2 Introduction Name Stereochemistry SAR Prodrugs Uses Side


- Slides: 14


• • 2 Introduction Name Stereochemistry SAR Prodrugs Uses Side effects

• Chloramphenicol is a semisynthetic, broadspectrum antibiotic derived from Streptomyces venezuelae with primarily bacteriostatic activity • . They bind to 50 S r-RNA and inhibit formation of peptide bond • 3

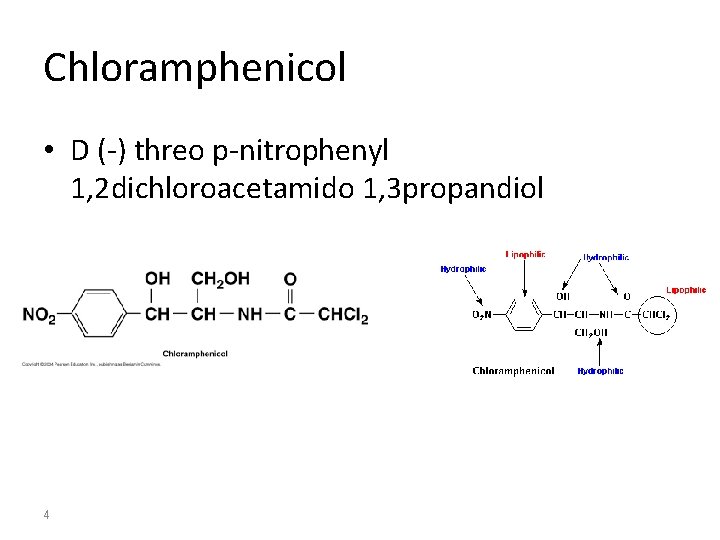
Chloramphenicol • D (-) threo p-nitrophenyl 1, 2 dichloroacetamido 1, 3 propandiol 4

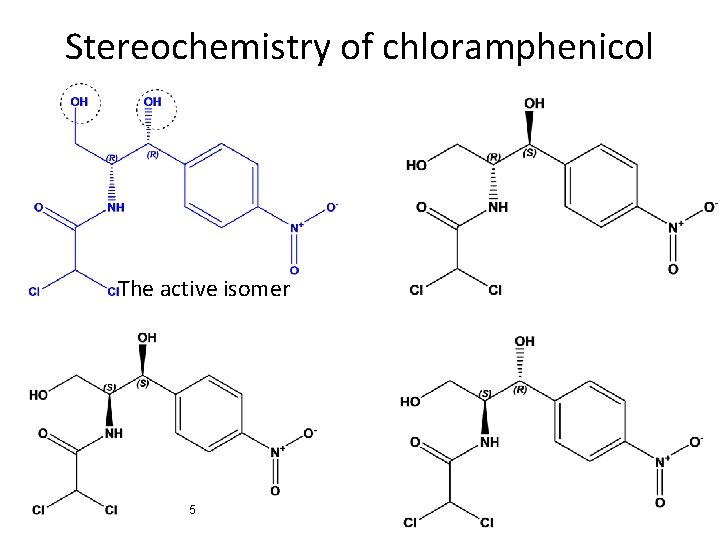
Stereochemistry of chloramphenicol The active isomer 5

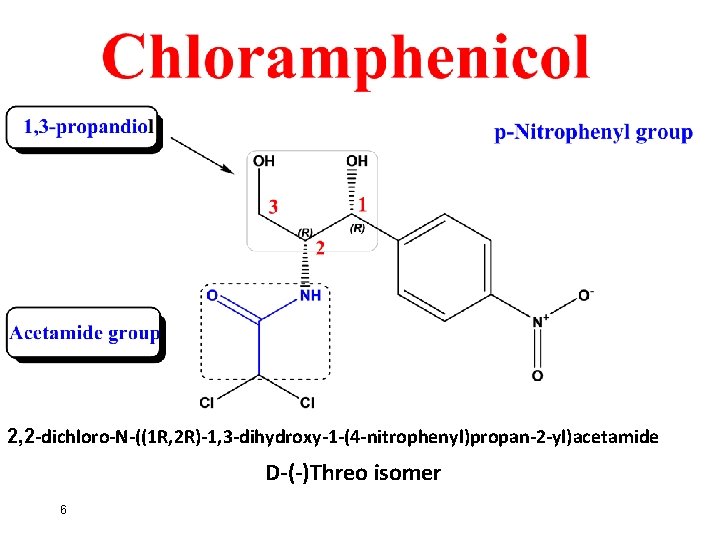
2, 2 -dichloro-N-((1 R, 2 R)-1, 3 -dihydroxy-1 -(4 -nitrophenyl)propan-2 -yl)acetamide D-(-)Threo isomer 6

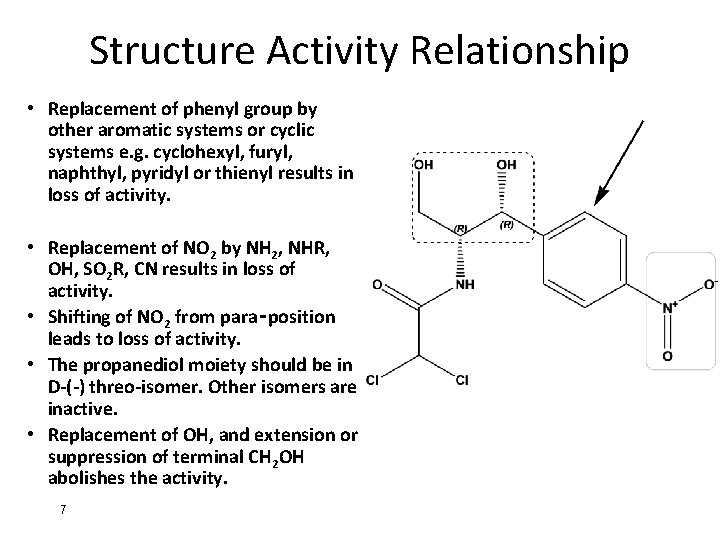
Structure Activity Relationship • Replacement of phenyl group by other aromatic systems or cyclic systems e. g. cyclohexyl, furyl, naphthyl, pyridyl or thienyl results in loss of activity. • Replacement of NO 2 by NH 2, NHR, OH, SO 2 R, CN results in loss of activity. • Shifting of NO 2 from para‑position leads to loss of activity. • The propanediol moiety should be in D-(-) threo-isomer. Other isomers are inactive. • Replacement of OH, and extension or suppression of terminal CH 2 OH abolishes the activity. 7

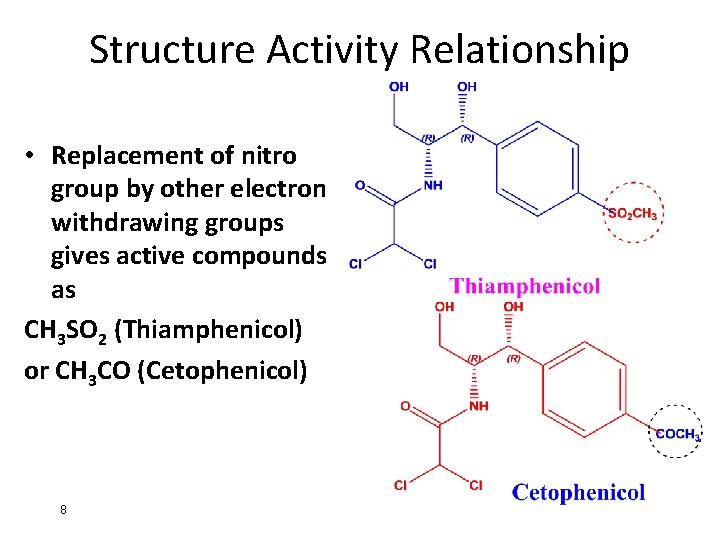
Structure Activity Relationship • Replacement of nitro group by other electron withdrawing groups gives active compounds as CH 3 SO 2 (Thiamphenicol) or CH 3 CO (Cetophenicol) 8

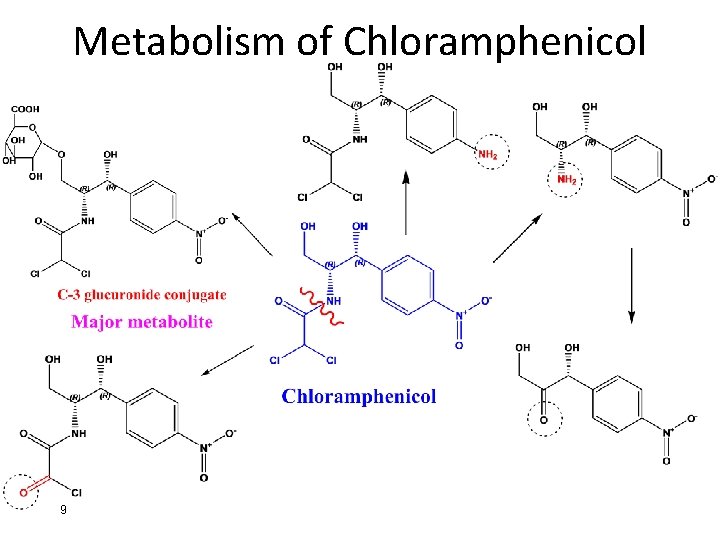
Metabolism of Chloramphenicol 9

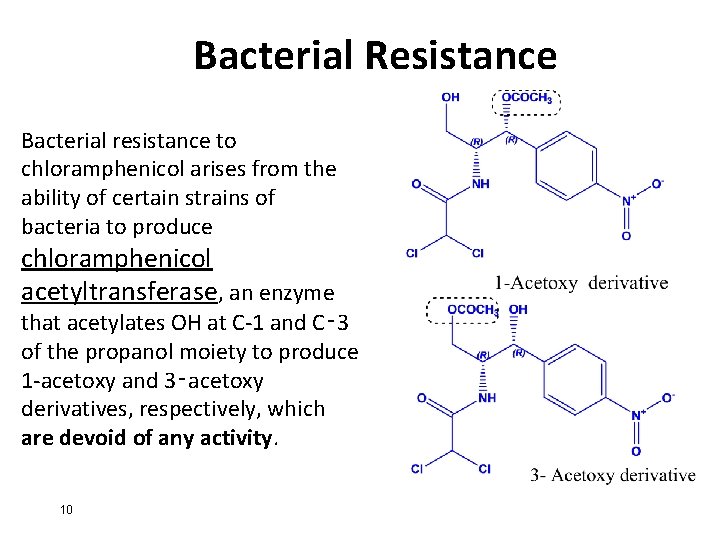
Bacterial Resistance Bacterial resistance to chloramphenicol arises from the ability of certain strains of bacteria to produce chloramphenicol acetyltransferase, an enzyme that acetylates OH at C-1 and C‑ 3 of the propanol moiety to produce 1 -acetoxy and 3‑acetoxy derivatives, respectively, which are devoid of any activity. 10

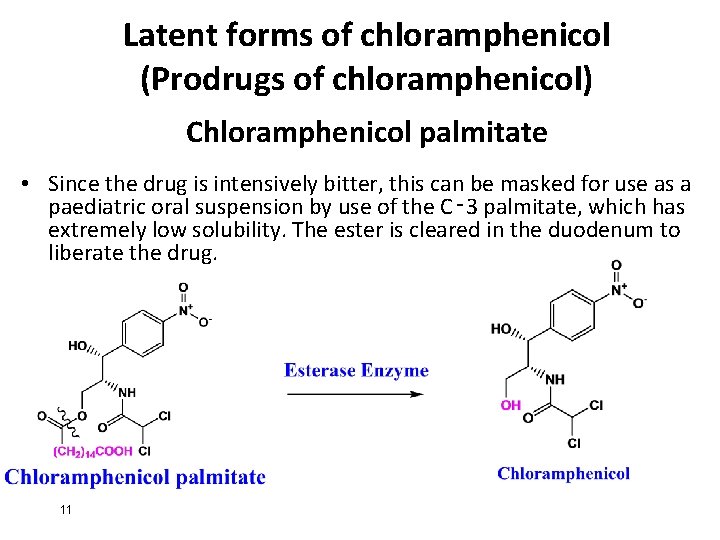
Latent forms of chloramphenicol (Prodrugs of chloramphenicol) Chloramphenicol palmitate • Since the drug is intensively bitter, this can be masked for use as a paediatric oral suspension by use of the C‑ 3 palmitate, which has extremely low solubility. The ester is cleared in the duodenum to liberate the drug. 11

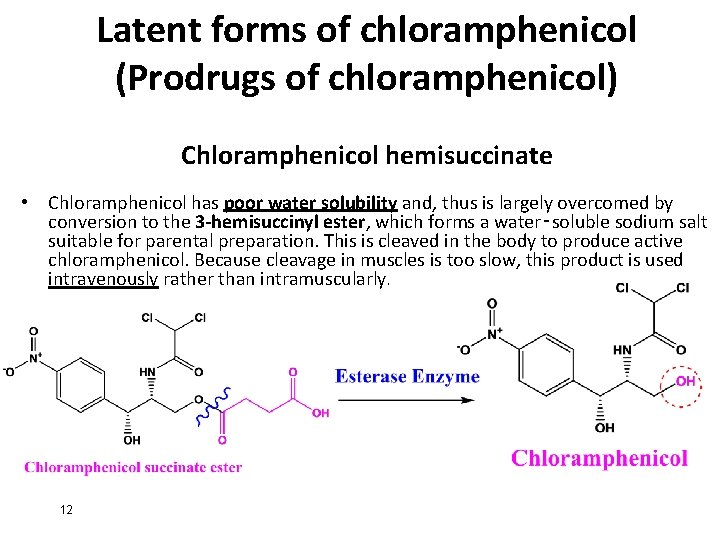
Latent forms of chloramphenicol (Prodrugs of chloramphenicol) Chloramphenicol hemisuccinate • Chloramphenicol has poor water solubility and, thus is largely overcomed by conversion to the 3 -hemisuccinyl ester, which forms a water‑soluble sodium salt suitable for parental preparation. This is cleaved in the body to produce active chloramphenicol. Because cleavage in muscles is too slow, this product is used intravenously rather than intramuscularly. 12

Uses of Chloramphenicol • Despite of potential serious limitations, chloramphenicol is an excellent drug when used carefully. It is of special value for treatment of typhoid and parathyroid fevers, haemophilus infections, pneumococcal and meningococcal meningitis in beta lactam allergic patients. Safer antibiotics should be used whenever possible. 13

Chloramphenicol ( cont. ) Side effects 1. Hypersensitivity- low incidence May ppt hemolysis in G 6 PD deficient pts 2. A plastic anaemia ( fatal ) 3. Grey baby syndrome 4. Suprainfections 5. Interaction with other drugs : Inhibits liver microsomal enzymes Phenytoin Tolbutamide Chlorpropamide Anticoagulants 14
 Head sar body sar
Head sar body sar Side side side similarity
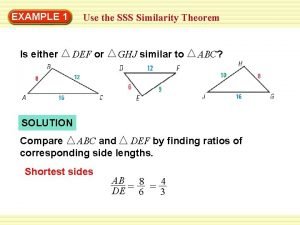
Side side side similarity Sas similarity theorem
Sas similarity theorem Sss similarity theorem examples
Sss similarity theorem examples What is aa similarity theorem
What is aa similarity theorem Introduction to stereochemistry
Introduction to stereochemistry Finding stereocenters
Finding stereocenters What is protein
What is protein Stereoisomers always possess handedness.
Stereoisomers always possess handedness. Trans-1-chloro-3-methylcyclohexane
Trans-1-chloro-3-methylcyclohexane Indicate the relationship of the pair of molecules shown.
Indicate the relationship of the pair of molecules shown. Father of stereochemistry
Father of stereochemistry Stereochemistry
Stereochemistry Ethane
Ethane Stereochemistry of cephalosporin
Stereochemistry of cephalosporin