STORM trial A randomized phase II trial for





- Slides: 9

STORM trial A randomized phase II trial for the Salvage Treatment of Oligo. Recurrent prostate cancer Metastases Kristine Engen Andreassen Urokyst 6. 9. 19

Sponsor: Prof. Dr. Piet Ost, Ghent University Hospital Co-PI: Dr. Thomas Zilli, Geneva University Hospital Local PI at OUS: Reino Heikkilä Number of participants: 178

Rationale • The entity regional nodal recurrence is not mentioned in the EAU guidelines 2016 but is an emerging clinical situation • There are no specific treatment recommendations for these type of patients, different treatment are currently used, and called coined metastasis directed therapy (MDT) • MDT in combination with or without temporary ADT could delay the subsequent risk of progression, and even cure limited regional nodal recurrences and allow to postponed lifelong palliative ADT. • The proposed trial randomizes patients with oligorecurrent nodal prostate cancer following primary PCa treatment to either metastasisdirected therapy (MDT) (s. LND or SBRT) or MDT plus WPRT (45 Gy in 25 fractions).

Objectives PRIMARY • To compare metastasis-free survival (MFS) between metastasisdirected therapy and metastasis-directed therapy with whole pelvis radiotherapy for oligorecurrent nodal prostate cancer SECONDARY • To compare clinical progression-free survival between arms • To compare biochemical progression between arms • To compare late toxicity between arms • To compare the quality of life between arms. • To compare the relapse pattern between arms • To compare time to start of palliative ADT, time to castration-resistant disease and overall survival between arms • Economical evaluation of different treatment arms • Sensitivity and specificity of imaging modality for patients receiving s. LND as MDT • Biomarker discovery: To develop a mi. RNA panel predictive for treatment response using whole genome mi. RNA expression profiling

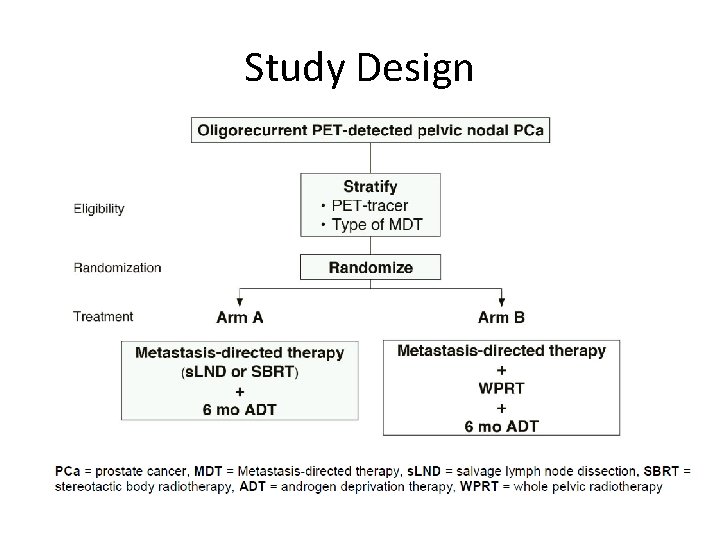
Study Design

Inclusion criteria – Histologically proven initial diagnosis of adenocarcinoma of the prostate – Biochemical relapse of prostate cancer following radical local prostate treatment (radical prostatectomy, primary radiotherapy or radical prostatectomy +/- prostate bed adjuvant/ salvage radiotherapy) according to the EAU guidelines 2016. – Following radical prostatectomy, patients with a biochemical relapse are eligible in case a nodal relapse is detected in the pelvis even in the absence of prior postoperative prostate bed radiotherapy (adjuvant or salvage). – In case of a suspected local recurrence following primary radiotherapy, a biopsy should confirm local recurrence and patients with a confirmed local recurrence are eligible in case they also undergo a local salvage therapy. If imaging rules out local relapse, patients are eligible. – Nodal relapse in the pelvis on choline, PSMA or FACBC PET-CT with a maximum of 3 (5) positive nodal lymph nodes. The upper limit of the pelvis is defined as the aortic bifurcation. – WHO performance state 0 -1 – Age 18 years – Absence of any psychological, familial, sociological or geographical condition potentially hampering compliance with the study protocol and follow-up schedule; those conditions should be discussed with the patient before registration in the trial – Before patient registration/randomization, written informed consent must be given according to ICH/GCP, and national/local regulations.

Exclusion criteria Ø Bone or visceral metastases Ø Para-aortic lymph node metastases (above the aortic bifurcation) Ø Local relapse in the prostate gland or prostate bed not suitable for a curative treatment Ø Previous irradiation of the pelvic and or para-aortic nodes Ø Serum testosterone level <50 ng/dl or 1. 7 nmol/L at time of randomization Ø Symptomatic metastases Ø Lymph node metastases in previously irradiated areas resulting in dose constraint violation as stipulated in part 5. 2 Ø Contraindications to pelvic radiotherapy (chronic pelvic inflammatory bowel disease) Ø Contraindications to androgen deprivation therapy Ø PSA rise while on active treatment with (LHRH-agonist, LHRH-antagonist, anti-androgen, estrogen Ø Previous treatment with cytotoxic agent for PCa Ø Treatment during the past month with products known to influence PSA levels (e. g. fluconazole, finasteride, corticosteroids, …) Ø Other active malignancy, except non-melanoma skin cancer or other malignancies with a documented disease-free survival for a minimum of 3 years before randomization.

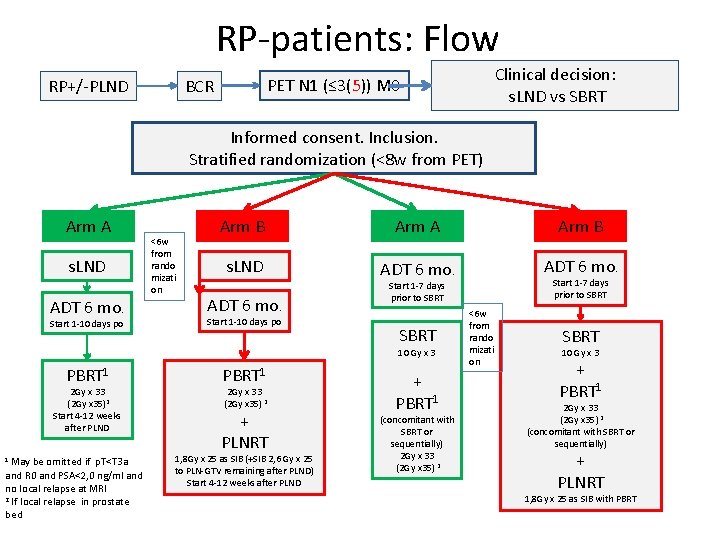
RP-patients: Flow Clinical decision: s. LND vs SBRT PET N 1 (≤ 3(5)) M 0 BCR RP+/-PLND Informed consent. Inclusion. Stratified randomization (<8 w from PET) Arm A s. LND ADT 6 mo. Start 1 -10 days po <6 w from rando mizati on Arm B Arm A Arm B s. LND ADT 6 mo. Start 1 -10 days po SBRT 10 Gy x 3 PBRT 1 2 Gy x 33 (2 Gy x 35)2 Start 4 -12 weeks after PLND 1 May be omitted if p. T<T 3 a and R 0 and PSA<2, 0 ng/ml and no local relapse at MRI 2 If local relapse in prostate bed PBRT 1 2 Gy x 33 (2 Gy x 35) 2 + PLNRT 1, 8 Gy x 25 as SIB (+SIB 2, 6 Gy x 25 to PLN-GTV remaining after PLND) Start 4 -12 weeks after PLND Start 1 -7 days prior to SBRT + PBRT 1 (concomitant with SBRT or sequentially) 2 Gy x 33 (2 Gy x 35) 2 <6 w from rando mizati on SBRT 10 Gy x 3 + PBRT 1 2 Gy x 33 (2 Gy x 35) 2 (concomitant with SBRT or sequentially) + PLNRT 1, 8 Gy x 25 as SIB with PBRT

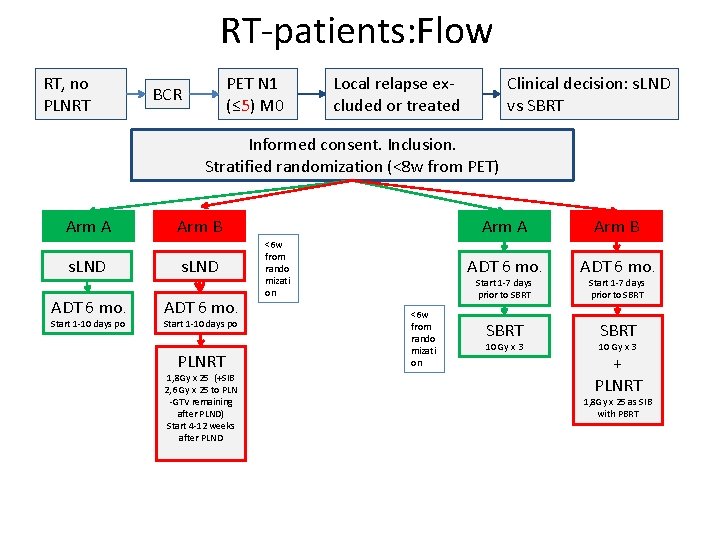
RT-patients: Flow RT, no PLNRT PET N 1 (≤ 5) M 0 BCR Clinical decision: s. LND vs SBRT Local relapse excluded or treated Informed consent. Inclusion. Stratified randomization (<8 w from PET) Arm A Arm B s. LND ADT 6 mo. Start 1 -10 days po PLNRT 1, 8 Gy x 25 (+SIB 2, 6 Gy x 25 to PLN -GTV remaining after PLND) Start 4 -12 weeks after PLND <6 w from rando mizati on Arm A Arm B ADT 6 mo. SBRT Start 1 -7 days prior to SBRT <6 w from rando mizati on 10 Gy x 3 Start 1 -7 days prior to SBRT 10 Gy x 3 + PLNRT 1, 8 Gy x 25 as SIB with PBRT