failure (confirmed VL>400 c/m. L at/after 24 weeks) Difference in failure probability by week 48 RAL - NRTI: -3. 4% (95% CI -8. 4, 2. 5) 1: RAL 2: NRTI Upper bound of CI <10% (non-inferiority boundary) RAL non-inferior Upper bound of CI > zero RAL not superior 7

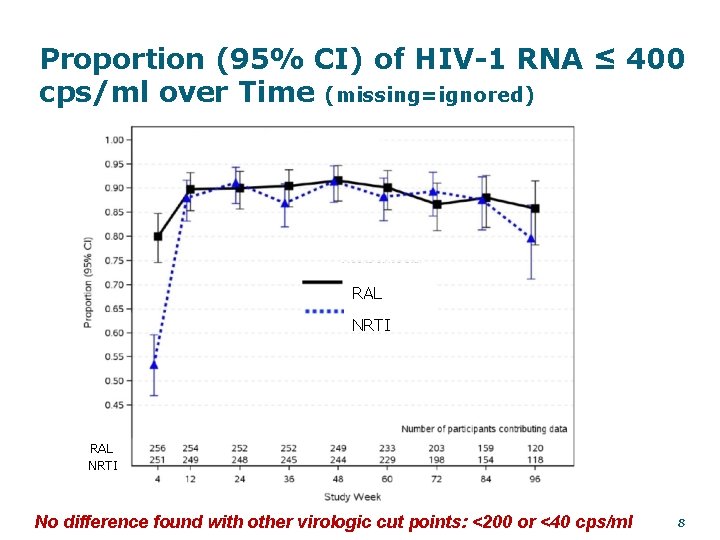
Proportion (95% CI) of HIV-1 RNA ≤ 400 cps/ml over Time (missing=ignored) RAL NRTI No difference found with other virologic cut points: <200 or <40 cps/ml 8

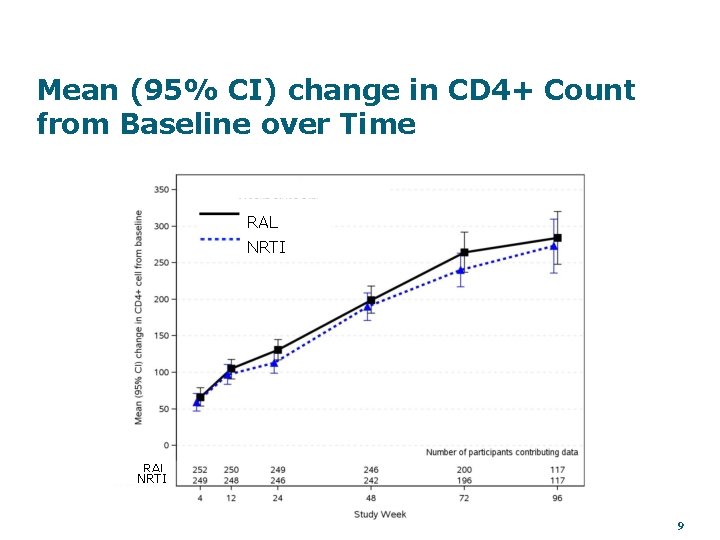
Mean (95% CI) change in CD 4+ Count from Baseline over Time RAL NRTI 9

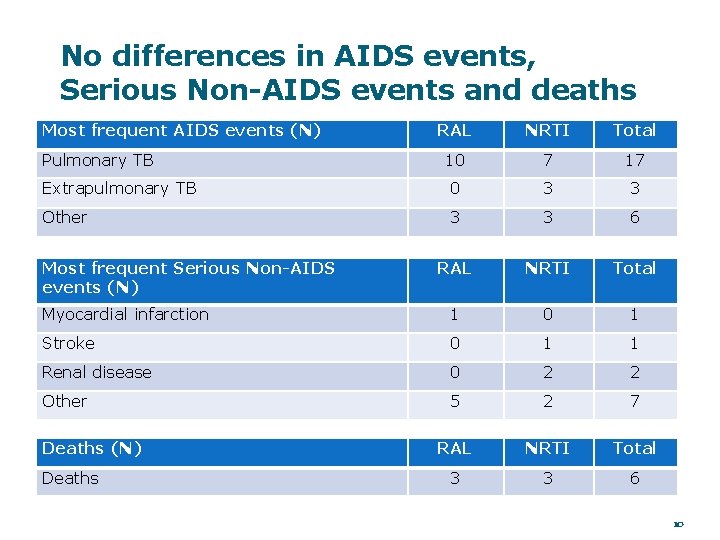
No differences in AIDS events, Serious Non-AIDS events and deaths Most frequent AIDS events (N) RAL NRTI Total 10 7 17 Extrapulmonary TB 0 3 3 Other 3 3 6 RAL NRTI Total Myocardial infarction 1 0 1 Stroke 0 1 1 Renal disease 0 2 2 Other 5 2 7 RAL NRTI Total 3 3 6 Pulmonary TB Most frequent Serious Non-AIDS events (N) Deaths 10

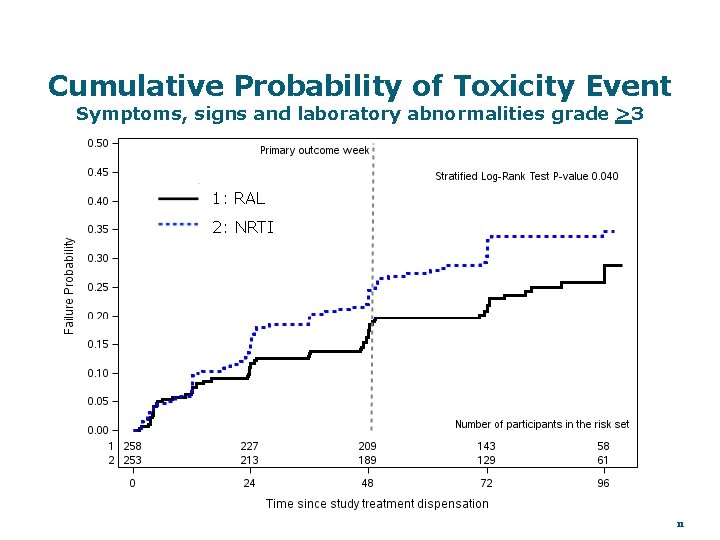
Cumulative Probability of Toxicity Event Symptoms, signs and laboratory abnormalities grade >3 1: RAL 2: NRTI 11

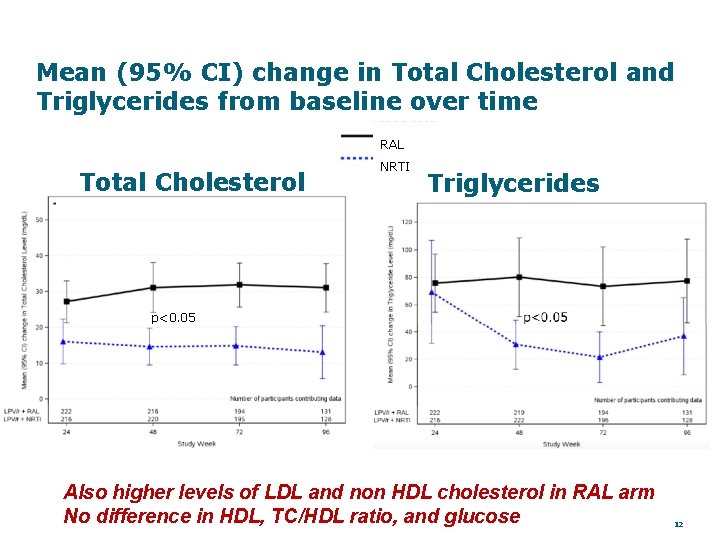
Mean (95% CI) change in Total Cholesterol and Triglycerides from baseline over time RAL Total Cholesterol NRTI Triglycerides p<0. 05 Also higher levels of LDL and non HDL cholesterol in RAL arm No difference in HDL, TC/HDL ratio, and glucose 12

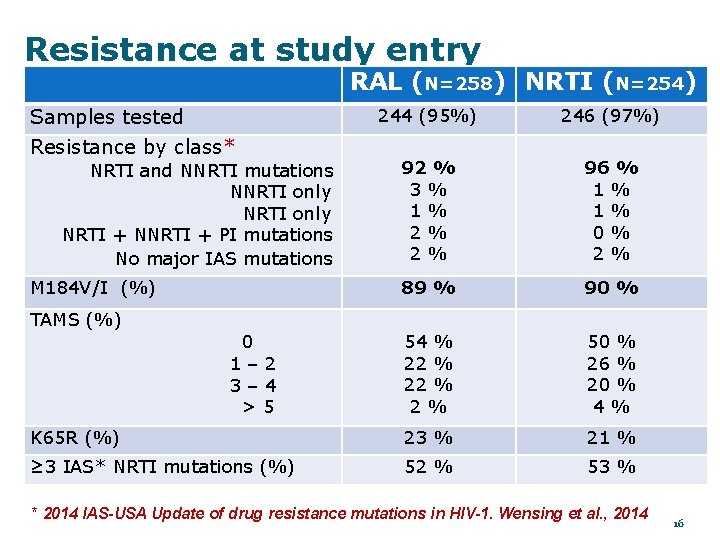
Resistance at study entry RAL (N=258) NRTI (N=254) Samples tested 244 (95%) 246 (97%) 92 % 3% 1% 2% 2% 96 % 1% 1% 0% 2% 89 % 90 % 54 % 22 % 2% 50 % 26 % 20 % 4% K 65 R (%) 23 % 21 % ≥ 3 IAS* NRTI mutations (%) 52 % 53 % Resistance by class* NRTI and NNRTI mutations NNRTI only NRTI + NNRTI + PI mutations No major IAS mutations M 184 V/I (%) TAMS (%) 0 1– 2 3– 4 >5 * 2014 IAS-USA Update of drug resistance mutations in HIV-1. Wensing et al. , 2014 16

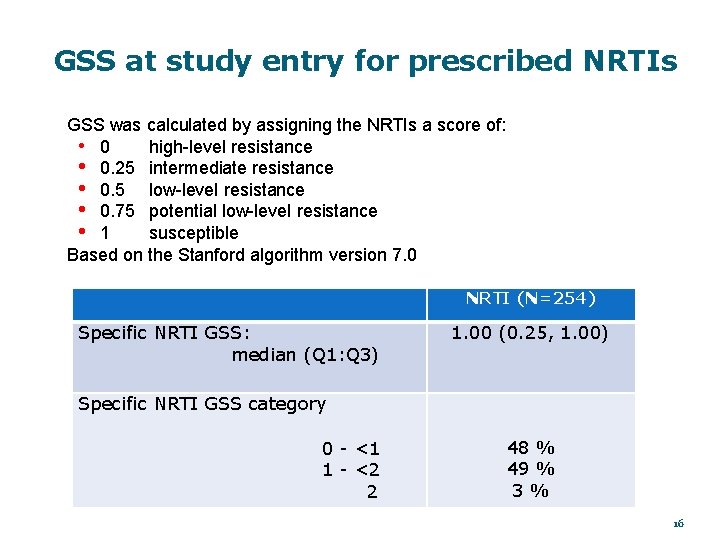
GSS at study entry for prescribed NRTIs GSS was calculated by assigning the NRTIs a score of: high-level resistance • 0. 25 intermediate resistance • 0. 5 low-level resistance • 0. 75 potential low-level resistance • 1 susceptible Based on the Stanford algorithm version 7. 0 NRTI (N=254) Specific NRTI GSS: median (Q 1: Q 3) 1. 00 (0. 25, 1. 00) Specific NRTI GSS category 0 - <1 1 - <2 2 48 % 49 % 3% 16

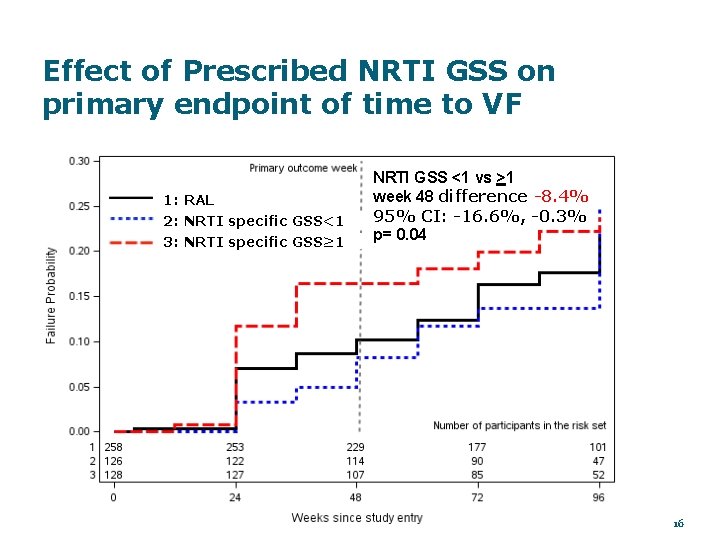
Effect of Prescribed NRTI GSS on primary endpoint of time to VF 1: RAL 2: NRTI specific GSS<1 3: NRTI specific GSS≥ 1 NRTI GSS <1 vs >1 week 48 difference -8. 4% 95% CI: -16. 6%, -0. 3% p= 0. 04 16

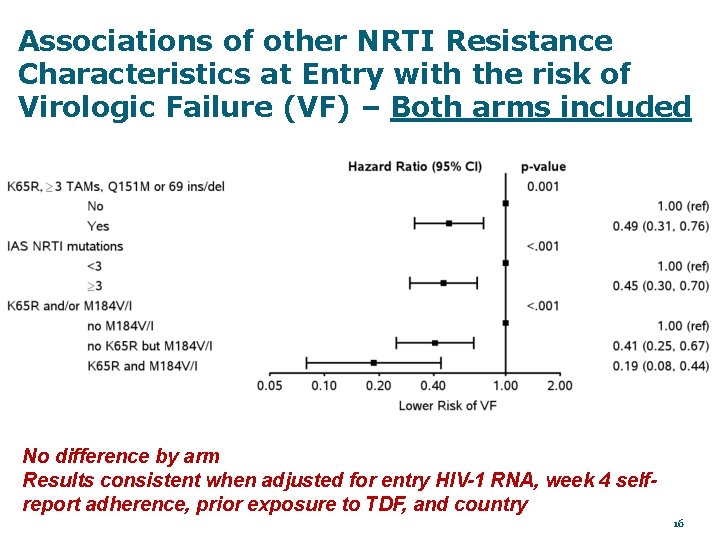
Associations of other NRTI Resistance Characteristics at Entry with the risk of Virologic Failure (VF) – Both arms included No difference by arm Results consistent when adjusted for entry HIV-1 RNA, week 4 selfreport adherence, prior exposure to TDF, and country 16

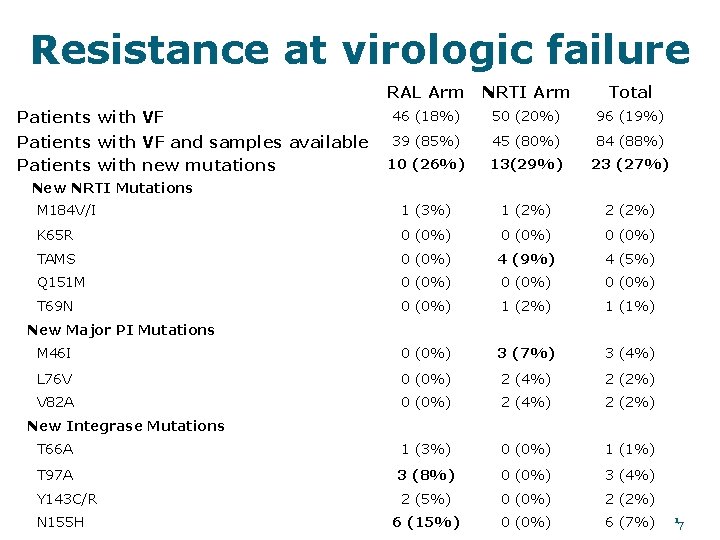
Resistance at virologic failure RAL Arm NRTI Arm Total Patients with VF 46 (18%) 50 (20%) 96 (19%) Patients with VF and samples available Patients with new mutations 39 (85%) 45 (80%) 84 (88%) 10 (26%) 13(29%) 23 (27%) M 184 V/I 1 (3%) 1 (2%) 2 (2%) K 65 R 0 (0%) TAMS 0 (0%) 4 (9%) 4 (5%) Q 151 M 0 (0%) T 69 N 0 (0%) 1 (2%) 1 (1%) M 46 I 0 (0%) 3 (7%) 3 (4%) L 76 V 0 (0%) 2 (4%) 2 (2%) V 82 A 0 (0%) 2 (4%) 2 (2%) T 66 A 1 (3%) 0 (0%) 1 (1%) T 97 A 3 (8%) 0 (0%) 3 (4%) Y 143 C/R 2 (5%) 0 (0%) 2 (2%) 6 (15%) 0 (0%) 6 (7%) New NRTI Mutations New Major PI Mutations New Integrase Mutations N 155 H 17

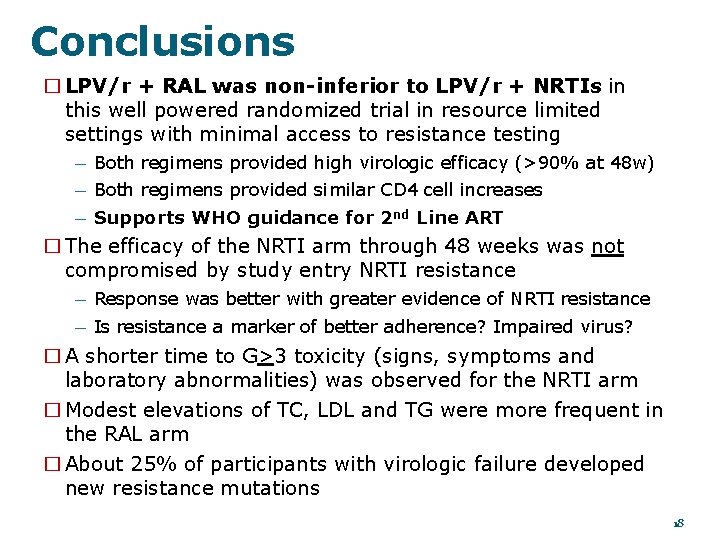
Conclusions � LPV/r + RAL was non-inferior to LPV/r + NRTIs in this well powered randomized trial in resource limited settings with minimal access to resistance testing — Both regimens provided high virologic efficacy (>90% at 48 w) — Both regimens provided similar CD 4 cell increases — Supports WHO guidance for 2 nd Line ART � The efficacy of the NRTI arm through 48 weeks was not compromised by study entry NRTI resistance — Response was better with greater evidence of NRTI resistance — Is resistance a marker of better adherence? Impaired virus? � A shorter time to G>3 toxicity (signs, symptoms and laboratory abnormalities) was observed for the NRTI arm � Modest elevations of TC, LDL and TG were more frequent in the RAL arm � About 25% of participants with virologic failure developed new resistance mutations 18

Team Members Acknowledgments Participating Sites Role Study Co. Chair Study Co-Chair Name Ann C. Collier Alberto La Rosa John Mellors Babafemi Taiwo Flavio Pontes Lara Hosey Study Vice-Chair CSS Representative Clinical Trials Specialist Clinical Trials Jennifer Specialist Rothenberg Data Manager Bernadette Drug Co. Jarocki Randi Representative Immunolog Michael Leavitt ist Co. Lederman Drug James Rooney Representative Industry Krishna Sewal Representative Jean van Wyk Drug Co. Brad Saget Industry Sandra Representative Drug Co. Wendy Snowden Representative Cardoso Mina Representative Nagalingeswaran Investigator Hosseinipour Field Representative Janet Nicotera. Cynthia Kumarasamy Investigator Laboratory Industry Asmeret Riviere Kidane Investigator. Data Manager (LDM) Representative Laura Hovind Laboratory Carmen Investigator Technologist Irizarry Medical Officer Peter Kim Pharmacologist Ed Acosta Protocol Pharmacist Ana Senior Statistician Martinez Statistician Lu (Summer) Virologi Zheng Linda st Harrison John Lancet/BARC Raquel Virologi Mellors Carole Site ID 1110 1 Site. Name Wits HIV CRS 11201 11302 11501 Durban Adult HIV CRS IMPACTA Barranco, CRS IMPACTA San Miguel, CRS Chiang Mai Univ. ACTG CRS 11601 NARI Pune CRS 11701 YRG CARE Medical Ctr. , VHS CRS 12001 3030 1 12101 3031 3 12301 3144 12601 1 12901 University of North Carolina Lilongwe College of Med. JHU CRS Instituto Pesquisa Clínica Evandro Blantyre de UZ-Parirenyatwa Chagas CRS - Harare Soweto ACTG CRS BJ Medical College CRS Moi University International CRS Study participants HIV Genotyping Labs/Staff Univ. of Pittsburgh Lancet/BARC Labs Lou Halvas Raquel Viana Industry Collaborators Abb. Vie Gilead Sciences, Inc Glaxo. Smith. Kline Merck and Company Kilimanjaro Christian Medical CRS 19
 Actg 2p12
Actg 2p12 Advantage of randomized controlled trial
Advantage of randomized controlled trial Randomized hill climbing
Randomized hill climbing Probabilistic analysis and randomized algorithms
Probabilistic analysis and randomized algorithms R o x o research design
R o x o research design Types of randomized algorithms
Types of randomized algorithms Randomized block design example
Randomized block design example Randomized polynomial time
Randomized polynomial time Cara menghitung ulangan rak
Cara menghitung ulangan rak Block design vs matched pairs
Block design vs matched pairs Randomized polynomial time
Randomized polynomial time Difference between rcbd and latin square design
Difference between rcbd and latin square design Factorial randomized block design
Factorial randomized block design Completely randomized design
Completely randomized design Statistical model for crd
Statistical model for crd Expected running time of randomized algorithm
Expected running time of randomized algorithm Rcbd design example
Rcbd design example Pronounce nivolumab
Pronounce nivolumab Randomized design
Randomized design Completely randomized design definition
Completely randomized design definition