17 2 Measuring and Expressing Enthalpy Changes Calorimetry










- Slides: 21

17. 2 Measuring and Expressing Enthalpy Changes > Calorimetry is the precise measurement of the heat flow into or out of a system for chemical and physical processes. Slide 1 of 33 © Copyright Pearson Prentice Hall

17. 2 Measuring and Expressing Enthalpy Changes > Calorimetry In calorimetry, the heat released by the system is equal to the heat absorbed by its surroundings. Conversely, the heat absorbed by a system is equal to the heat released by its surroundings. Slide 2 of 33 © Copyright Pearson Prentice Hall

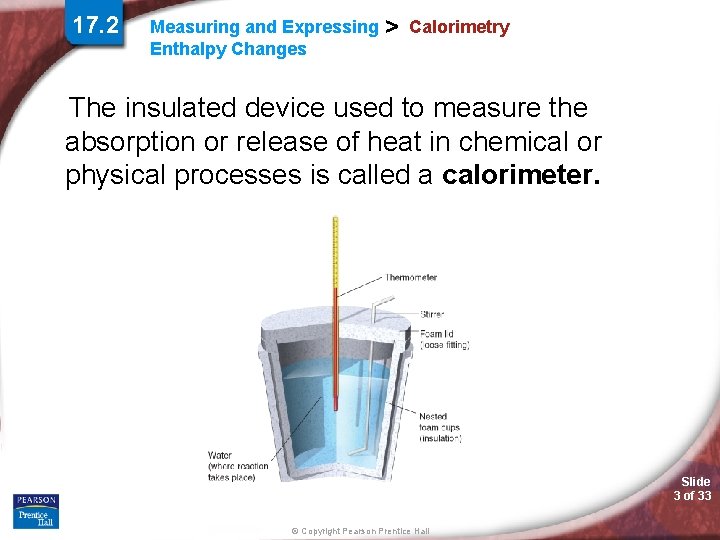
17. 2 Measuring and Expressing Enthalpy Changes > Calorimetry The insulated device used to measure the absorption or release of heat in chemical or physical processes is called a calorimeter. Slide 3 of 33 © Copyright Pearson Prentice Hall

17. 2 Measuring and Expressing Enthalpy Changes > Calorimetry Constant-Pressure Calorimeters The heat content of a system at constant pressure is the same as a property called the enthalpy (H) of the system. Slide 4 of 33 © Copyright Pearson Prentice Hall

17. 2 Measuring and Expressing Enthalpy Changes > Calorimetry Constant-Volume Calorimeters Calorimetry experiments can be performed at a constant volume using a bomb calorimeter. Slide 5 of 33 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 17. 2 Slide 6 of 33 © Copyright Pearson Prentice Hall

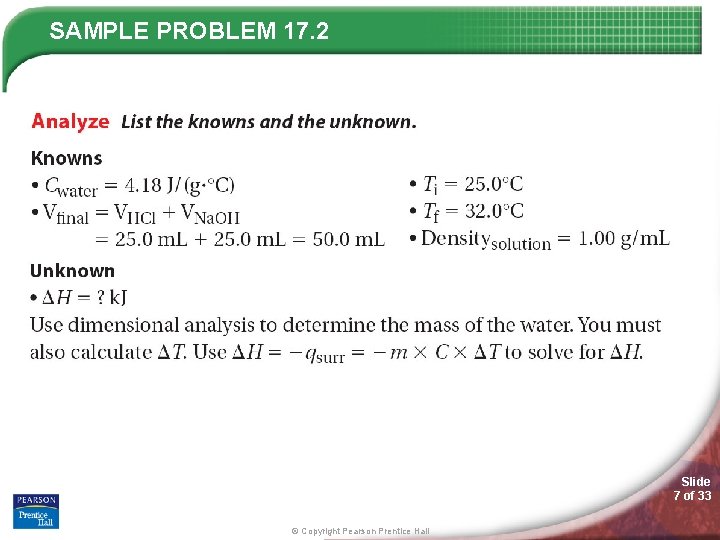
SAMPLE PROBLEM 17. 2 Slide 7 of 33 © Copyright Pearson Prentice Hall

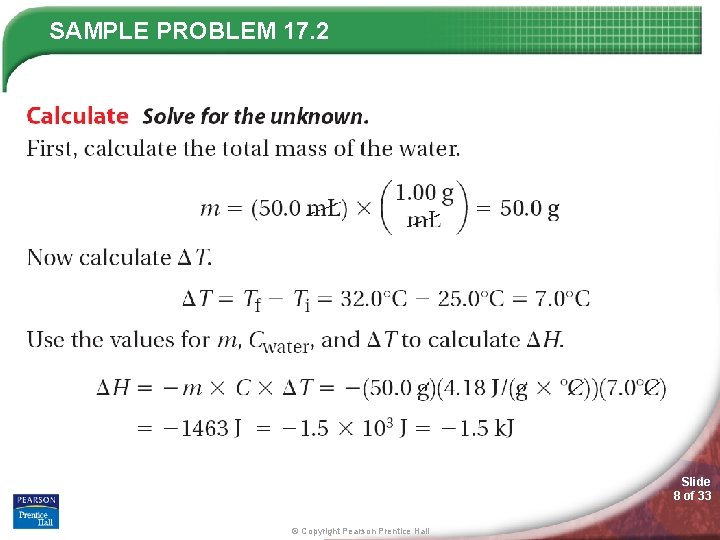
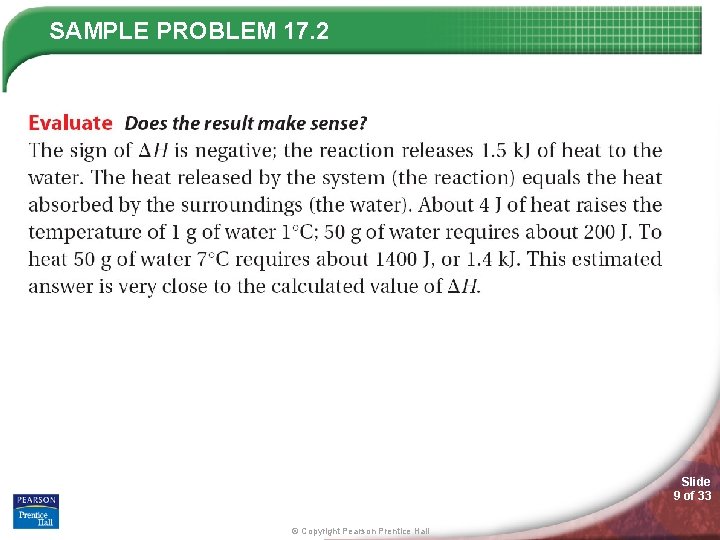
SAMPLE PROBLEM 17. 2 Slide 8 of 33 © Copyright Pearson Prentice Hall

SAMPLE PROBLEM 17. 2 Slide 9 of 33 © Copyright Pearson Prentice Hall

Practice Problems for Sample Problem 17. 2 Problem Solving 17. 13 Solve Problem 13 with the help of an interactive guided tutorial. © Copyright Pearson Prentice Hall Slide 10 of 33

17. 2 Measuring and Expressing Enthalpy Changes > Thermochemical Equations How can you express the enthalpy change for a reaction in a chemical equation? Slide 11 of 33 © Copyright Pearson Prentice Hall

17. 2 Measuring and Expressing Enthalpy Changes > Thermochemical Equations In a chemical equation, the enthalpy change for the reaction can be written as either a reactant or a product. Slide 12 of 33 © Copyright Pearson Prentice Hall

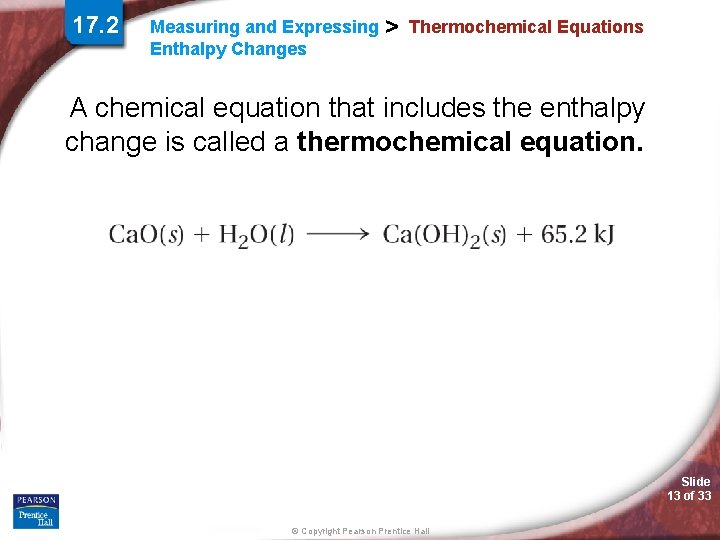
17. 2 Measuring and Expressing Enthalpy Changes > Thermochemical Equations A chemical equation that includes the enthalpy change is called a thermochemical equation. Slide 13 of 33 © Copyright Pearson Prentice Hall

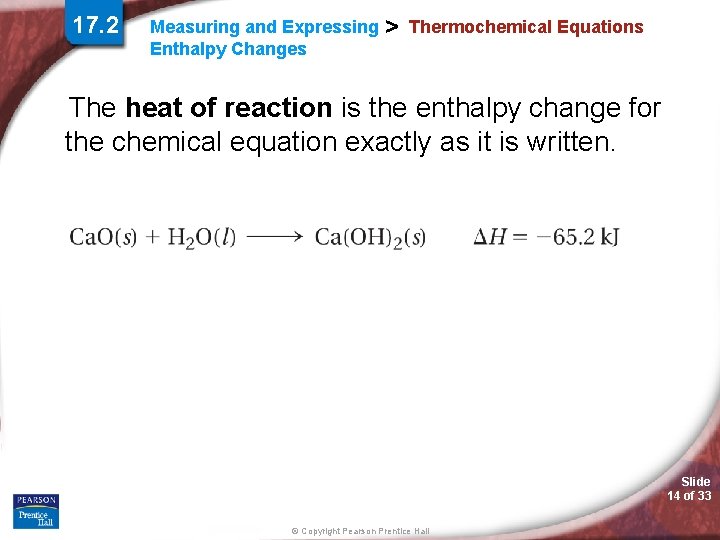
17. 2 Measuring and Expressing Enthalpy Changes > Thermochemical Equations The heat of reaction is the enthalpy change for the chemical equation exactly as it is written. Slide 14 of 33 © Copyright Pearson Prentice Hall

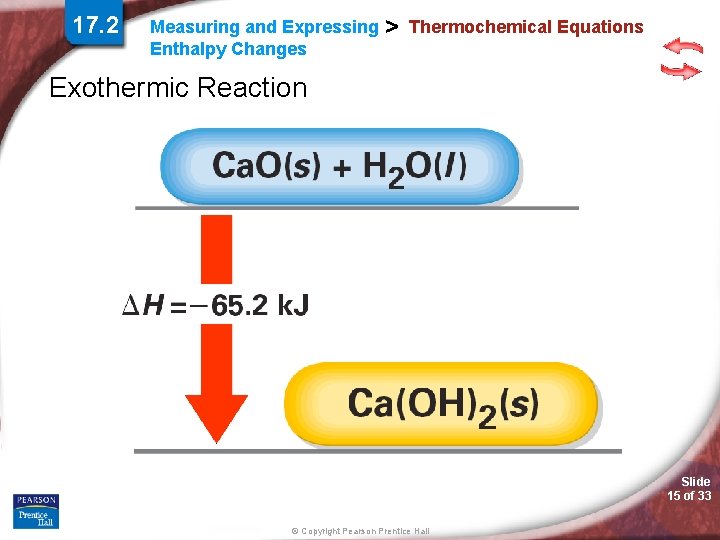
17. 2 Measuring and Expressing Enthalpy Changes > Thermochemical Equations Exothermic Reaction Slide 15 of 33 © Copyright Pearson Prentice Hall

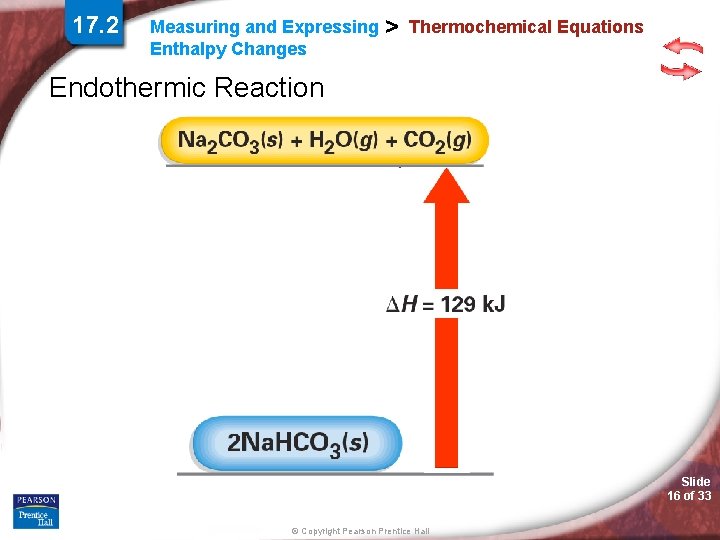
17. 2 Measuring and Expressing Enthalpy Changes > Thermochemical Equations Endothermic Reaction Slide 16 of 33 © Copyright Pearson Prentice Hall

17. 2 Measuring and Expressing Enthalpy Changes > Thermochemical Equations The heat of combustion is the heat of reaction for the complete burning of one mole of a substance. Slide 17 of 33 © Copyright Pearson Prentice Hall

17. 2 Section Quiz. 1. The change in temperature recorded by thermometer in a calorimeter is a measurement of a. the enthalpy change of the reaction in the calorimeter. b. the specific heat of each compound in a calorimeter. c. the physical states of the reactants in a colorimeter. d. the heat of combustion for one substance in a calorimeter. Slide 18 of 33 © Copyright Pearson Prentice Hall

17. 2 Section Quiz. 2. For the reaction Ca. O(s) + H 2 O(l) Ca(OH)2(s), H = 65. 2 k. J. This means that 62. 5 k. J of heat is _____ during the process. a. absorbed b. destroyed c. changed to mass d. released Slide 19 of 33 © Copyright Pearson Prentice Hall

17. 2 Section Quiz. 3. How much heat is absorbed by 325 g of water if its temperature changes from 17. 0°C to 43. 5°C? The specific heat of water is 4. 18 J/g°C. a. 2. 00 k. J b. 3. 60 k. J c. 36. 0 k. J d. 360 k. J Slide 20 of 33 © Copyright Pearson Prentice Hall

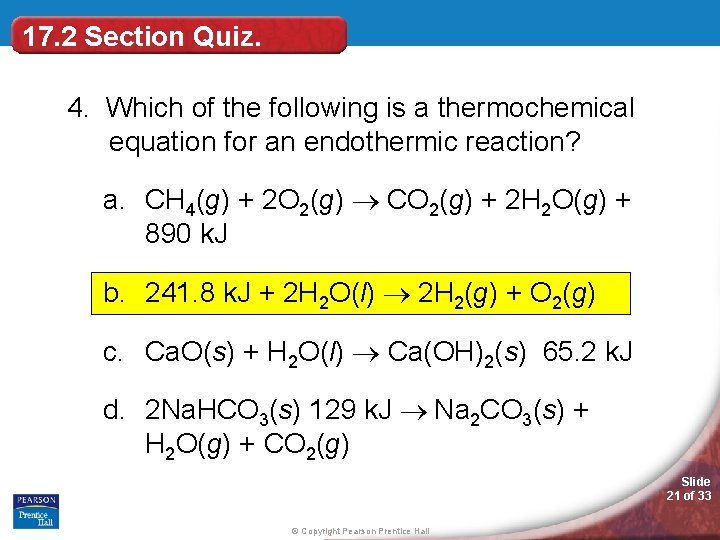
17. 2 Section Quiz. 4. Which of the following is a thermochemical equation for an endothermic reaction? a. CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) + 890 k. J b. 241. 8 k. J + 2 H 2 O(l) 2 H 2(g) + O 2(g) c. Ca. O(s) + H 2 O(l) Ca(OH)2(s) 65. 2 k. J d. 2 Na. HCO 3(s) 129 k. J Na 2 CO 3(s) + H 2 O(g) + CO 2(g) Slide 21 of 33 © Copyright Pearson Prentice Hall