A Prospective Randomized Evaluation of Supersaturated Oxygen Therapy



- Slides: 12

A Prospective, Randomized Evaluation of Supersaturated Oxygen Therapy After Percutaneous Coronary Intervention in Acute Anterior Myocardial Infarction Gregg W. Stone MD For the AMIHOT II Investigators

Disclosures • Gregg W. Stone MD § Research support from Ther. Ox Inc.

Background • Despite successful reperfusion in AMI, myocardial recovery is often suboptimal, resulting in extensive infarction. • In experimental infarct models, hyperbaric oxygen reduces myocardial tissue damage, in part by reducing reperfusion injury and improving microcirculatory perfusion. • Regional hyperoxemia in the infarct zone can be achieved by infusion of supersaturated blood into the infarct artery after successful primary PCI. • This concept was tested in the AMIHOT I trial.

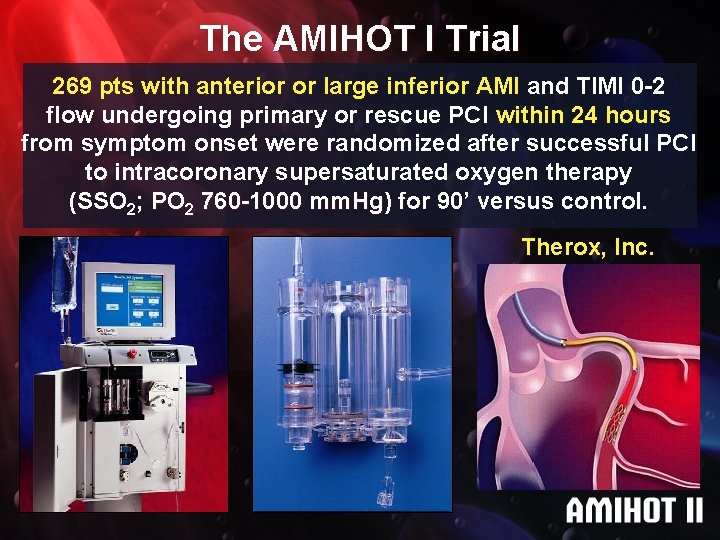
The AMIHOT I Trial 269 pts with anterior or large inferior AMI and TIMI 0 -2 flow undergoing primary or rescue PCI within 24 hours from symptom onset were randomized after successful PCI to intracoronary supersaturated oxygen therapy (SSO 2; PO 2 760 -1000 mm. Hg) mm. Hg for 90’ versus control. Therox, Inc.

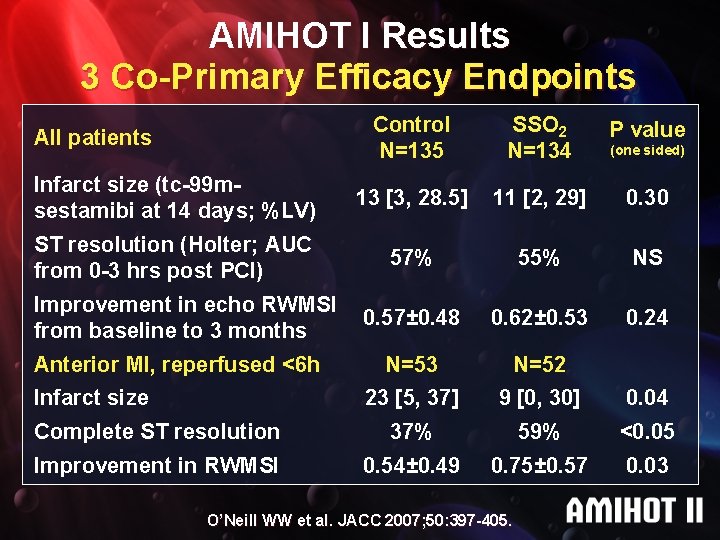
AMIHOT I Results 3 Co-Primary Efficacy Endpoints Control N=135 SSO 2 N=134 P value Infarct size (tc-99 msestamibi at 14 days; %LV) 13 [3, 28. 5] 11 [2, 29] 0. 30 ST resolution (Holter; AUC from 0 -3 hrs post PCI) 57% 55% NS 0. 57± 0. 48 0. 62± 0. 53 0. 24 N=53 N=52 23 [5, 37] 9 [0, 30] 0. 04 Complete ST resolution 37% 59% <0. 05 Improvement in RWMSI 0. 54± 0. 49 0. 75± 0. 57 0. 03 All patients Improvement in echo RWMSI from baseline to 3 months Anterior MI, reperfused <6 h Infarct size O’Neill WW et al. JACC 2007; 50: 397 -405. (one sided)

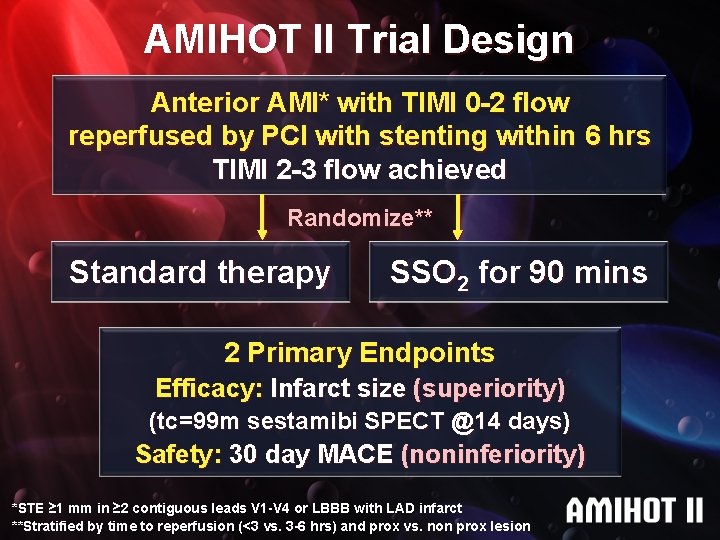
AMIHOT II Trial Design Anterior AMI* with TIMI 0 -2 flow reperfused by PCI with stenting within 6 hrs TIMI 2 -3 flow achieved Randomize** Standard therapy SSO 2 for 90 mins 2 Primary Endpoints Efficacy: Infarct size (superiority) (tc=99 m sestamibi SPECT @14 days) Safety: 30 day MACE (noninferiority) *STE ≥ 1 mm in ≥ 2 contiguous leads V 1 -V 4 or LBBB with LAD infarct **Stratified by time to reperfusion (<3 vs. 3 -6 hrs) and prox vs. non prox lesion

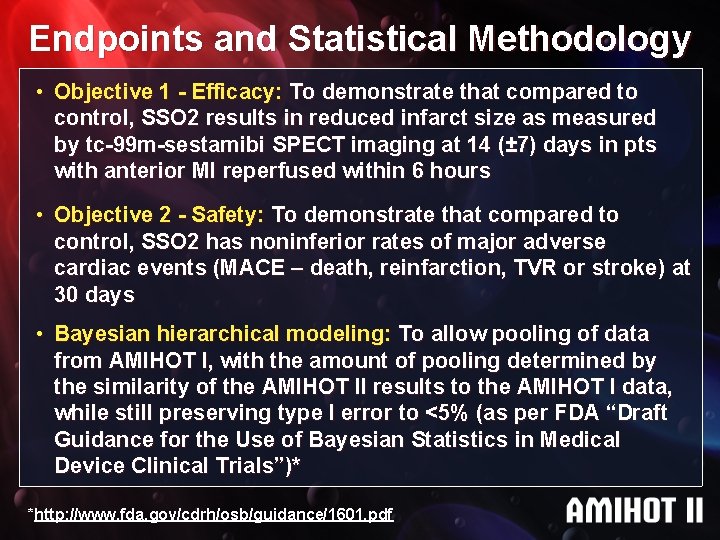
Endpoints and Statistical Methodology • Objective 1 - Efficacy: To demonstrate that compared to control, SSO 2 results in reduced infarct size as measured by tc-99 m-sestamibi SPECT imaging at 14 (± 7) days in pts with anterior MI reperfused within 6 hours • Objective 2 - Safety: To demonstrate that compared to control, SSO 2 has noninferior rates of major adverse cardiac events (MACE – death, reinfarction, TVR or stroke) at 30 days • Bayesian hierarchical modeling: To allow pooling of data from AMIHOT I, with the amount of pooling determined by the similarity of the AMIHOT II results to the AMIHOT I data, while still preserving type I error to <5% (as per FDA “Draft Guidance for the Use of Bayesian Statistics in Medical Device Clinical Trials”)* *http: //www. fda. gov/cdrh/osb/guidance/1601. pdf

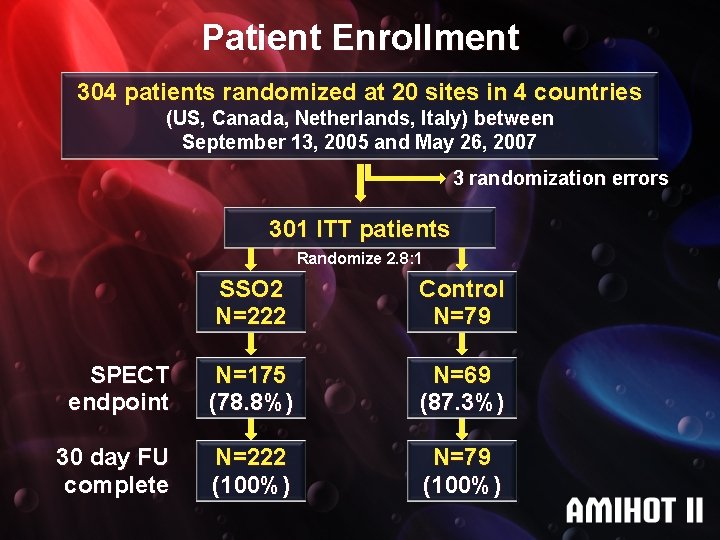
Patient Enrollment 304 patients randomized at 20 sites in 4 countries (US, Canada, Netherlands, Italy) between September 13, 2005 and May 26, 2007 3 randomization errors 301 ITT patients Randomize 2. 8: 1 SSO 2 N=222 Control N=79 SPECT endpoint N=175 (78. 8%) N=69 (87. 3%) 30 day FU complete N=222 (100%) N=79 (100%)

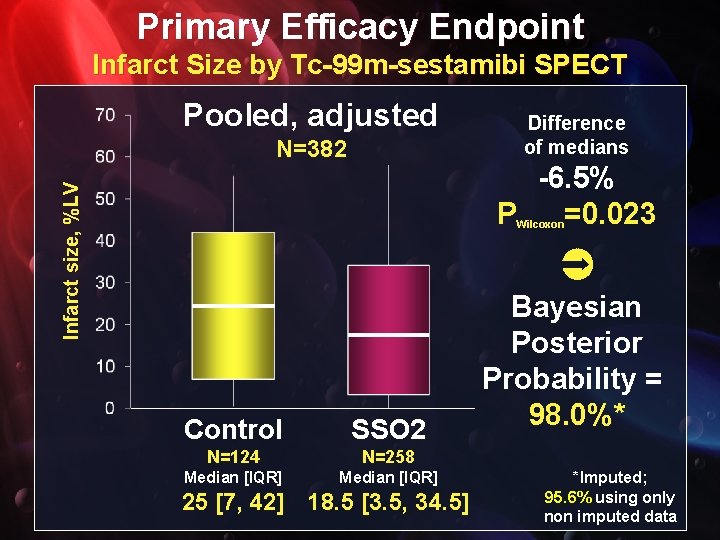
Primary Efficacy Endpoint Infarct Size by Tc-99 m-sestamibi SPECT Pooled, adjusted N=382 Difference of medians Infarct size, %LV -6. 5% P =0. 023 Wilcoxon Control SSO 2 N=124 N=258 Median [IQR] 25 [7, 42] 18. 5 [3. 5, 34. 5] Bayesian Posterior Probability = 98. 0%* *Imputed; 95. 6% using only non imputed data

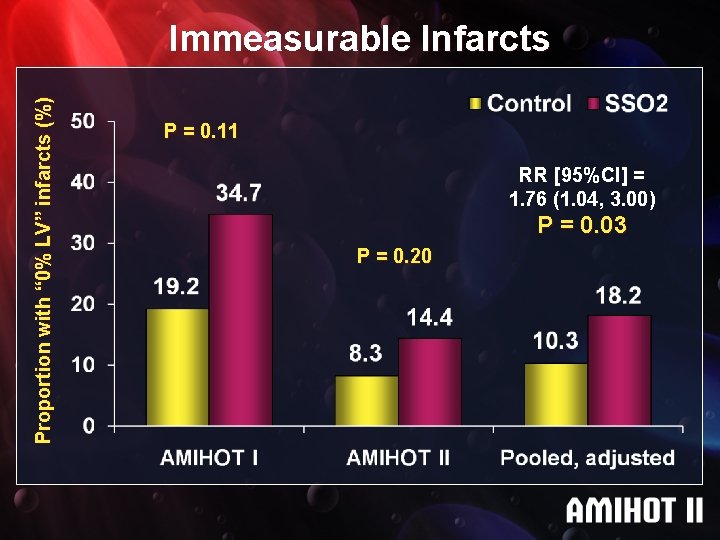
Proportion with “ 0% LV” infarcts (%) Immeasurable Infarcts P = 0. 11 RR [95%CI] = 1. 76 (1. 04, 3. 00) P = 0. 03 P = 0. 20

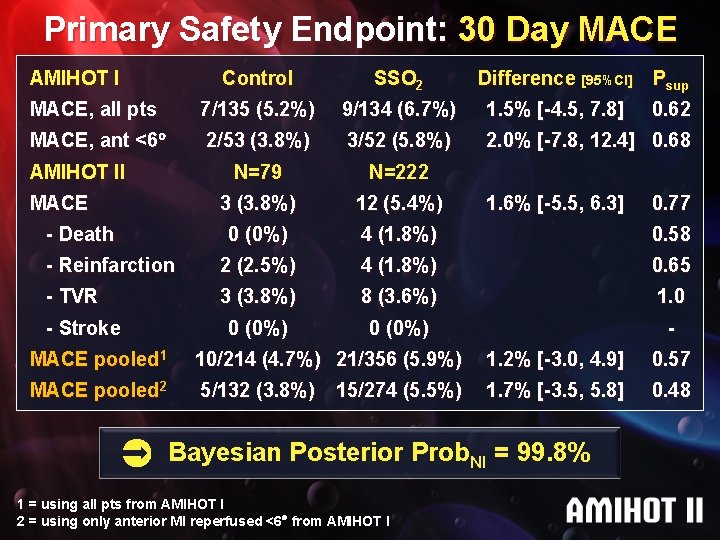
Primary Safety Endpoint: 30 Day MACE AMIHOT I Control SSO 2 MACE, all pts 7/135 (5. 2%) 9/134 (6. 7%) 1. 5% [-4. 5, 7. 8] MACE, ant <6 2/53 (3. 8%) 3/52 (5. 8%) 2. 0% [-7. 8, 12. 4] 0. 68 N=79 N=222 3 (3. 8%) 12 (5. 4%) 0 (0%) 4 (1. 8%) 0. 58 - Reinfarction 2 (2. 5%) 4 (1. 8%) 0. 65 - TVR 3 (3. 8%) 8 (3. 6%) 1. 0 0 (0%) - AMIHOT II MACE - Death - Stroke Difference [95%CI] Psup 1. 6% [-5. 5, 6. 3] 0. 62 0. 77 MACE pooled 1 10/214 (4. 7%) 21/356 (5. 9%) 1. 2% [-3. 0, 4. 9] 0. 57 MACE pooled 2 5/132 (3. 8%) 1. 7% [-3. 5, 5. 8] 0. 48 15/274 (5. 5%) Bayesian Posterior Prob. NI = 99. 8% 1 = using all pts from AMIHOT I 2 = using only anterior MI reperfused <6 from AMIHOT I

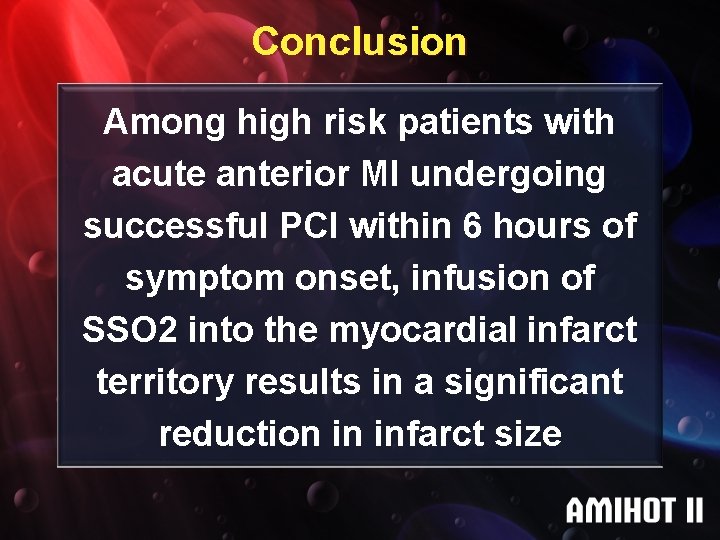
Conclusion Among high risk patients with acute anterior MI undergoing successful PCI within 6 hours of symptom onset, infusion of SSO 2 into the myocardial infarct territory results in a significant reduction in infarct size
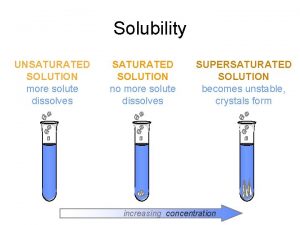
 Gas solubility curve
Gas solubility curve Saturated unsaturated and supersaturated
Saturated unsaturated and supersaturated Saturated unsaturated and supersaturated
Saturated unsaturated and supersaturated A supersaturated solution
A supersaturated solution Solid
Solid Saturated vs unsaturated vs supersaturated
Saturated vs unsaturated vs supersaturated Oxalic acid is saturated or unsaturated
Oxalic acid is saturated or unsaturated Chemistry
Chemistry A supersaturated solution
A supersaturated solution Supersaturated solution
Supersaturated solution Types of solubility
Types of solubility Solubility curve saturated unsaturated supersaturated
Solubility curve saturated unsaturated supersaturated Indications of oxygen therapy
Indications of oxygen therapy