1 ALLHAT U S Department of Health and

























- Slides: 50

1 ALLHAT U. S. Department of Health and Human Services Major Outcomes in High Risk Hypertensive Patients Randomized to Angiotensin. Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic National Institutes of Health The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) The ALLHAT Collaborative Research Group National Heart, Lung, and Blood Institute Sponsored by the National Heart, Lung, and Blood Institute (NHLBI) JAMA. 2002; 288: 2981 -2997

2 ALLHAT Antihypertensive Trial Design • Randomized, double-blind, multi-center clinical trial • Determine whether occurrence of fatal CHD or nonfatal MI is lower for high-risk hypertensive patients treated with newer agents (CCB, ACEI, alpha-blocker) compared with a diuretic • 42, 418 high-risk hypertensive patients ≥ 55 years

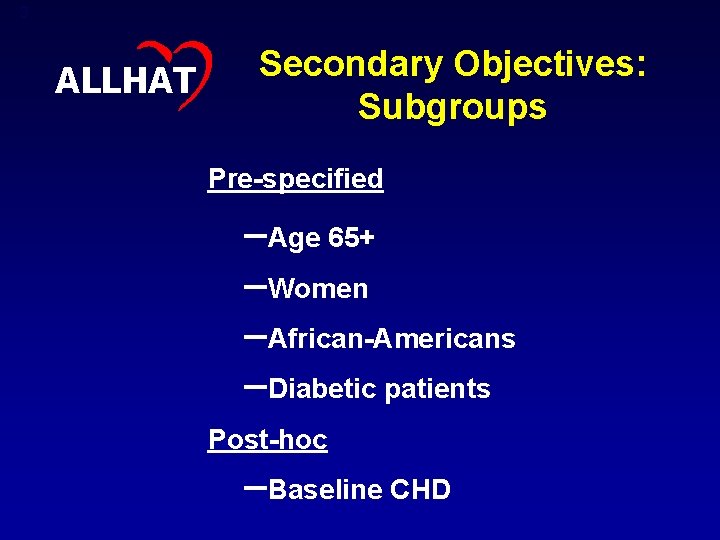
3 ALLHAT Secondary Objectives: Subgroups Pre-specified –Age 65+ –Women –African-Americans –Diabetic patients Post-hoc –Baseline CHD

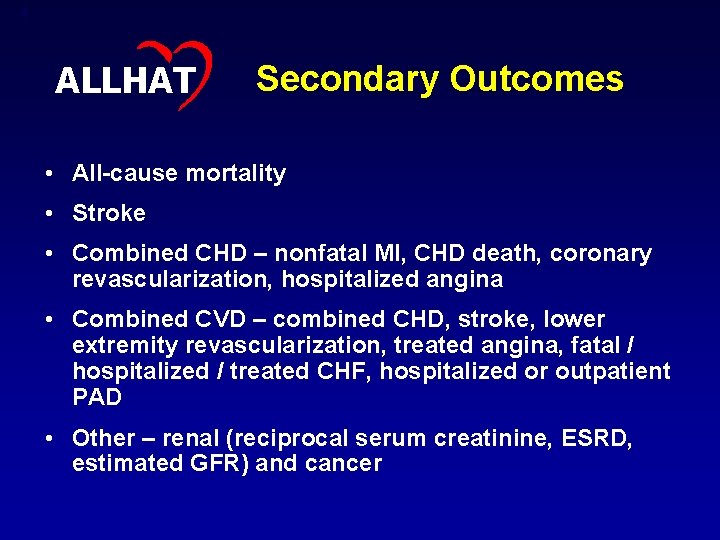
4 ALLHAT Secondary Outcomes • All-cause mortality • Stroke • Combined CHD – nonfatal MI, CHD death, coronary revascularization, hospitalized angina • Combined CVD – combined CHD, stroke, lower extremity revascularization, treated angina, fatal / hospitalized / treated CHF, hospitalized or outpatient PAD • Other – renal (reciprocal serum creatinine, ESRD, estimated GFR) and cancer

5 ALLHAT Sites in ALLHAT • 623 clinical sites • United States, Canada, Puerto Rico, US Virgin Islands • VA, private & group general medicine practices, community health centers, HMOs, specialty practices • Variety of research experience

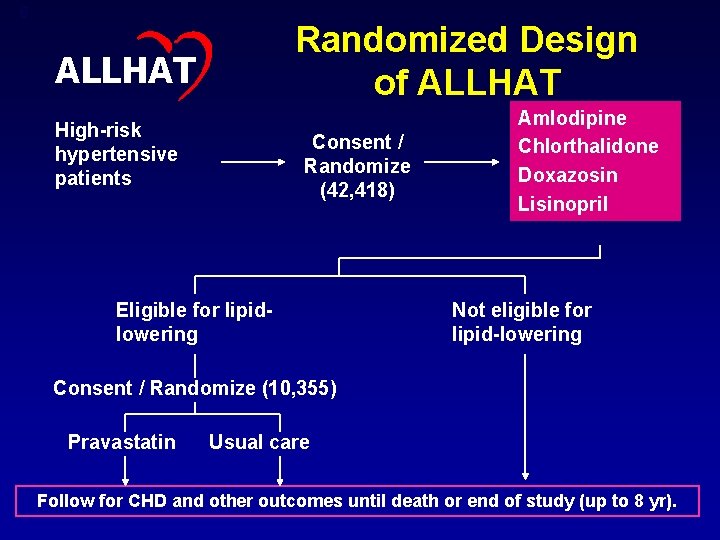
6 Randomized Design of ALLHAT High-risk hypertensive patients Consent / Randomize (42, 418) Eligible for lipidlowering Amlodipine Chlorthalidone Doxazosin Lisinopril Not eligible for lipid-lowering Consent / Randomize (10, 355) Pravastatin Usual care Follow for CHD and other outcomes until death or end of study (up to 8 yr).

7 ALLHAT Inclusion Criteria for Antihypertensive Trial • Age/sex: men and women aged > 55 years • BP eligibility: – Untreated systolic and/or diastolic hypertension ( 140/90 mm Hg but 180/110 mm Hg at two visits) – Treated hypertension • ≤ 160/100 mm Hg on 1 -2 antihypertensive drugs at Visit 1 • ≤ 180/110 mm Hg at Visit 2, when medication may have been partially withdrawn –No washout period was required in ALLHAT.

8 ALLHAT Inclusion Criteria: Risk Factors At least one of the following: • Myocardial infarction or stroke: at least 6 months old, or age-indeterminate • History of revascularization procedure • Major ST segment depression or T-wave inversion • Other documented ASCVD

9 ALLHAT Inclusion Criteria: Risk Factors At least one of the following (cont. ) • Type 2 diabetes mellitus • HDL cholesterol < 35 mg/d. L on any 2 or more determinations in past 5 years • Left ventricular hypertrophy (past 2 years) – ECG, or echo (septum + posterior wall thickness 25 mm) • Current cigarette smoking

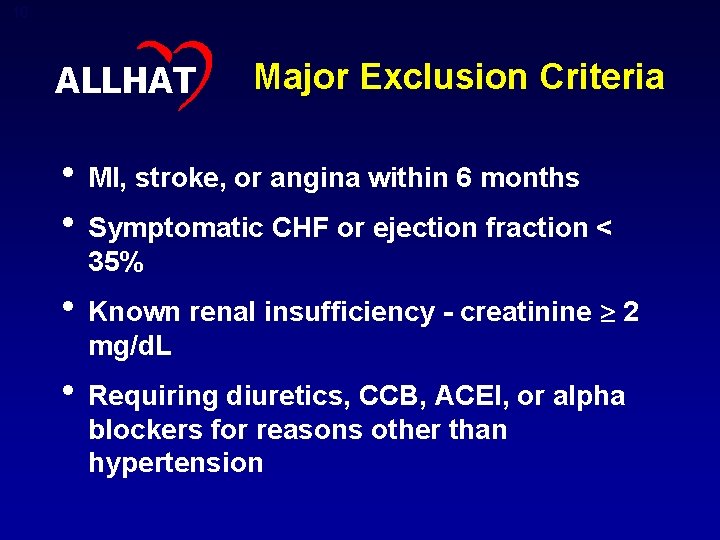
10 ALLHAT Major Exclusion Criteria • MI, stroke, or angina within 6 months • Symptomatic CHF or ejection fraction < 35% • Known renal insufficiency - creatinine 2 mg/d. L • Requiring diuretics, CCB, ACEI, or alpha blockers for reasons other than hypertension

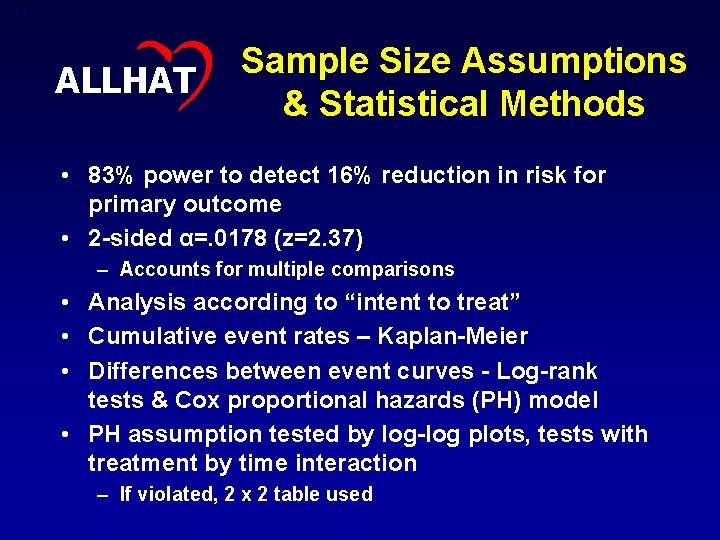
11 ALLHAT Sample Size Assumptions & Statistical Methods • 83% power to detect 16% reduction in risk for primary outcome • 2 -sided α=. 0178 (z=2. 37) – Accounts for multiple comparisons • Analysis according to “intent to treat” • Cumulative event rates – Kaplan-Meier • Differences between event curves - Log-rank tests & Cox proportional hazards (PH) model • PH assumption tested by log-log plots, tests with treatment by time interaction – If violated, 2 x 2 table used

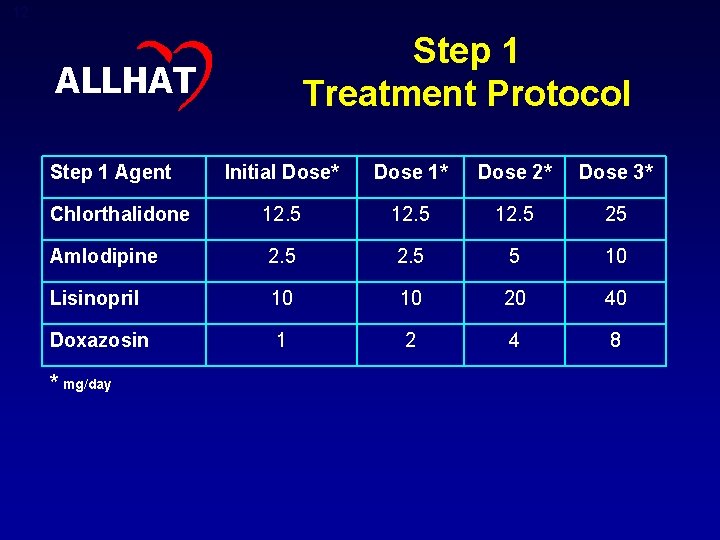
12 Step 1 Treatment Protocol ALLHAT Step 1 Agent Initial Dose* Dose 1* Dose 2* Dose 3* Chlorthalidone 12. 5 25 Amlodipine 2. 5 5 10 Lisinopril 10 10 20 40 Doxazosin 1 2 4 8 * mg/day

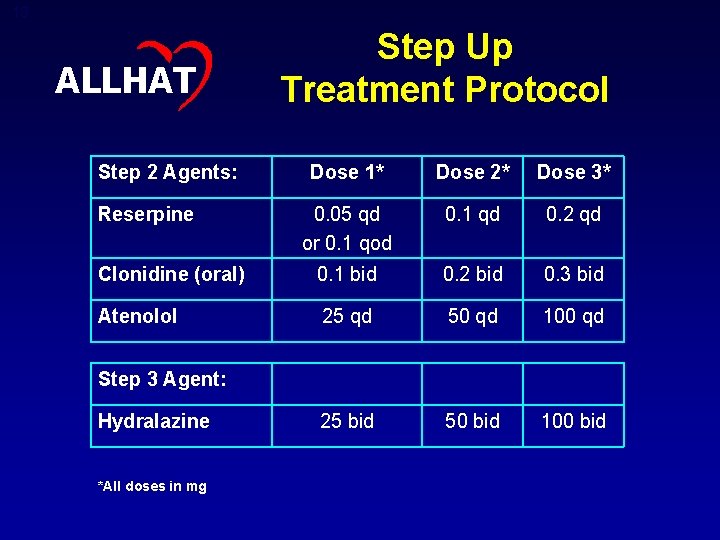
13 ALLHAT Step 2 Agents: Step Up Treatment Protocol Dose 1* Dose 2* Dose 3* 0. 05 qd or 0. 1 qod 0. 1 qd 0. 2 qd Clonidine (oral) 0. 1 bid 0. 2 bid 0. 3 bid Atenolol 25 qd 50 qd 100 qd 25 bid 50 bid 100 bid Reserpine Step 3 Agent: Hydralazine *All doses in mg

14 ALLHAT Safety Outcomes • Angioedema • Hospitalization for gastrointestinal bleeding – Records from the VA hospitalization database – Records from the Center for Medicare & Medicaid Services (CMS) database (participants age 65 or older)

15 ALLHAT Decision to Drop an ALLHAT Arm • January 24, 2000 – NHLBI Director accepts the recommendation of an independent review group to terminate doxazosin arm – Futility of finding a significant difference for primary outcome – Statistically significant 25 percent higher rate of major secondary endpoint, combined CVD outcomes

16 Cardiovascular Disease Cumulative Event Rate Rel Risk 1. 25 95% CI 1. 17 -1. 33 ALLHAT z = 6. 77, p < 0. 0001 doxazosin chlorthalidone 12, 990 7, 382 C: 15, 268 D: 9, 067 9, 443 5, 285 4, 827 2, 654 2, 010 1, 083 Years of Follow-up JAMA. 2000; 283: 1967 -1975

17 Heart Failure Cumulative Event Rate Rel Risk 2. 04 95% CI 1. 79 -2. 32 ALLHAT z = 10. 95, p < 0. 0001 doxazosin 13, 644 7, 845 C: 15, 268 D: 9, 067 chlorthalidone 9, 541 5, 457 5, 531 3, 089 2, 427 1, 351 Years of Follow-up JAMA. 2000; 283: 1967 -1975

18 ALLHAT Comparison of Doxazosin with Chlorthalidone Conclusions • Doxazosin is not recommended as firstline therapy in hypertension. • ALLHAT does not allow an assessment of the effect of doxazosin compared with placebo on the incidence of CVD. • The use of doxazosin as a step-up drug for treating hypertension was not tested in this trial. • These findings are likely to apply to all alpha-blockers. JAMA. 2000; 283: 1967 -1975

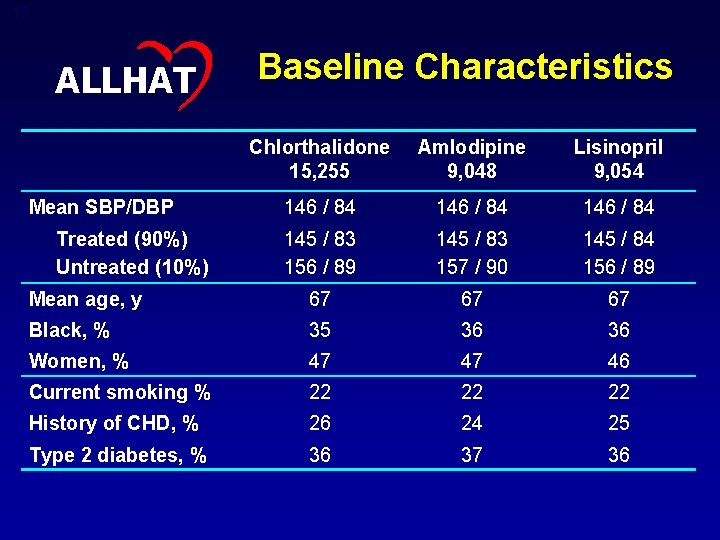
19 ALLHAT Baseline Characteristics Chlorthalidone 15, 255 Amlodipine 9, 048 Lisinopril 9, 054 146 / 84 145 / 83 156 / 89 145 / 83 157 / 90 145 / 84 156 / 89 Mean age, y 67 67 67 Black, % 35 36 36 Women, % 47 47 46 Current smoking % 22 22 22 History of CHD, % 26 24 25 Type 2 diabetes, % 36 37 36 Mean SBP/DBP Treated (90%) Untreated (10%)

20 ALLHAT On Step 1 or Equivalent Treatment by Antihypertensive Treatment Group

21 ALLHAT Full Crossovers by Antihypertensive Treatment Group Chlorthalidone: not on assigned medicine or open-label diuretic, but on open-label calcium channel blocker or ACE-inhibitor Amlodipine: not on assigned medicine or open-label calcium channel blocker, but on open-label diuretic Lisinopril: not on assigned medicine or open-label ACE-inhibitor, but on open-label diuretic

22 ALLHAT On Step 2 or Step 3 Treatment by Antihypertensive Treatment Group

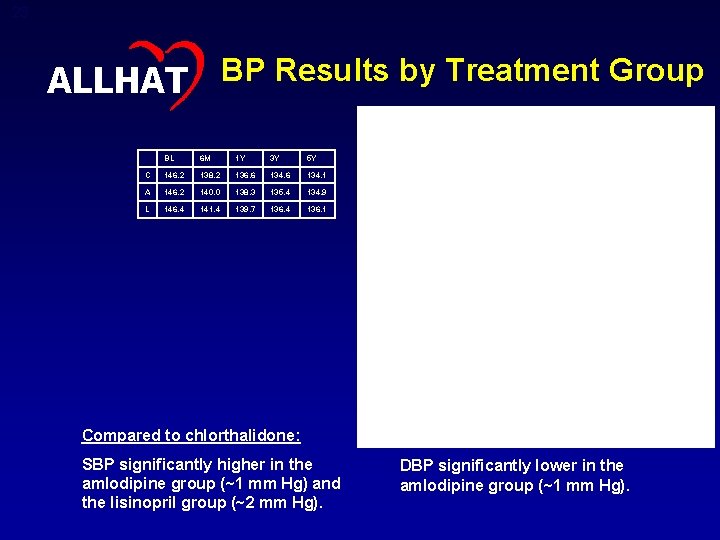
23 ALLHAT BP Results by Treatment Group BL 6 M 1 Y 3 Y 5 Y C 146. 2 138. 2 136. 6 134. 1 A 146. 2 140. 0 138. 3 135. 4 134. 9 L 146. 4 141. 4 139. 7 136. 4 136. 1 BL 6 M 1 Y 3 Y 5 Y C 84. 0 80. 1 79. 2 77. 1 75. 4 A 83. 9 79. 7 78. 5 76. 1 74. 5 L 84. 1 80. 8 79. 7 77. 2 75. 4 Compared to chlorthalidone: SBP significantly higher in the amlodipine group (~1 mm Hg) and the lisinopril group (~2 mm Hg). DBP significantly lower in the amlodipine group (~1 mm Hg).

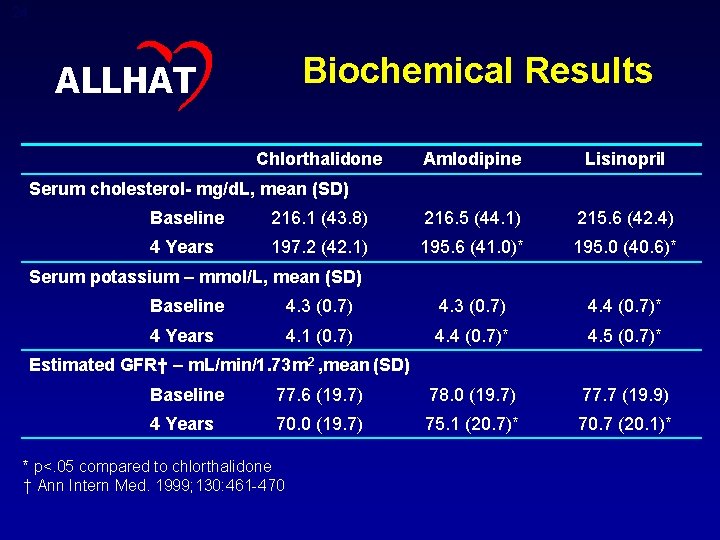
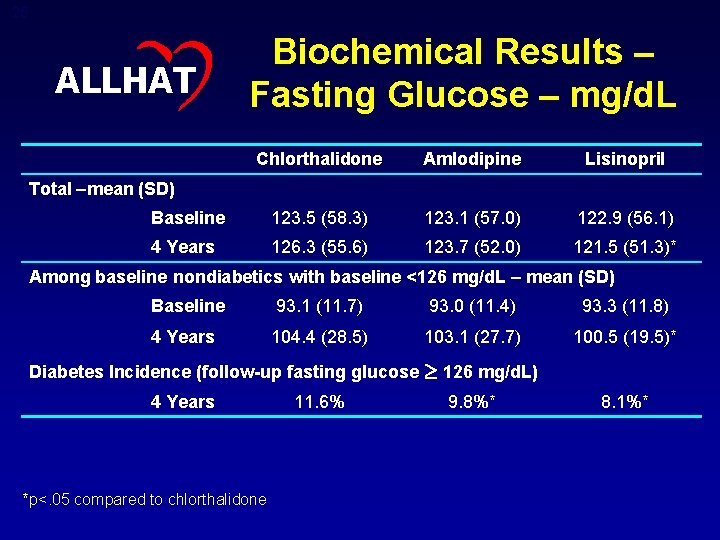
24 Biochemical Results ALLHAT Chlorthalidone Amlodipine Lisinopril Serum cholesterol- mg/d. L, mean (SD) Baseline 216. 1 (43. 8) 216. 5 (44. 1) 215. 6 (42. 4) 4 Years 197. 2 (42. 1) 195. 6 (41. 0)* 195. 0 (40. 6)* Serum potassium – mmol/L, mean (SD) Baseline 4. 3 (0. 7) 4. 4 (0. 7)* 4 Years 4. 1 (0. 7) 4. 4 (0. 7)* 4. 5 (0. 7)* Estimated GFR† – m. L/min/1. 73 m 2 , mean (SD) Baseline 77. 6 (19. 7) 78. 0 (19. 7) 77. 7 (19. 9) 4 Years 70. 0 (19. 7) 75. 1 (20. 7)* 70. 7 (20. 1)* * p<. 05 compared to chlorthalidone † Ann Intern Med. 1999; 130: 461 -470

25 ALLHAT Biochemical Results – Fasting Glucose – mg/d. L Chlorthalidone Amlodipine Lisinopril Baseline 123. 5 (58. 3) 123. 1 (57. 0) 122. 9 (56. 1) 4 Years 126. 3 (55. 6) 123. 7 (52. 0) 121. 5 (51. 3)* Total –mean (SD) Among baseline nondiabetics with baseline <126 mg/d. L – mean (SD) Baseline 93. 1 (11. 7) 93. 0 (11. 4) 93. 3 (11. 8) 4 Years 104. 4 (28. 5) 103. 1 (27. 7) 100. 5 (19. 5)* Diabetes Incidence (follow-up fasting glucose 4 Years *p<. 05 compared to chlorthalidone 11. 6% 126 mg/d. L) 9. 8%* 8. 1%*

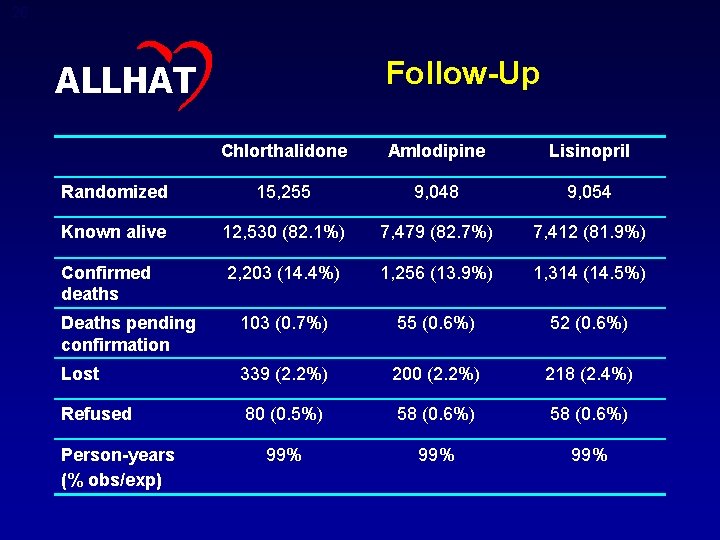
26 Follow-Up ALLHAT Chlorthalidone Amlodipine Lisinopril Randomized 15, 255 9, 048 9, 054 Known alive 12, 530 (82. 1%) 7, 479 (82. 7%) 7, 412 (81. 9%) Confirmed deaths 2, 203 (14. 4%) 1, 256 (13. 9%) 1, 314 (14. 5%) Deaths pending confirmation 103 (0. 7%) 55 (0. 6%) 52 (0. 6%) Lost 339 (2. 2%) 200 (2. 2%) 218 (2. 4%) Refused 80 (0. 5%) 58 (0. 6%) 99% 99% Person-years (% obs/exp)

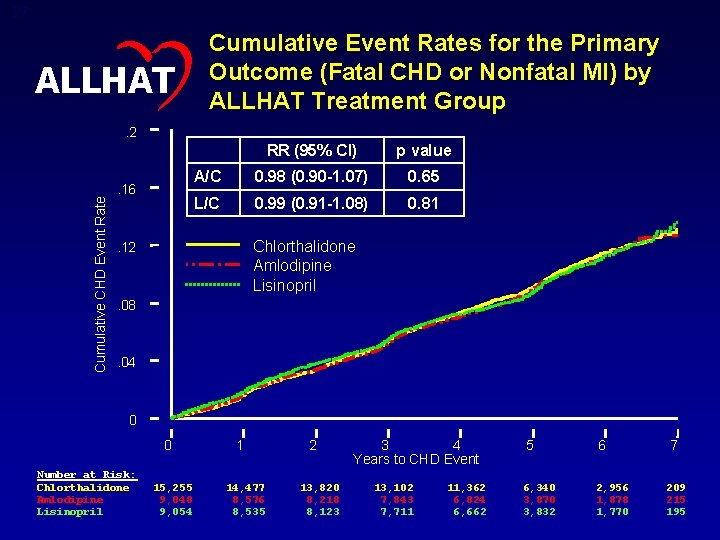
27 ALLHAT Cumulative Event Rates for the Primary Outcome (Fatal CHD or Nonfatal MI) by ALLHAT Treatment Group Cumulative CHD Event Rate . 2 . 16 RR (95% CI) p value A/C 0. 98 (0. 90 -1. 07) 0. 65 L/C 0. 99 (0. 91 -1. 08) 0. 81 Chlorthalidone Amlodipine Lisinopril . 12 . 08 . 04 0 0 Number at Risk: Chlorthalidone Amlodipine Lisinopril 15, 255 9, 048 9, 054 1 14, 477 8, 576 8, 535 2 13, 820 8, 218 8, 123 3 4 Years to CHD Event 13, 102 7, 843 7, 711 11, 362 6, 824 6, 662 5 6 6, 340 3, 870 3, 832 2, 956 1, 878 1, 770 7 209 215 195

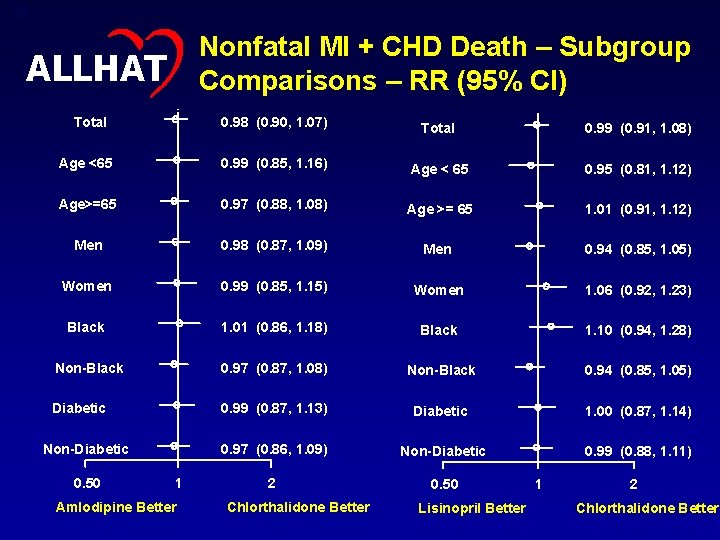
28 Nonfatal MI + CHD Death – Subgroup Comparisons – RR (95% CI) ALLHAT Total 0. 98 (0. 90, 1. 07) Total 0. 99 (0. 91, 1. 08) Age <65 0. 99 (0. 85, 1. 16) Age < 65 0. 95 (0. 81, 1. 12) Age>=65 0. 97 (0. 88, 1. 08) Age >= 65 1. 01 (0. 91, 1. 12) Men 0. 98 (0. 87, 1. 09) Men 0. 94 (0. 85, 1. 05) Women 0. 99 (0. 85, 1. 15) Women 1. 06 (0. 92, 1. 23) Black 1. 01 (0. 86, 1. 18) Black 1. 10 (0. 94, 1. 28) Non-Black 0. 97 (0. 87, 1. 08) Non-Black 0. 94 (0. 85, 1. 05) Diabetic 0. 99 (0. 87, 1. 13) Diabetic 1. 00 (0. 87, 1. 14) 0. 97 (0. 86, 1. 09) Non-Diabetic 0. 99 (0. 88, 1. 11) 2 0. 50 Non-Diabetic 0. 50 1 Amlodipine Better Chlorthalidone Better Lisinopril Better 1 2 Chlorthalidone Better

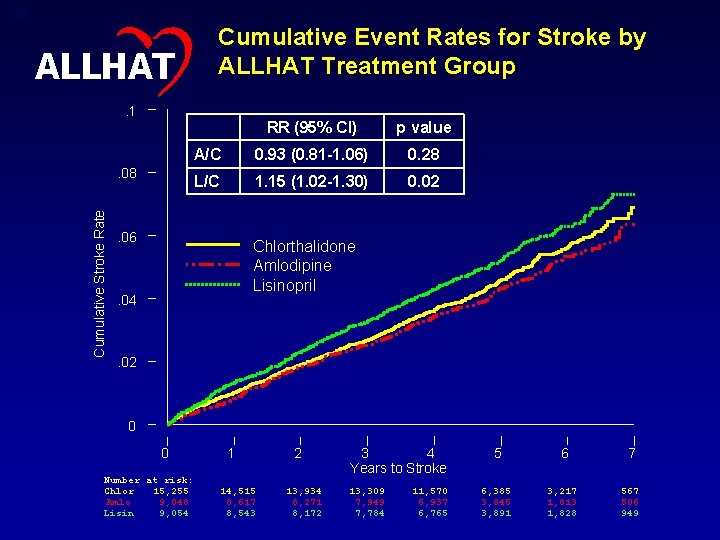
29 Cumulative Event Rates for Stroke by ALLHAT Treatment Group ALLHAT. 1 Cumulative Stroke Rate . 08 RR (95% CI) p value A/C 0. 93 (0. 81 -1. 06) 0. 28 L/C 1. 15 (1. 02 -1. 30) 0. 02 . 06 Chlorthalidone Amlodipine Lisinopril . 04 . 02 0 0 Number at risk: Chlor 15, 255 Amlo 9, 048 Lisin 9, 054 1 14, 515 8, 617 8, 543 2 13, 934 8, 271 8, 172 3 4 Years to Stroke 13, 309 7, 949 7, 784 11, 570 6, 937 6, 765 5 6 7 6, 385 3, 845 3, 891 3, 217 1, 813 1, 828 567 506 949

30 ALLHAT Stroke – Subgroup Comparisons – RR (95% CI) Total 0. 93 (0. 82, 1. 06) Total 1. 15 (1. 02, 1. 30) Age < 65 0. 93 (0. 73, 1. 19) Age < 65 1. 21 (0. 97, 1. 52) Age >= 65 0. 93 (0. 81, 1. 08) Age >= 65 1. 13 (0. 98, 1. 30) Men 1. 00 (0. 85, 1. 18) Men 1. 10 (0. 94, 1. 29) Women 0. 84 (0. 69, 1. 03) Women 1. 22 (1. 01, 1. 46) Black 0. 93 (0. 76, 1. 14) Black 1. 40 (1. 17, 1. 68) Non-Black 0. 93 (0. 79, 1. 10) Non-Black 1. 00 (0. 85, 1. 17) Diabetic 0. 90 (0. 75, 1. 08) Diabetic 1. 07 (0. 90, 1. 28) Non-Diabetic 0. 96 (0. 81, 1. 14) Non-Diabetic 2 0. 50 1 Amlodipine Better Chlorthalidone Better 1. 23 (1. 05, 1. 44) 1 Lisinopril Better 2 Chlorthalidone Better P =. 01 for interaction

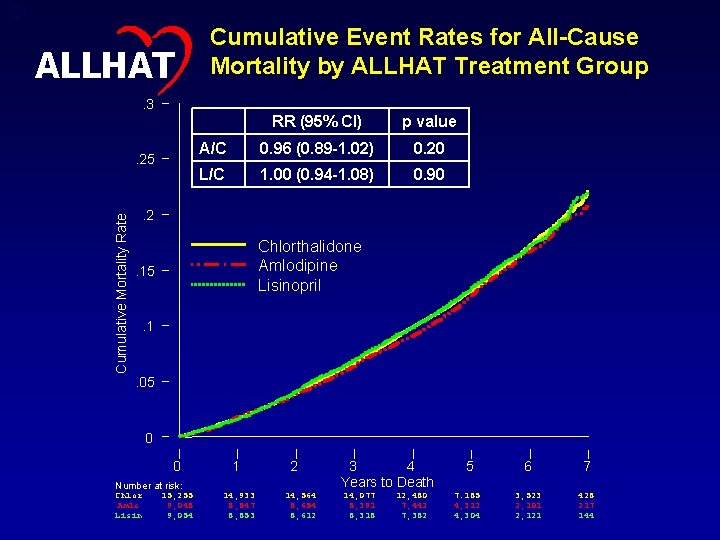
31 ALLHAT Cumulative Event Rates for All-Cause Mortality by ALLHAT Treatment Group . 3 Cumulative Mortality Rate . 25 RR (95% CI) p value A/C 0. 96 (0. 89 -1. 02) 0. 20 L/C 1. 00 (0. 94 -1. 08) 0. 90 . 2 Chlorthalidone Amlodipine Lisinopril . 15 . 1 . 05 0 0 Number at risk: Chlor Amlo Lisin 15, 255 9, 048 9, 054 1 14, 933 8, 847 8, 853 2 14, 564 8, 654 8, 612 3 4 Years to Death 14, 077 8, 391 8, 318 12, 480 7, 442 7, 382 5 6 7 7. 185 4, 312 4, 304 3, 523 2, 101 2, 121 428 217 144

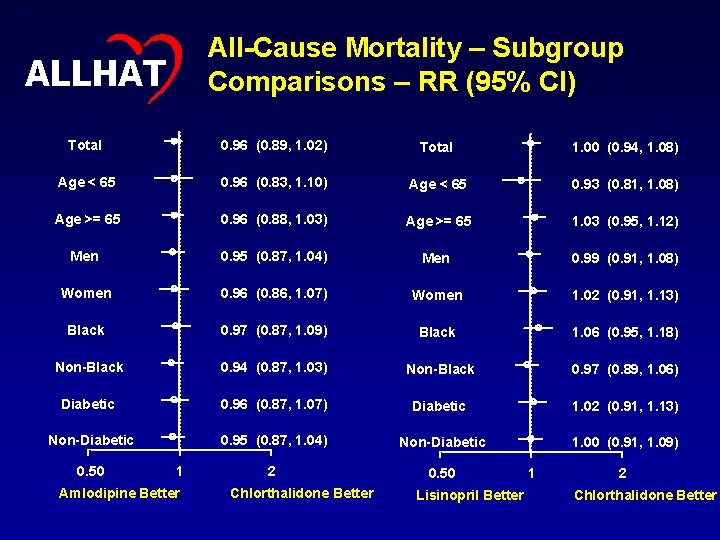
32 All-Cause Mortality – Subgroup Comparisons – RR (95% CI) ALLHAT Total 0. 96 (0. 89, 1. 02) Total 1. 00 (0. 94, 1. 08) Age < 65 0. 96 (0. 83, 1. 10) Age < 65 0. 93 (0. 81, 1. 08) Age >= 65 0. 96 (0. 88, 1. 03) Age >= 65 1. 03 (0. 95, 1. 12) Men 0. 95 (0. 87, 1. 04) Men 0. 99 (0. 91, 1. 08) Women 0. 96 (0. 86, 1. 07) Women 1. 02 (0. 91, 1. 13) Black 0. 97 (0. 87, 1. 09) Black 1. 06 (0. 95, 1. 18) Non-Black 0. 94 (0. 87, 1. 03) Non-Black 0. 97 (0. 89, 1. 06) Diabetic 0. 96 (0. 87, 1. 07) Diabetic 1. 02 (0. 91, 1. 13) Non-Diabetic 0. 95 (0. 87, 1. 04) Non-Diabetic 1. 00 (0. 91, 1. 09) 2 0. 50 1 Amlodipine Better Chlorthalidone Better Lisinopril Better 1 2 Chlorthalidone Better

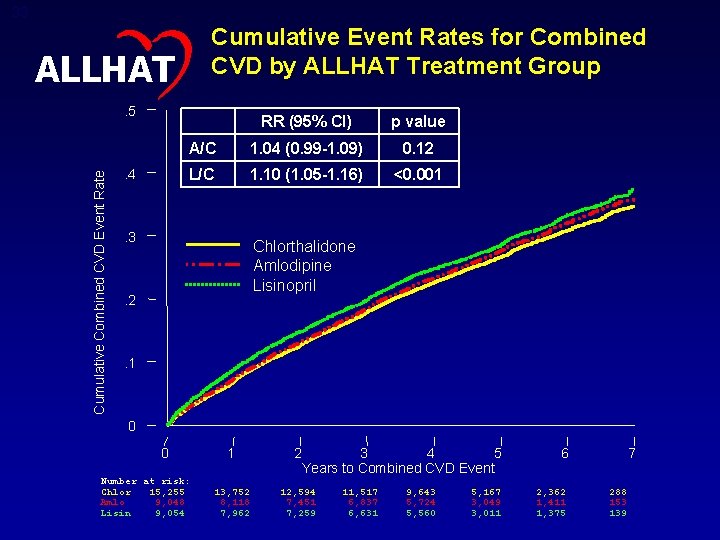
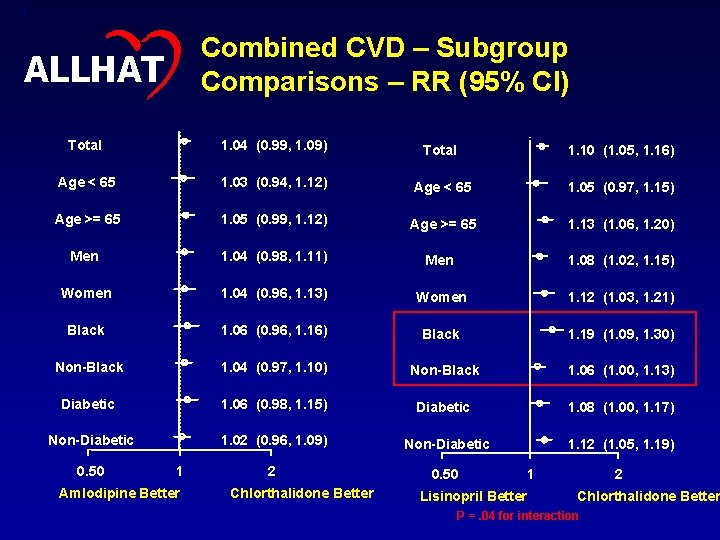
33 Cumulative Event Rates for Combined CVD by ALLHAT Treatment Group ALLHAT Cumulative Combined CVD Event Rate . 5 . 4 RR (95% CI) p value A/C 1. 04 (0. 99 -1. 09) 0. 12 L/C 1. 10 (1. 05 -1. 16) <0. 001 . 3 Chlorthalidone Amlodipine Lisinopril . 2 . 1 0 0 Number at risk: Chlor 15, 255 Amlo 9, 048 Lisin 9, 054 1 2 3 4 5 Years to Combined CVD Event 13, 752 8, 118 7, 962 12, 594 7, 451 7, 259 11, 517 6, 837 6, 631 9, 643 5, 724 5, 560 5, 167 3, 049 3, 011 6 2, 362 1, 411 1, 375 7 288 153 139

34 Combined CVD – Subgroup Comparisons – RR (95% CI) ALLHAT Total 1. 04 (0. 99, 1. 09) Total 1. 10 (1. 05, 1. 16) Age < 65 1. 03 (0. 94, 1. 12) Age < 65 1. 05 (0. 97, 1. 15) Age >= 65 1. 05 (0. 99, 1. 12) Age >= 65 1. 13 (1. 06, 1. 20) Men 1. 04 (0. 98, 1. 11) Men 1. 08 (1. 02, 1. 15) Women 1. 04 (0. 96, 1. 13) Women 1. 12 (1. 03, 1. 21) Black 1. 06 (0. 96, 1. 16) Black 1. 19 (1. 09, 1. 30) Non-Black 1. 04 (0. 97, 1. 10) Non-Black 1. 06 (1. 00, 1. 13) Diabetic 1. 06 (0. 98, 1. 15) Diabetic 1. 08 (1. 00, 1. 17) Non-Diabetic 1. 02 (0. 96, 1. 09) Non-Diabetic 1. 12 (1. 05, 1. 19) 2 0. 50 1 Amlodipine Better Chlorthalidone Better Lisinopril Better 1 2 Chlorthalidone Better P =. 04 for interaction

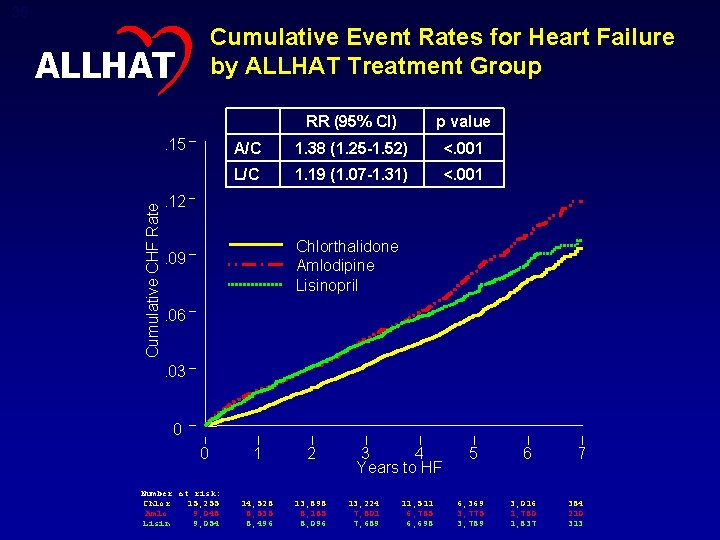
35 Cumulative Event Rates for Heart Failure by ALLHAT Treatment Group ALLHAT Cumulative CHF Rate . 15 RR (95% CI) p value A/C 1. 38 (1. 25 -1. 52) <. 001 L/C 1. 19 (1. 07 -1. 31) <. 001 . 12 Chlorthalidone Amlodipine Lisinopril . 09. 06. 03 0 0 Number at risk: Chlor 15, 255 Amlo 9, 048 Lisin 9, 054 1 2 14, 528 8, 535 8, 496 13, 898 8, 185 8, 096 3 4 Years to HF 13, 224 7, 801 7, 689 11, 511 6, 785 6, 698 5 6, 369 3, 775 3, 789 6 3, 016 1, 780 1, 837 7 384 210 313

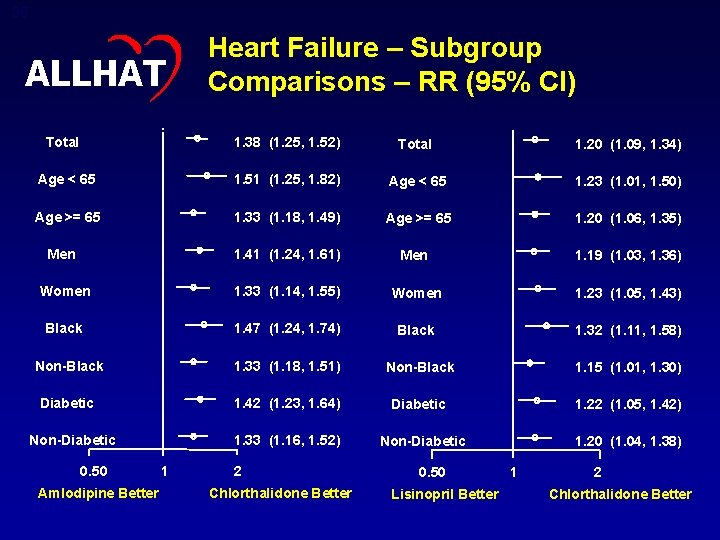
36 ALLHAT Heart Failure – Subgroup Comparisons – RR (95% CI) Total 1. 38 (1. 25, 1. 52) Total 1. 20 (1. 09, 1. 34) Age < 65 1. 51 (1. 25, 1. 82) Age < 65 1. 23 (1. 01, 1. 50) Age >= 65 1. 33 (1. 18, 1. 49) Age >= 65 1. 20 (1. 06, 1. 35) Men 1. 41 (1. 24, 1. 61) Men 1. 19 (1. 03, 1. 36) Women 1. 33 (1. 14, 1. 55) Women 1. 23 (1. 05, 1. 43) Black 1. 47 (1. 24, 1. 74) Black 1. 32 (1. 11, 1. 58) Non-Black 1. 33 (1. 18, 1. 51) Non-Black 1. 15 (1. 01, 1. 30) Diabetic 1. 42 (1. 23, 1. 64) Diabetic 1. 22 (1. 05, 1. 42) Non-Diabetic 0. 50 Amlodipine Better 1. 33 (1. 16, 1. 52) 1 2 Chlorthalidone Better Non-Diabetic 0. 50 Lisinopril Better 1. 20 (1. 04, 1. 38) 1 2 Chlorthalidone Better

37 ALLHAT Overall Conclusions Because of the superiority of thiazide-type diuretics in preventing one or more major forms of CVD and their lower cost, they should be the drugs of choice for first-step antihypertensive drug therapy.

38 ALLHAT Other Conclusions • Neither amlodipine (representing CCB) nor lisinopril (representing ACEI) was superior to chlorthalidone (representing thiazide-type diuretics) in preventing major coronary events or increasing overall survival. • Although chlorthalidone did not differ from amlodipine in overall CVD event prevention, it was superior to amlodipine (by about onefourth) in preventing heart failure, overall and for hospitalized or fatal cases.

39 ALLHAT Other Conclusions • Chlorthalidone was superior to lisinopril in preventing aggregate CV events, principally stroke, HF, angina, and coronary revascularization • Chlorthalidone was superior to doxazosin (representing alpha-blockers) in preventing CV events, including both HF and other CVD.

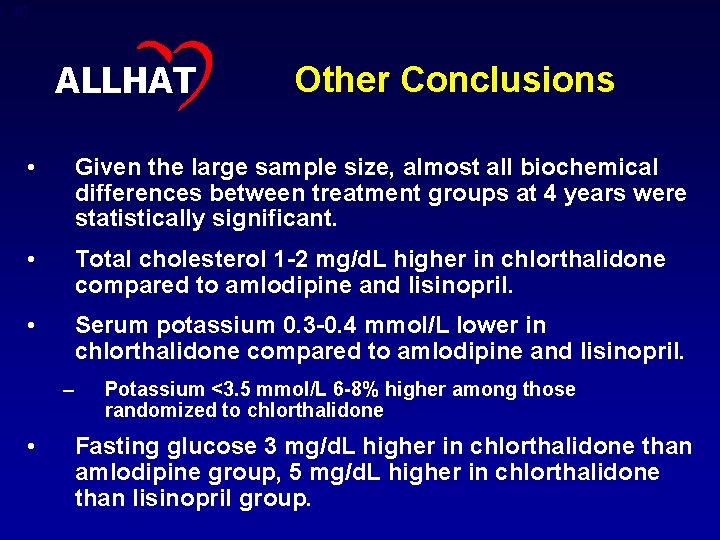
40 ALLHAT Other Conclusions • Given the large sample size, almost all biochemical differences between treatment groups at 4 years were statistically significant. • Total cholesterol 1 -2 mg/d. L higher in chlorthalidone compared to amlodipine and lisinopril. • Serum potassium 0. 3 -0. 4 mmol/L lower in chlorthalidone compared to amlodipine and lisinopril. – • Potassium <3. 5 mmol/L 6 -8% higher among those randomized to chlorthalidone Fasting glucose 3 mg/d. L higher in chlorthalidone than amlodipine group, 5 mg/d. L higher in chlorthalidone than lisinopril group.

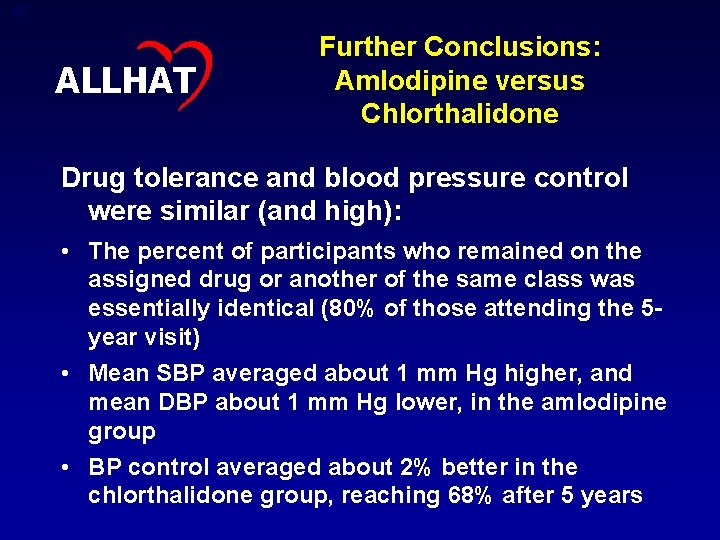
41 ALLHAT Other Conclusions • Among nondiabetic participants, incidence of fasting glucose 126 mg/d. L at 4 years was 1. 8% higher in chlorthalidone vs amlodipine, and 3. 5% higher in chlorthalidone vs lisinopril. • Estimated GFR decreased by 7 -8 units at 4 years in chlorthalidone and lisinopril arms, but decreased only by about 3 units in the amlodipine arm. • Overall, metabolic differences did not translate into more adverse cardiovascular events, or into higher all-cause mortality, with chlorthalidone.

42 ALLHAT Further Conclusions: Amlodipine versus Chlorthalidone Drug tolerance and blood pressure control were similar (and high): • The percent of participants who remained on the assigned drug or another of the same class was essentially identical (80% of those attending the 5 year visit) • Mean SBP averaged about 1 mm Hg higher, and mean DBP about 1 mm Hg lower, in the amlodipine group • BP control averaged about 2% better in the chlorthalidone group, reaching 68% after 5 years

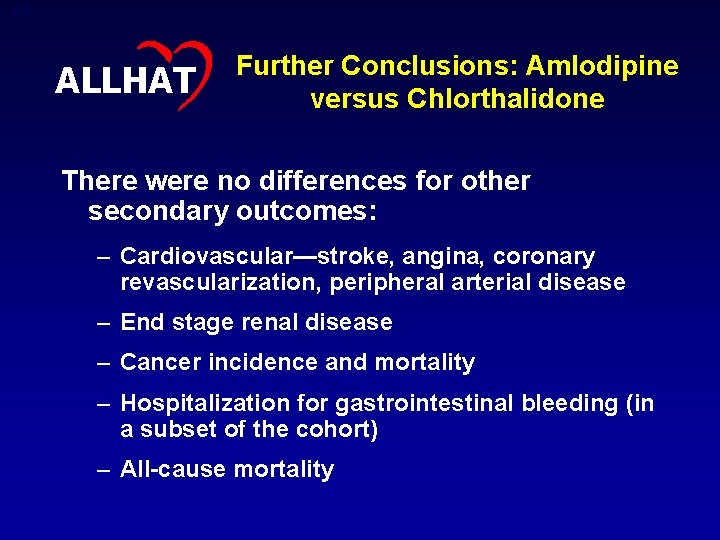
43 ALLHAT Further Conclusions: Amlodipine versus Chlorthalidone There were no differences for other secondary outcomes: – Cardiovascular—stroke, angina, coronary revascularization, peripheral arterial disease – End stage renal disease – Cancer incidence and mortality – Hospitalization for gastrointestinal bleeding (in a subset of the cohort) – All-cause mortality

44 ALLHAT Further Conclusions: Amlodipine versus Chlorthalidone Results for all cited outcomes were consistent for major (pre-specified) subgroups: – Men and women – Black and nonblack participants – Older and younger participants (<65 and 65+) – Diabetic and non-diabetic participants

45 ALLHAT Further Conclusions: Lisinopril versus Chlorthalidone Drug tolerance and blood pressure control were better with chlorthalidone, especially for black patients: • The percent of participants remaining on lisinopril or another ACEI averaged about 5 -6% less than participants assigned to the diuretic • About 6 -8% more of the participants in the lisinopril group than those in the chlorthalidone group required additional antihypertensive drugs

46 ALLHAT Further Conclusions: Lisinopril versus Chlorthalidone • Mean SBP averaged about 2 mm Hg higher in the lisinopril than the chlorthalidone group (4 mm Hg for blacks); mean DBPs were equivalent • BP control averaged about 4 -7% better in the chlorthalidone group • Of patients in the lisinopril group who remained on an ACEI, 19% were also on a diuretic at 5 years

47 ALLHAT Further Conclusions: Lisinopril versus Chlorthalidone There were no differences for other secondary outcomes – peripheral arterial disease – end stage renal disease – cancer incidence and mortality – all-cause mortality

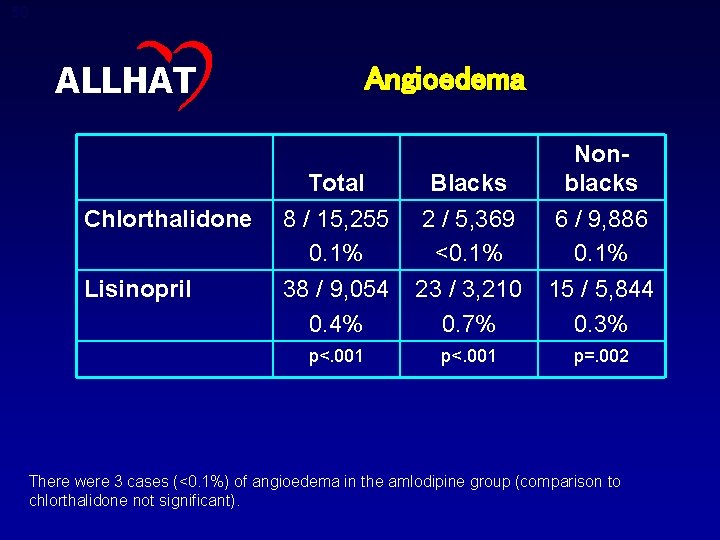
48 ALLHAT Further Conclusions: Lisinopril versus Chlorthalidone • Results were consistent for all outcomes by age, gender, race, and diabetic status, except for stroke and CVD, where there was significant heterogeneity by race (p=. 01 and p=. 04, respectively) – Among black participants assigned to lisinopril, the stroke rate was increased 40% compared to the chlorthalidone group. (No difference among nonblack participants. ) – The combined CVD rate was increased 19% in blacks and by 6% in whites. • Angiodema, a rare adverse effect, was more frequent with lisinopril, especially in blacks

49 ALLHAT Antihypertensive Trial: Implications • Diuretics should be the drug of choice for first step therapy of hypertension • For the patient who cannot take a diuretic (which should be an unusual circumstance), CCB’s and ACEI’s may be considered. • Most hypertensive patients require more than one drug. Diuretics should generally be part of the antihypertensive regimen. Lifestyle advice should also be provided.

50 ALLHAT Chlorthalidone Lisinopril Angioedema Total Blacks Nonblacks 8 / 15, 255 0. 1% 38 / 9, 054 0. 4% 2 / 5, 369 <0. 1% 23 / 3, 210 0. 7% 6 / 9, 886 0. 1% 15 / 5, 844 0. 3% p<. 001 p=. 002 There were 3 cases (<0. 1%) of angioedema in the amlodipine group (comparison to chlorthalidone not significant).
 Louisiana department of health health standards section
Louisiana department of health health standards section Iowa department of health and human services
Iowa department of health and human services Washington state department of social and health services
Washington state department of social and health services Department of health and senior services missouri
Department of health and senior services missouri Milwaukee county department of health and human services
Milwaukee county department of health and human services Maine department of health and human services
Maine department of health and human services Barnstable county department of health and environment
Barnstable county department of health and environment Health and social component 3
Health and social component 3 Sotch orange
Sotch orange What are the national core standards
What are the national core standards Calvert health department
Calvert health department Gloucester county health department nj
Gloucester county health department nj Cowlitz county health department
Cowlitz county health department Tuscarawas county health department
Tuscarawas county health department Tennessee department of health licensure
Tennessee department of health licensure Sussex county board of health
Sussex county board of health Oklahoma department of mental health
Oklahoma department of mental health Georgia department of community health
Georgia department of community health Fishers health department
Fishers health department Worcester electrical inspector
Worcester electrical inspector Chickasaw nation department of health
Chickasaw nation department of health Unified government public health department
Unified government public health department Menasha health department
Menasha health department Georgia department of public health
Georgia department of public health Victorian department of health
Victorian department of health San diego department of environmental health
San diego department of environmental health Guthrie health department
Guthrie health department Montclair health department
Montclair health department Pueblo city county health department
Pueblo city county health department Vermont department of health
Vermont department of health Local department of health
Local department of health Ridgefield health department
Ridgefield health department Local department of health
Local department of health Department health
Department health Clean water branch
Clean water branch Department of health
Department of health Ennis health department
Ennis health department Ridgefield department of health
Ridgefield department of health Department of managed health care california
Department of managed health care california North central district health department ct
North central district health department ct Ridgefield department of health
Ridgefield department of health Jalgaon health department
Jalgaon health department Dc department of health
Dc department of health Fishers health department
Fishers health department Erie county department of mental health
Erie county department of mental health Utah daycare licensing
Utah daycare licensing Butte public health department
Butte public health department Benzie-leelanau county health department
Benzie-leelanau county health department Difference between health education and health promotion
Difference between health education and health promotion Chapter 3 health wellness and health disparities
Chapter 3 health wellness and health disparities Health propaganda definition
Health propaganda definition