16 Dielectrics and Ferroelectrics Maxwell Equations Polarization Macroscopic

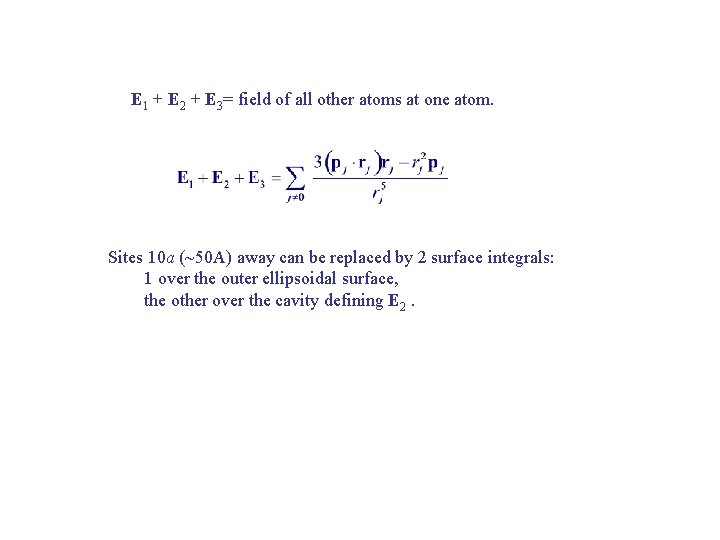
- Slides: 29

16. Dielectrics and Ferroelectrics Maxwell Equations Polarization Macroscopic Electric Field Depolarization Field, E 1 Local Electric Field at An atom Lorentz Field, E 2 Field of Dipoles Inside Cavity, E 3 Dielectric Constant And Polarizability Electronic Polarizability Classical Theory Examples Structural Phase Transitions Ferroelectric Crystals Classification of Ferroelectric Crystals Displacive Transitions Soft Optical Phonons Landau theory of the Phase Transition Second-Order Transition First-Order Transition Antiferroelectricity Ferroelectric Domains Piezoelectricity

Maxwell Equations

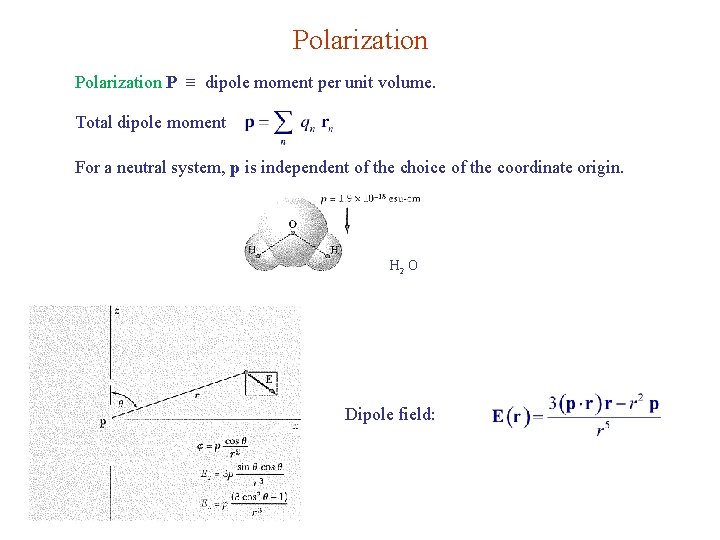
Polarization P dipole moment per unit volume. Total dipole moment For a neutral system, p is independent of the choice of the coordinate origin. H 2 O Dipole field:

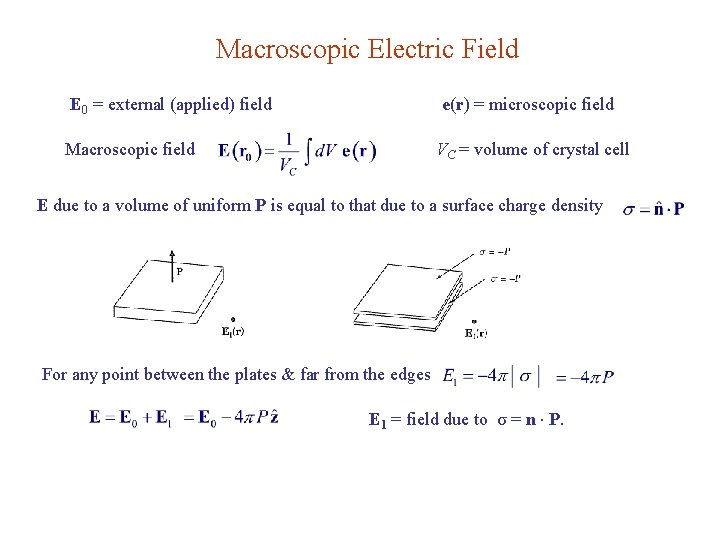
Macroscopic Electric Field E 0 = external (applied) field e(r) = microscopic field Macroscopic field VC = volume of crystal cell E due to a volume of uniform P is equal to that due to a surface charge density For any point between the plates & far from the edges E 1 = field due to σ = n P.

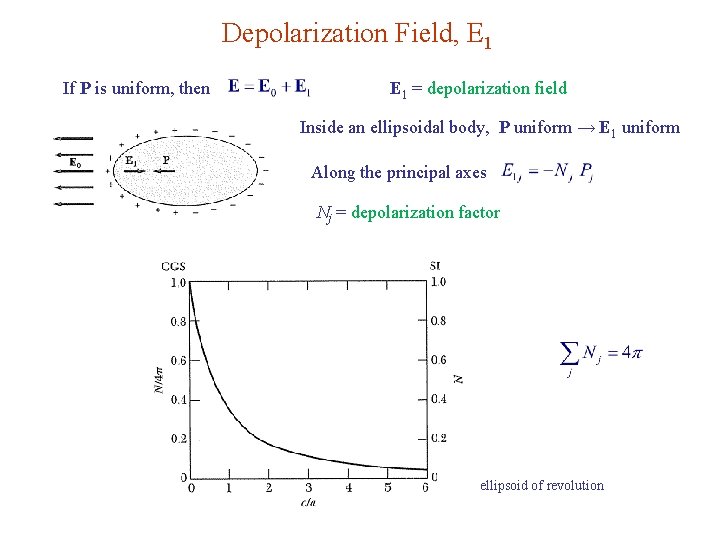
Depolarization Field, E 1 If P is uniform, then E 1 = depolarization field Inside an ellipsoidal body, P uniform → E 1 uniform Along the principal axes Nj = depolarization factor ellipsoid of revolution

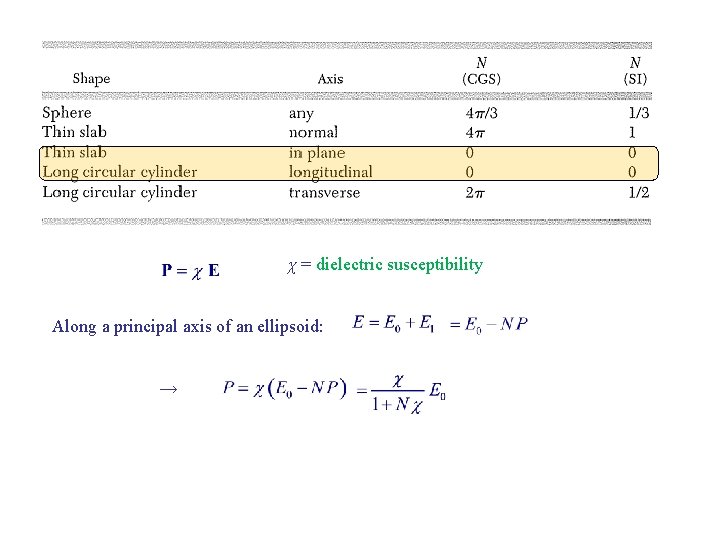
χ = dielectric susceptibility Along a principal axis of an ellipsoid: →

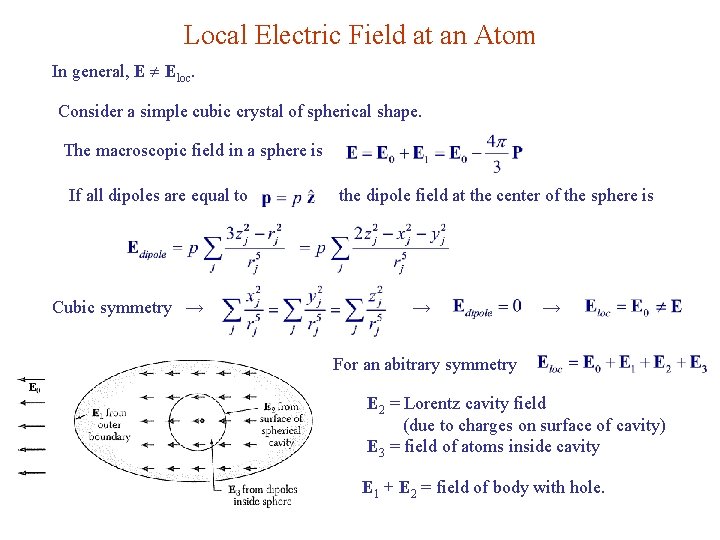
Local Electric Field at an Atom In general, E Eloc. Consider a simple cubic crystal of spherical shape. The macroscopic field in a sphere is If all dipoles are equal to Cubic symmetry → the dipole field at the center of the sphere is → → For an abitrary symmetry E 2 = Lorentz cavity field (due to charges on surface of cavity) E 3 = field of atoms inside cavity E 1 + E 2 = field of body with hole.
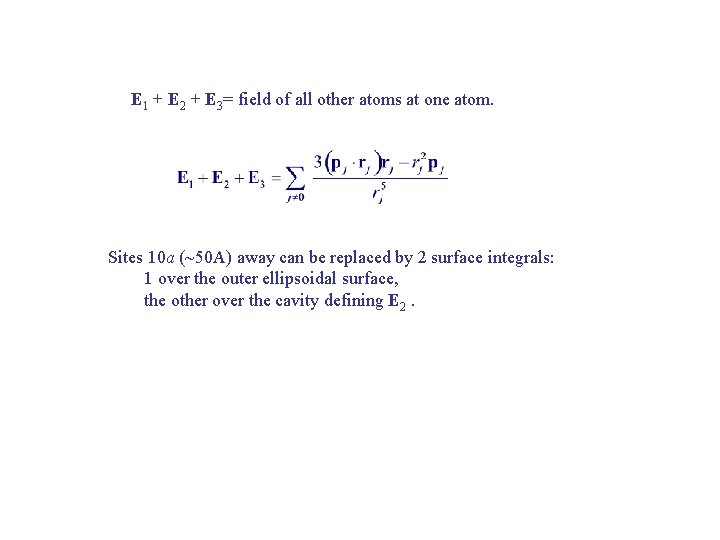
E 1 + E 2 + E 3= field of all other atoms at one atom. Sites 10 a (~50 A) away can be replaced by 2 surface integrals: 1 over the outer ellipsoidal surface, the other over the cavity defining E 2.

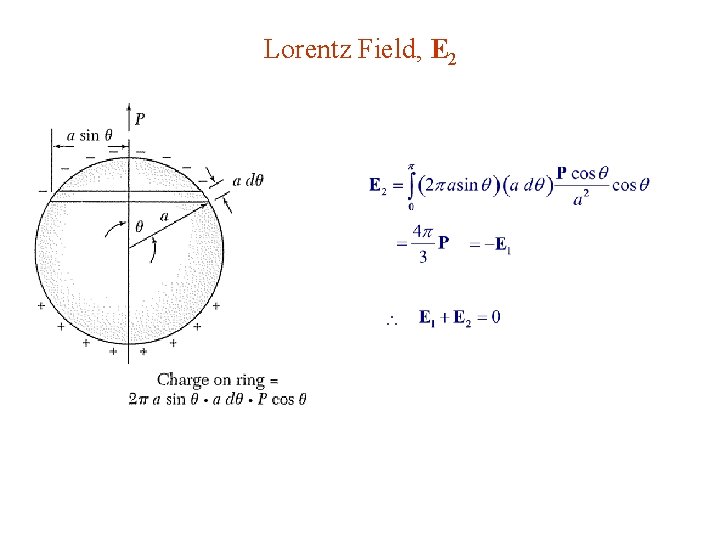
Lorentz Field, E 2

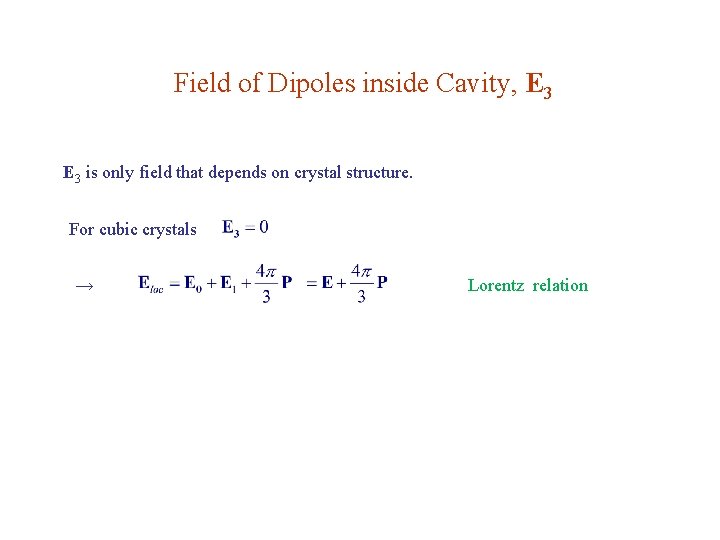
Field of Dipoles inside Cavity, E 3 is only field that depends on crystal structure. For cubic crystals → Lorentz relation

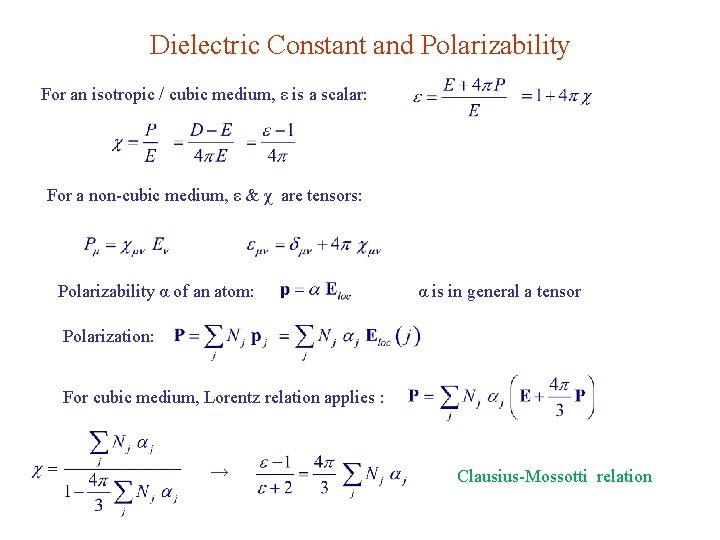
Dielectric Constant and Polarizability For an isotropic / cubic medium, ε is a scalar: For a non-cubic medium, ε & χ are tensors: Polarizability α of an atom: α is in general a tensor Polarization: For cubic medium, Lorentz relation applies : → Clausius-Mossotti relation

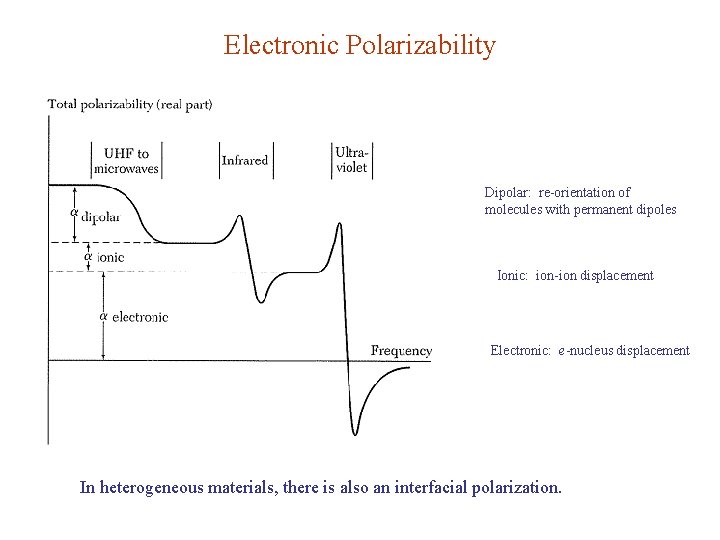
Electronic Polarizability Dipolar: re-orientation of molecules with permanent dipoles Ionic: ion-ion displacement Electronic: e-nucleus displacement In heterogeneous materials, there is also an interfacial polarization.

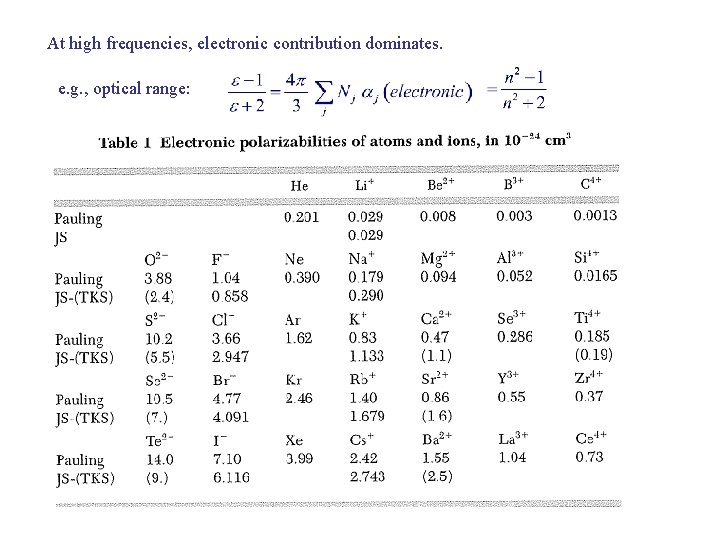
At high frequencies, electronic contribution dominates. e. g. , optical range:

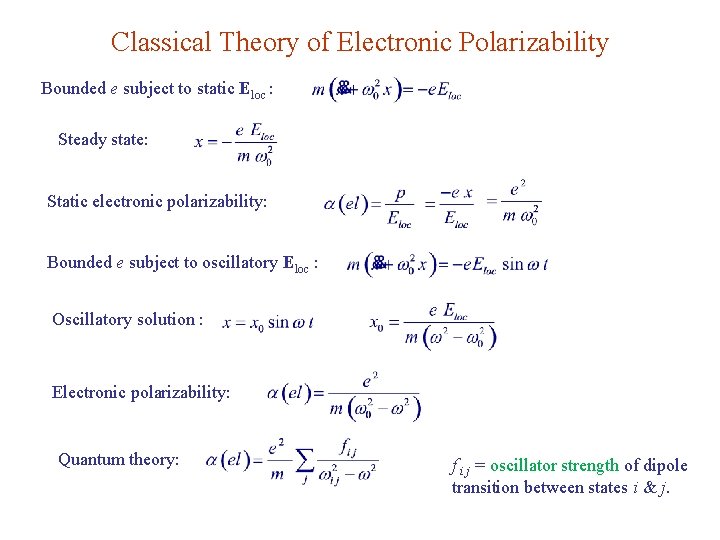
Classical Theory of Electronic Polarizability Bounded e subject to static Eloc : Steady state: Static electronic polarizability: Bounded e subject to oscillatory Eloc : Oscillatory solution : Electronic polarizability: Quantum theory: f i j = oscillator strength of dipole transition between states i & j.

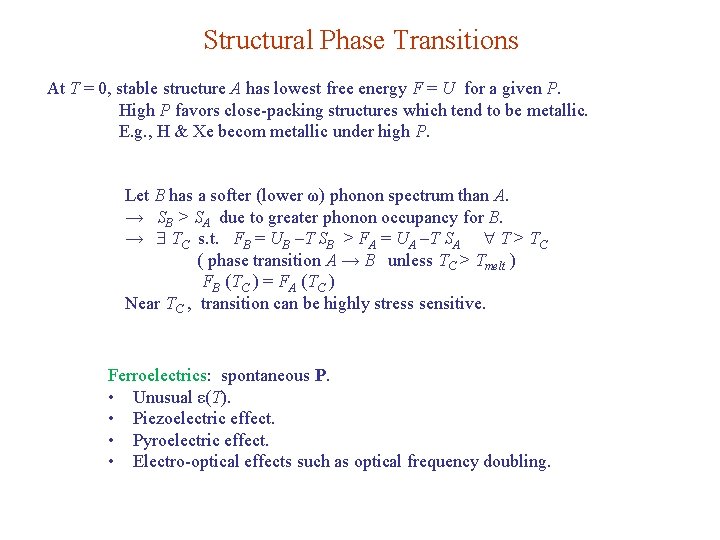
Structural Phase Transitions At T = 0, stable structure A has lowest free energy F = U for a given P. High P favors close-packing structures which tend to be metallic. E. g. , H & Xe becom metallic under high P. Let B has a softer (lower ω) phonon spectrum than A. → SB > SA due to greater phonon occupancy for B. → TC s. t. FB = UB –T SB > FA = UA –T SA T > TC ( phase transition A → B unless TC > Tmelt ) FB (TC ) = FA (TC ) Near TC , transition can be highly stress sensitive. Ferroelectrics: spontaneous P. • Unusual ε(T). • Piezoelectric effect. • Pyroelectric effect. • Electro-optical effects such as optical frequency doubling.

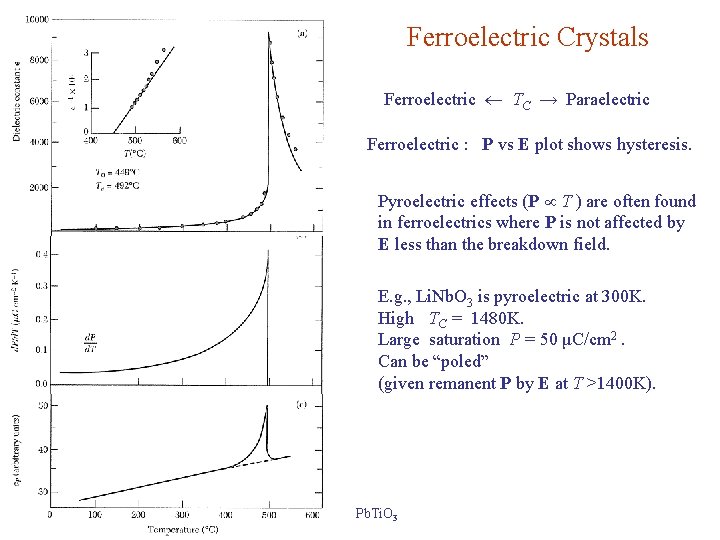
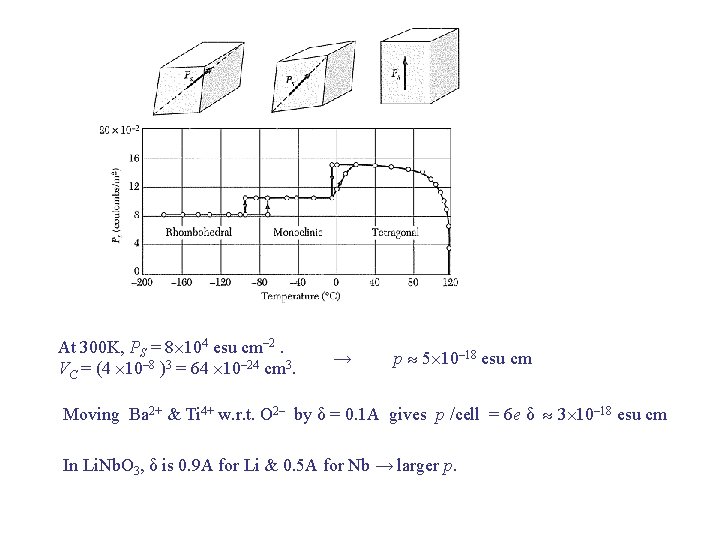
Ferroelectric Crystals Ferroelectric TC → Paraelectric Ferroelectric : P vs E plot shows hysteresis. Pyroelectric effects (P T ) are often found in ferroelectrics where P is not affected by E less than the breakdown field. E. g. , Li. Nb. O 3 is pyroelectric at 300 K. High TC = 1480 K. Large saturation P = 50 μC/cm 2. Can be “poled” (given remanent P by E at T >1400 K). Pb. Ti. O 3

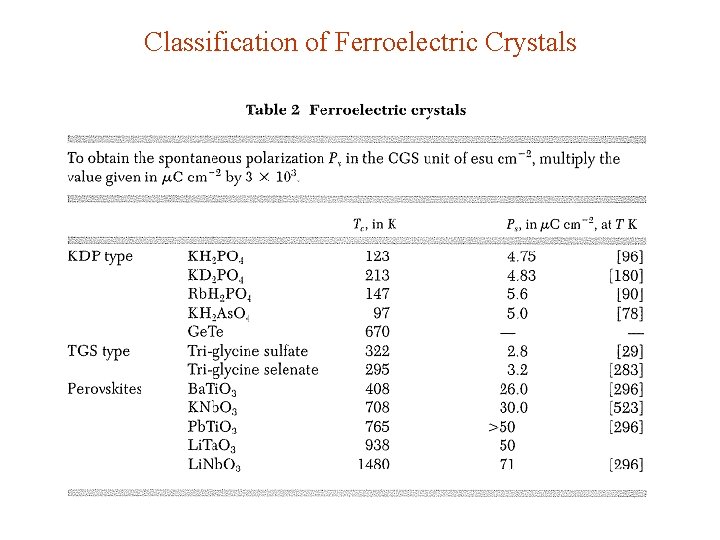
Classification of Ferroelectric Crystals

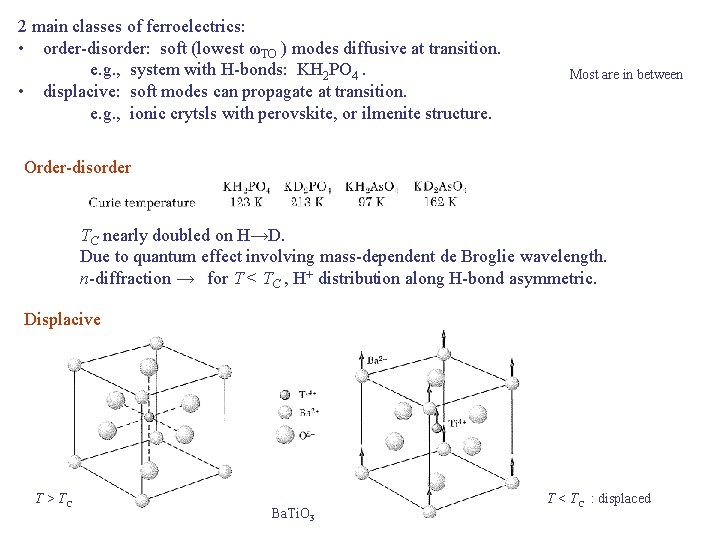
2 main classes of ferroelectrics: • order-disorder: soft (lowest ωTO ) modes diffusive at transition. e. g. , system with H-bonds: KH 2 PO 4. • displacive: soft modes can propagate at transition. e. g. , ionic crytsls with perovskite, or ilmenite structure. Most are in between Order-disorder TC nearly doubled on H→D. Due to quantum effect involving mass-dependent de Broglie wavelength. n-diffraction → for T < TC , H+ distribution along H-bond asymmetric. Displacive T > TC Ba. Ti. O 3 T < TC : displaced

At 300 K, PS = 8 104 esu cm– 2. VC = (4 10– 8 )3 = 64 10– 24 cm 3. → p 5 10– 18 esu cm Moving Ba 2+ & Ti 4+ w. r. t. O 2– by δ = 0. 1 A gives p /cell = 6 e δ 3 10– 18 esu cm In Li. Nb. O 3, δ is 0. 9 A for Li & 0. 5 A for Nb → larger p.

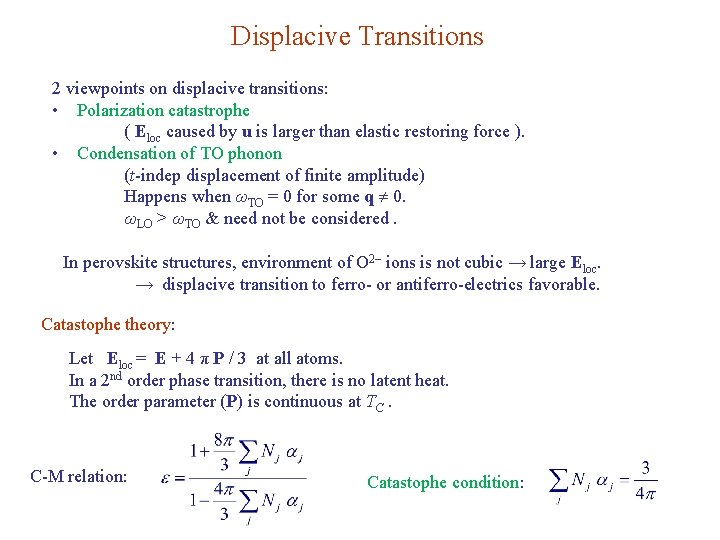
Displacive Transitions 2 viewpoints on displacive transitions: • Polarization catastrophe ( Eloc caused by u is larger than elastic restoring force ). • Condensation of TO phonon (t-indep displacement of finite amplitude) Happens when ωTO = 0 for some q 0. ωLO > ωTO & need not be considered. In perovskite structures, environment of O 2– ions is not cubic → large Eloc. → displacive transition to ferro- or antiferro-electrics favorable. Catastophe theory: Let Eloc = E + 4 π P / 3 at all atoms. In a 2 nd order phase transition, there is no latent heat. The order parameter (P) is continuous at TC. C-M relation: Catastophe condition:

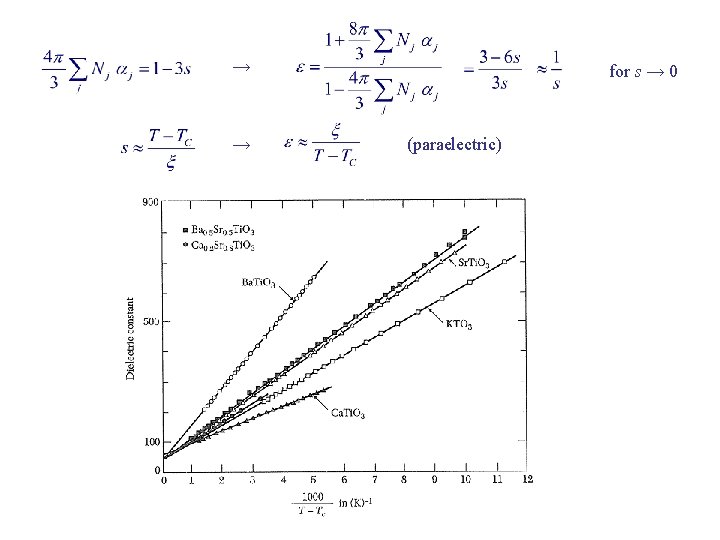
→ → for s → 0 (paraelectric)

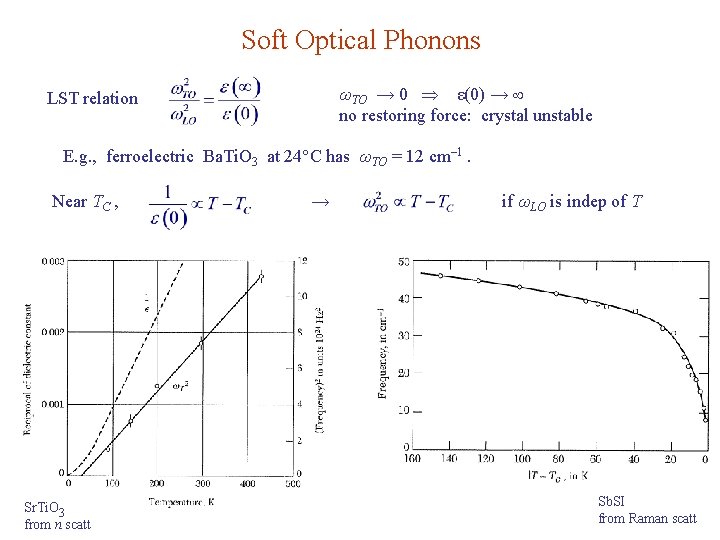
Soft Optical Phonons ωTO → 0 ε(0) → no restoring force: crystal unstable LST relation E. g. , ferroelectric Ba. Ti. O 3 at 24 C has ωTO = 12 cm– 1. Near TC , Sr. Ti. O 3 from n scatt → if ωLO is indep of T Sb. SI from Raman scatt

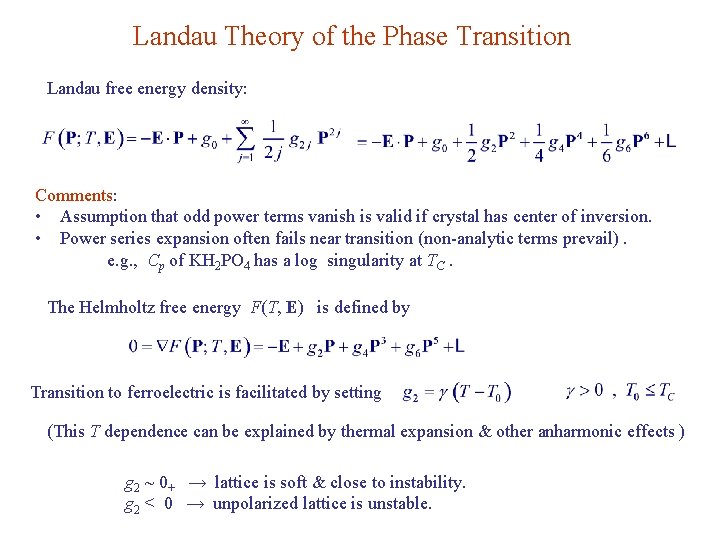
Landau Theory of the Phase Transition Landau free energy density: Comments: • Assumption that odd power terms vanish is valid if crystal has center of inversion. • Power series expansion often fails near transition (non-analytic terms prevail). e. g. , Cp of KH 2 PO 4 has a log singularity at TC. The Helmholtz free energy F(T, E) is defined by Transition to ferroelectric is facilitated by setting (This T dependence can be explained by thermal expansion & other anharmonic effects ) g 2 ~ 0+ → lattice is soft & close to instability. g 2 < 0 → unpolarized lattice is unstable.

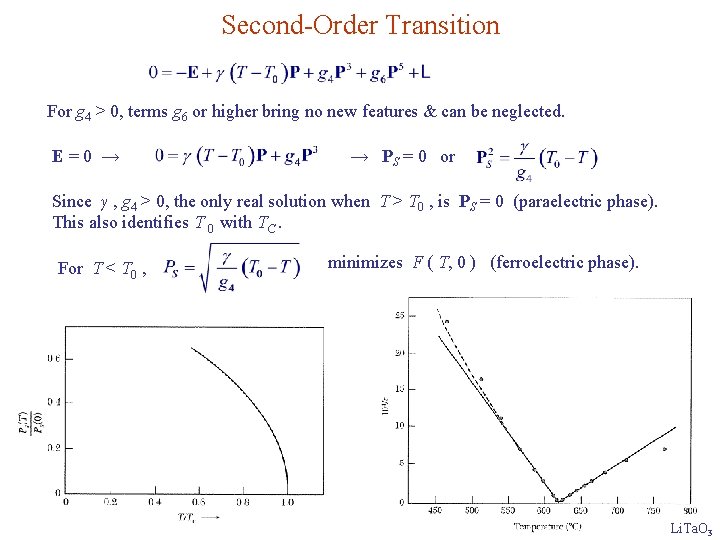
Second-Order Transition For g 4 > 0, terms g 6 or higher bring no new features & can be neglected. E=0 → → PS = 0 or Since γ , g 4 > 0, the only real solution when T > T 0 , is PS = 0 (paraelectric phase). This also identifies T 0 with TC. For T < T 0 , minimizes F ( T, 0 ) (ferroelectric phase). Li. Ta. O 3

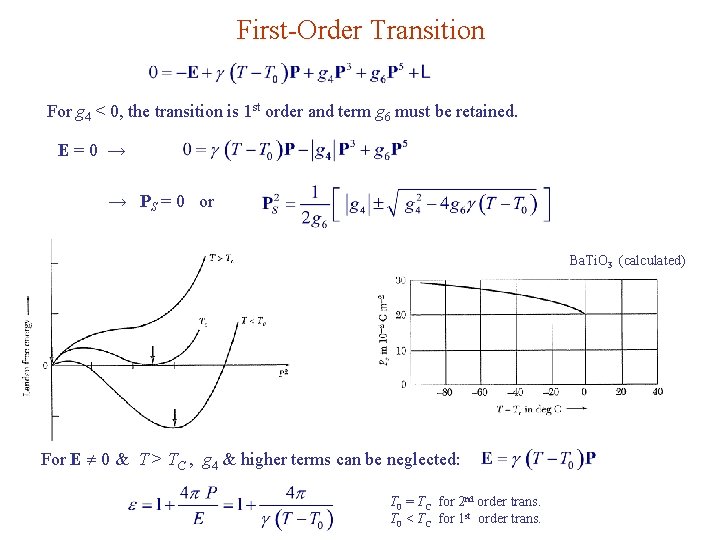
First-Order Transition For g 4 < 0, the transition is 1 st order and term g 6 must be retained. E=0 → → PS = 0 or Ba. Ti. O 3 (calculated) For E 0 & T > TC , g 4 & higher terms can be neglected: T 0 = TC for 2 nd order trans. T 0 < TC for 1 st order trans.

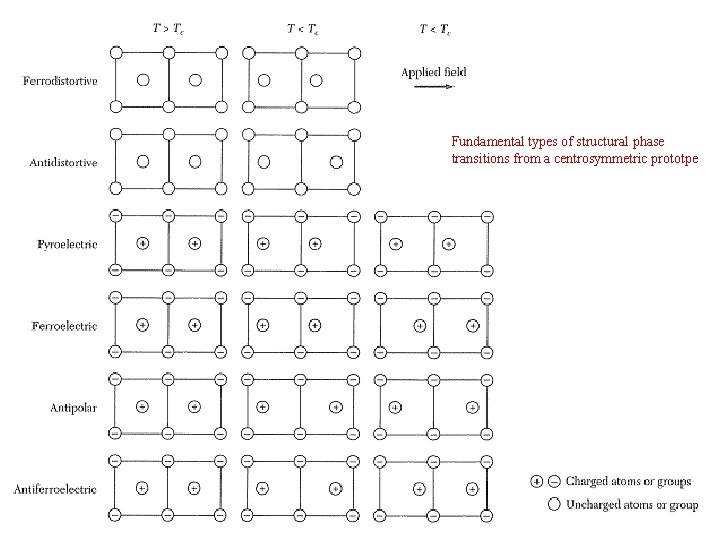
Fundamental types of structural phase transitions from a centrosymmetric prototpe

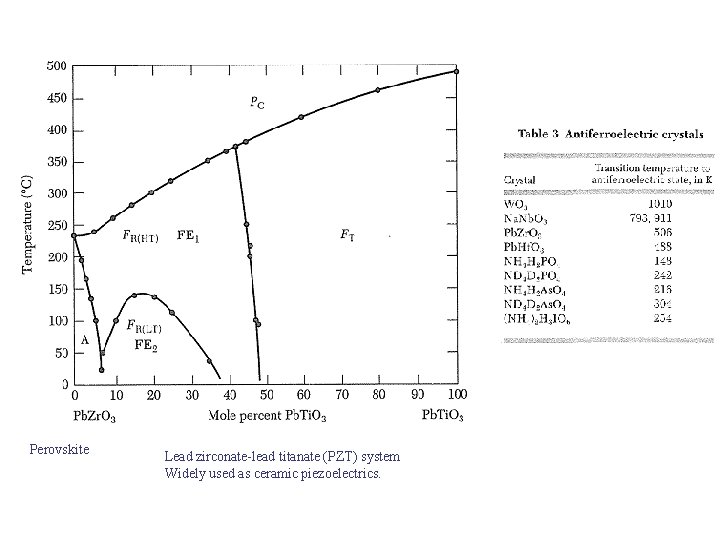
Perovskite Lead zirconate-lead titanate (PZT) system Widely used as ceramic piezoelectrics.

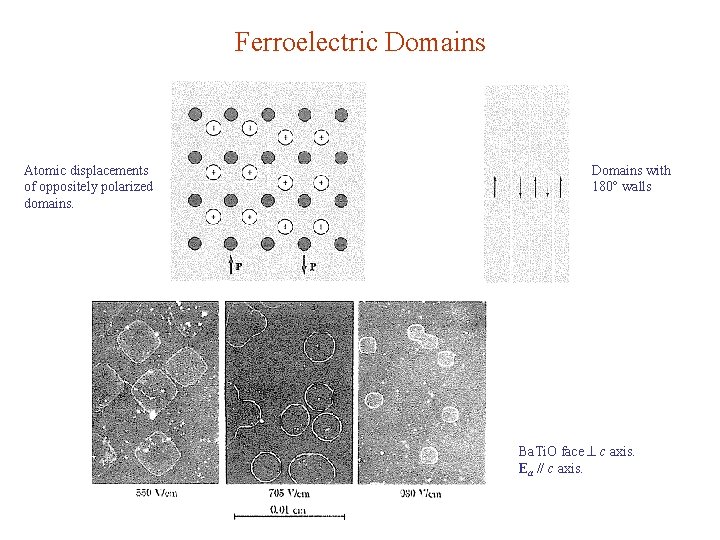
Ferroelectric Domains Atomic displacements of oppositely polarized domains. Domains with 180 walls Ba. Ti. O face c axis. Ea // c axis.

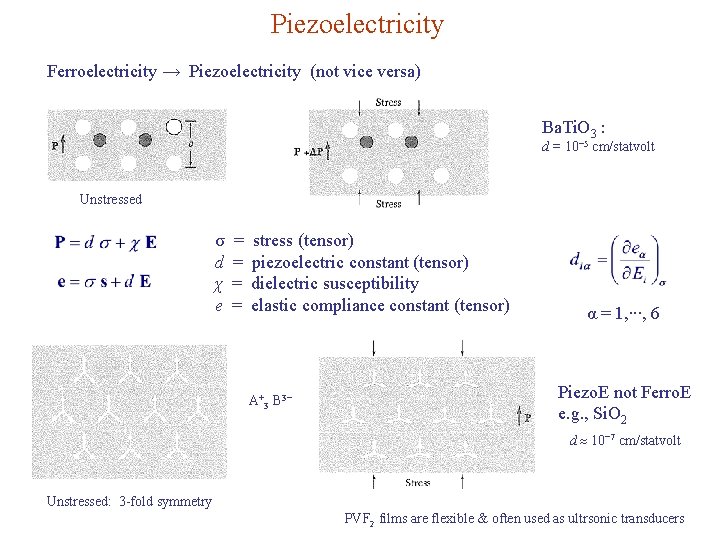
Piezoelectricity Ferroelectricity → Piezoelectricity (not vice versa) Ba. Ti. O 3 : d = 10− 5 cm/statvolt Unstressed σ d χ e = = stress (tensor) piezoelectric constant (tensor) dielectric susceptibility elastic compliance constant (tensor) A+3 B 3− α = 1, ∙∙∙, 6 Piezo. E not Ferro. E e. g. , Si. O 2 d 10− 7 cm/statvolt Unstressed: 3 -fold symmetry PVF 2 films are flexible & often used as ultrsonic transducers