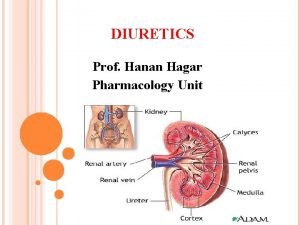
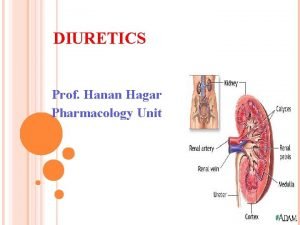
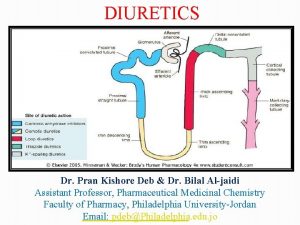
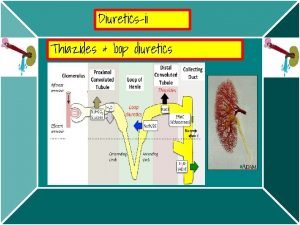
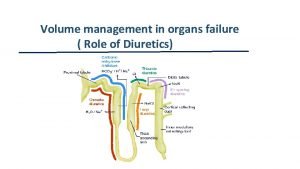
1 ALLHAT Role of Diuretics in the Prevention























- Slides: 44

1 ALLHAT Role of Diuretics in the Prevention of Heart Failure The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Davis BR, Piller LB, Cutler JA, et al. Circulation 2006. 113: 2201 -2210.

2 ALLHAT Introduction and Background l Heart failure is a major public health problem, especially in persons 65 years of age and older (= number one reason for hospitalizations in this age group). l Age-adjusted incidence per 100, 000 personyears during 1990 -1999 was 564 for men and 327 for women, age 65 -74 years (NEJM, 2002, Framingham) l Five-year age-adjusted survival rate was only 59% among men and 45% for women. l In 91% of HF cases, hypertension is an antecedent (Framingham, JAMA, 1996)

3 ALLHAT Hypertension Control and Heart Failure • In a meta-analysis of 12 trials of patients with hypertension it was found that, compared to placebo, drug therapy for hypertension prevents over 50% of HF events (Moser, JACC, 1996). • In another meta-analysis, diuretics and betablockers (BB) were equally effective in preventing HF events (Psaty, JAMA, 1997).

4 ALLHAT Hypertension Control and Heart Failure • A meta–analysis of active comparator trials found no significant difference between ACEinhibitors and diuretics for preventing HF; ACEinhibitors were more efficacious than CCBs (BPLTT Collaboration, Lancet, 2002). • The INSIGHT trial found that a long-acting nifedipine regimen was associated with a > 2 x higher incidence of HF events compared to a diuretic combination (HCTZ/amiolride) (Brown, Lancet, 2000).

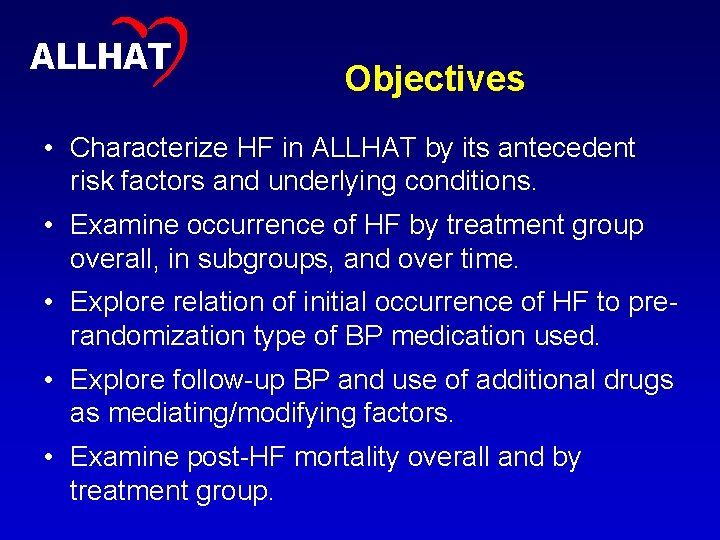
5 ALLHAT Objectives • Characterize HF in ALLHAT by its antecedent risk factors and underlying conditions. • Examine occurrence of HF by treatment group overall, in subgroups, and over time. • Explore relation of initial occurrence of HF to prerandomization type of BP medication used. • Explore follow-up BP and use of additional drugs as mediating/modifying factors. • Examine post-HF mortality overall and by treatment group.

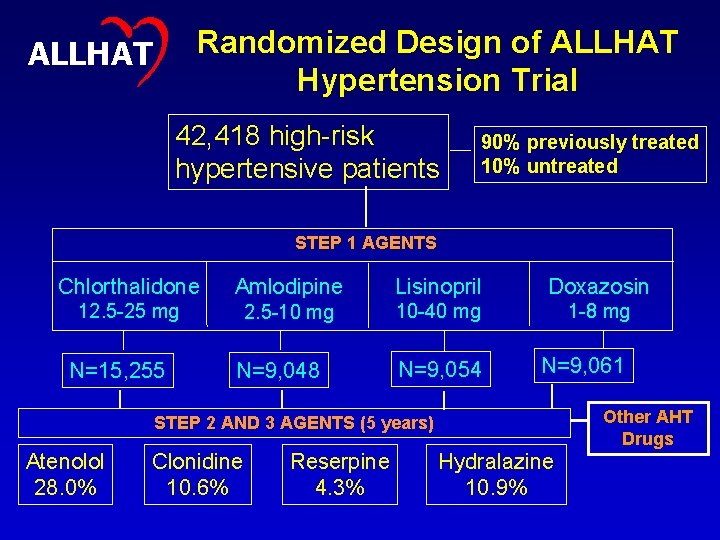
Randomized Design of ALLHAT Hypertension Trial ALLHAT 42, 418 high-risk hypertensive patients 90% previously treated 10% untreated STEP 1 AGENTS Chlorthalidone Amlodipine Lisinopril Doxazosin 12. 5 -25 mg 2. 5 -10 mg 10 -40 mg 1 -8 mg N=9, 054 N=9, 061 N=15, 255 N=9, 048 Other AHT Drugs STEP 2 AND 3 AGENTS (5 years) Atenolol 28. 0% Clonidine 10. 6% Reserpine 4. 3% Hydralazine 10. 9% 6

7 ALLHAT Decision to Stop Doxazosin Arm • NHLBI Director accepted recommendation of independent review group to terminate doxazosin arm (early in year 2000), due to: – Futility of finding a significant difference for primary outcome – Statistically significant 25 percent higher rate of major secondary endpoint, combined CVD outcomes, along with twofold higher rate of HF • Detailed HF analyses published (Davis et al. Ann Intern Med 2002).

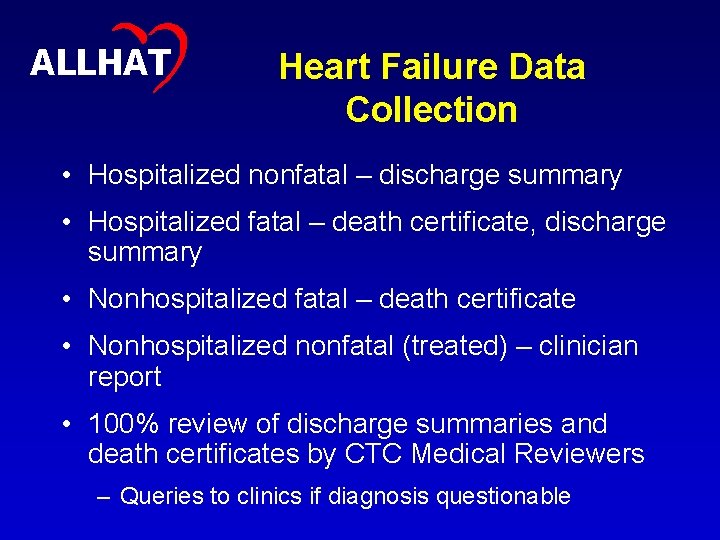
8 ALLHAT Heart Failure Data Collection • Hospitalized nonfatal – discharge summary • Hospitalized fatal – death certificate, discharge summary • Nonhospitalized fatal – death certificate • Nonhospitalized nonfatal (treated) – clinician report • 100% review of discharge summaries and death certificates by CTC Medical Reviewers – Queries to clinics if diagnosis questionable

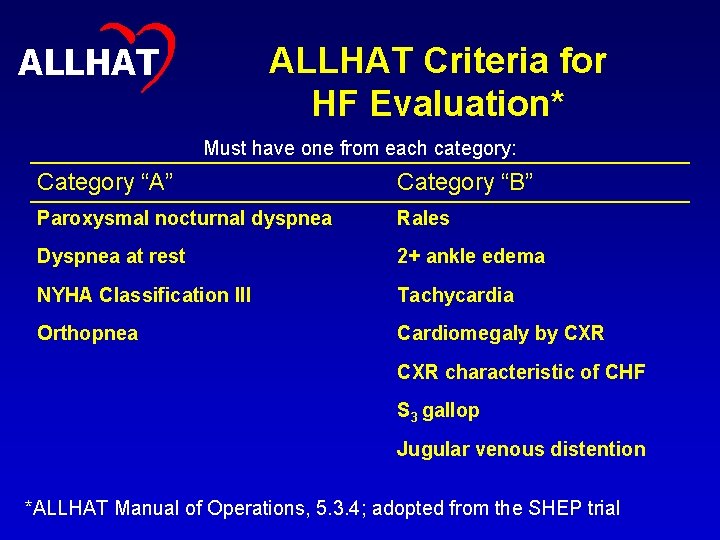
9 ALLHAT Criteria for HF Evaluation* Must have one from each category: Category “A” Category “B” Paroxysmal nocturnal dyspnea Rales Dyspnea at rest 2+ ankle edema NYHA Classification III Tachycardia Orthopnea Cardiomegaly by CXR characteristic of CHF S 3 gallop Jugular venous distention *ALLHAT Manual of Operations, 5. 3. 4; adopted from the SHEP trial

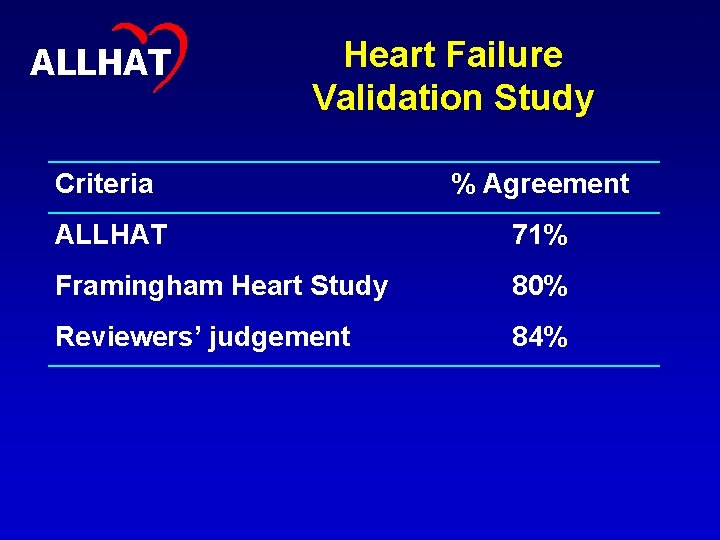
10 ALLHAT Validity of HF Outcome Verified • Traditional risk factors in agreement with previous studies, e. g. , Framingham • HF Validation Study confirmed original observed treatment differences – Independent central review using both ALLHAT and Framingham criteria

11 ALLHAT Heart Failure Validation Study Criteria % Agreement ALLHAT 71% Framingham Heart Study 80% Reviewers’ judgement 84%

12 ALLHAT Inclusion/Exclusion Criteria for Antihypertensive Trial • Men and women > 55 years old • If untreated: 140/90, 180/110 mm Hg (2 visits) • If treated: ≤ 160/100 mm Hg (visit 1), ≤ 180/110 mm Hg (visit 2) – No washout required • At least one additional cardiovascular risk factor • Exclude if symptomatic HF or EF < 35%, creatinine 2 mg/d. L, require diuretics, CCB, ACEI, or AB’s for non-BP indication

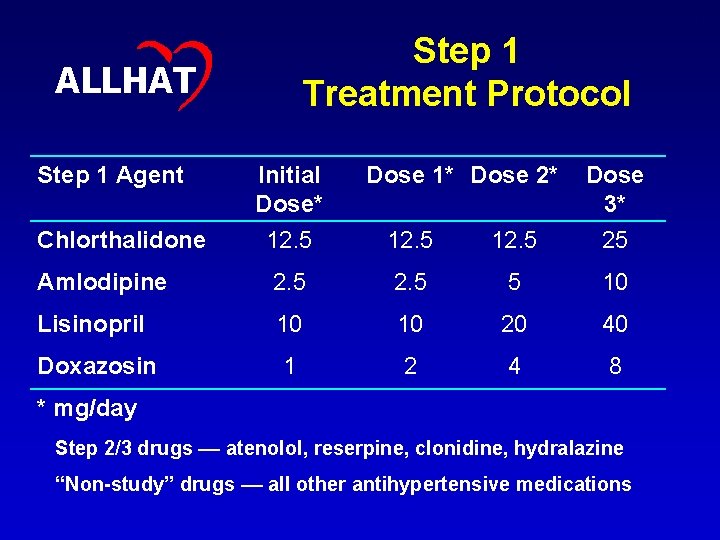
13 Step 1 Treatment Protocol ALLHAT Step 1 Agent Initial Dose* Dose 1* Dose 2* Dose 3* Chlorthalidone 12. 5 25 Amlodipine 2. 5 5 10 Lisinopril 10 10 20 40 Doxazosin 1 2 4 8 * mg/day Step 2/3 drugs –– atenolol, reserpine, clonidine, hydralazine “Non-study” drugs –– all other antihypertensive medications

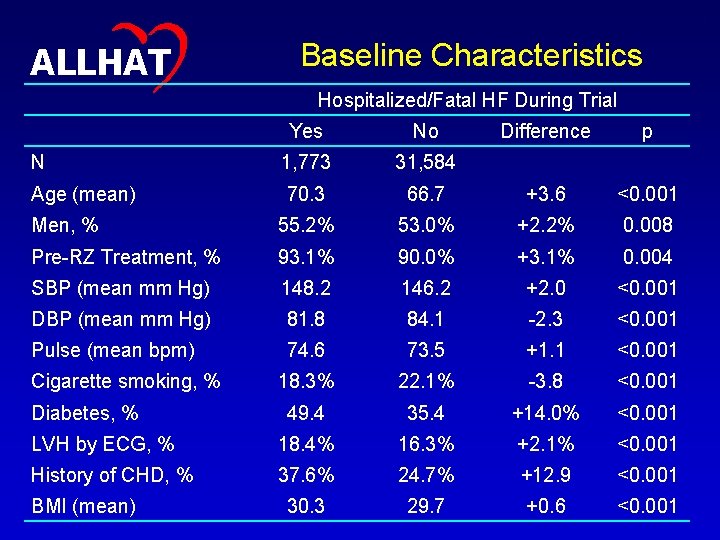
14 ALLHAT Baseline Characteristics Hospitalized/Fatal HF During Trial Yes No Difference p N 1, 773 31, 584 Age (mean) 70. 3 66. 7 +3. 6 <0. 001 Men, % 55. 2% 53. 0% +2. 2% 0. 008 Pre-RZ Treatment, % 93. 1% 90. 0% +3. 1% 0. 004 SBP (mean mm Hg) 148. 2 146. 2 +2. 0 <0. 001 DBP (mean mm Hg) 81. 8 84. 1 -2. 3 <0. 001 Pulse (mean bpm) 74. 6 73. 5 +1. 1 <0. 001 18. 3% 22. 1% -3. 8 <0. 001 49. 4 35. 4 +14. 0% <0. 001 LVH by ECG, % 18. 4% 16. 3% +2. 1% <0. 001 History of CHD, % 37. 6% 24. 7% +12. 9 <0. 001 30. 3 29. 7 +0. 6 <0. 001 Cigarette smoking, % Diabetes, % BMI (mean)

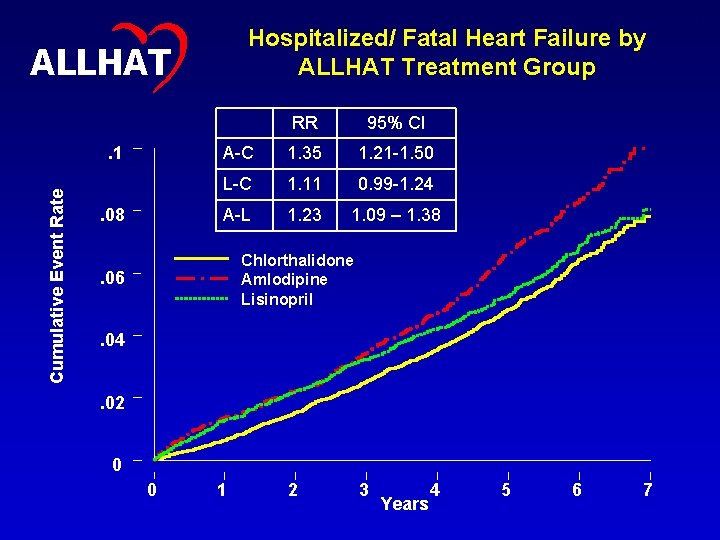
Hospitalized/ Fatal Heart Failure by ALLHAT Treatment Group ALLHAT Cumulative Event Rate . 1. 08 RR 95% CI A-C 1. 35 1. 21 -1. 50 L-C 1. 11 0. 99 -1. 24 A-L 1. 23 1. 09 – 1. 38 Chlorthalidone Amlodipine Lisinopril . 06. 04. 02 0 0 1 2 3 Years 4 5 6 7 15

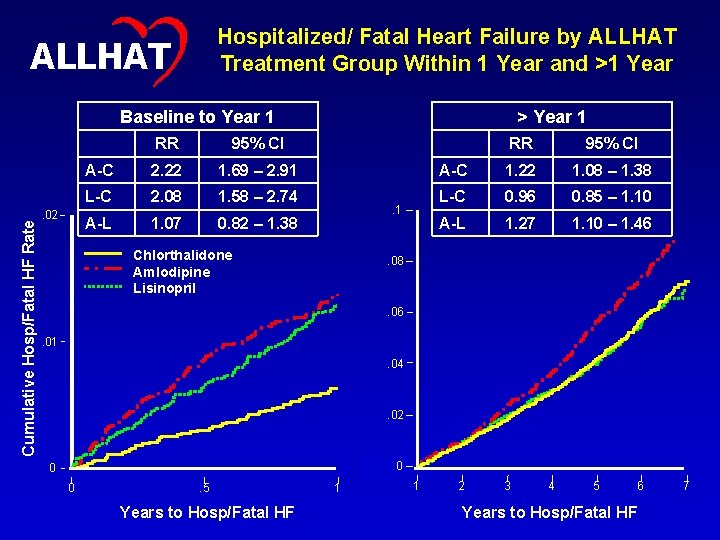
16 ALLHAT Heart Failure Before and After 1 Year • Observed HF differences were larger earlier in the follow-up. • The lisinopril group had a lower HF rate than the amlodipine group, but event curves did not separate until later. • A test of the proportional hazards assumption for Cox regression revealed that RRs were not constant over time. Therefore, a Cox regression that used a time-dependent indicator variable (<=1 year versus >1 year) was utilized.

Hospitalized/ Fatal Heart Failure by ALLHAT Treatment Group Within 1 Year and >1 Year ALLHAT Cumulative Hosp/Fatal HF Rate Baseline to Year 1 . 02 17 RR 95% CI A-C 2. 22 1. 69 – 2. 91 L-C 2. 08 1. 58 – 2. 74 A-L 1. 07 0. 82 – 1. 38 > Year 1 . 1 Chlorthalidone Amlodipine Lisinopril RR 95% CI A-C 1. 22 1. 08 – 1. 38 L-C 0. 96 0. 85 – 1. 10 A-L 1. 27 1. 10 – 1. 46 . 08 . 06. 01. 04 . 02 00 0 0 . 5 Years to Hosp/Fatal HF 1 1 2 3 4 5 6 Years to Hosp/Fatal HF 7

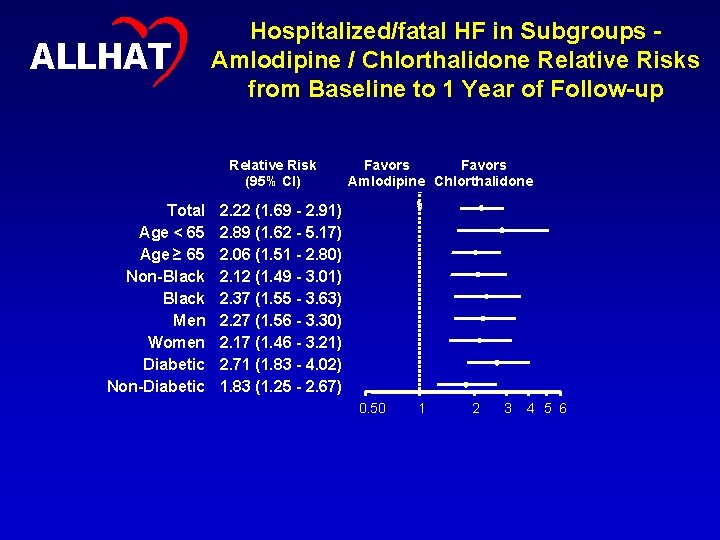
18 ALLHAT Hospitalized/fatal HF in Subgroups Amlodipine / Chlorthalidone Relative Risks from Baseline to 1 Year of Follow-up Relative Risk (95% CI) Total Age < 65 Age ≥ 65 Non-Black Men Women Diabetic Non-Diabetic Favors Amlodipine Chlorthalidone 2. 22 (1. 69 - 2. 91) 2. 89 (1. 62 - 5. 17) 2. 06 (1. 51 - 2. 80) 2. 12 (1. 49 - 3. 01) 2. 37 (1. 55 - 3. 63) 2. 27 (1. 56 - 3. 30) 2. 17 (1. 46 - 3. 21) 2. 71 (1. 83 - 4. 02) 1. 83 (1. 25 - 2. 67) 0. 50 1 2 3 4 5 6

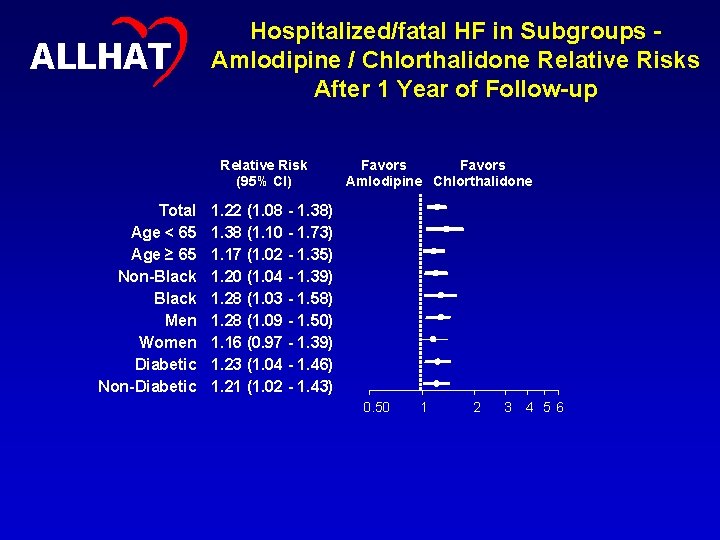
19 ALLHAT Hospitalized/fatal HF in Subgroups Amlodipine / Chlorthalidone Relative Risks After 1 Year of Follow-up Relative Risk (95% CI) Total Age < 65 Age ≥ 65 Non-Black Men Women Diabetic Non-Diabetic Favors Amlodipine Chlorthalidone 1. 22 (1. 08 - 1. 38) 1. 38 (1. 10 - 1. 73) 1. 17 (1. 02 - 1. 35) 1. 20 (1. 04 - 1. 39) 1. 28 (1. 03 - 1. 58) 1. 28 (1. 09 - 1. 50) 1. 16 (0. 97 - 1. 39) 1. 23 (1. 04 - 1. 46) 1. 21 (1. 02 - 1. 43) 0. 50 1 2 3 4 5 6

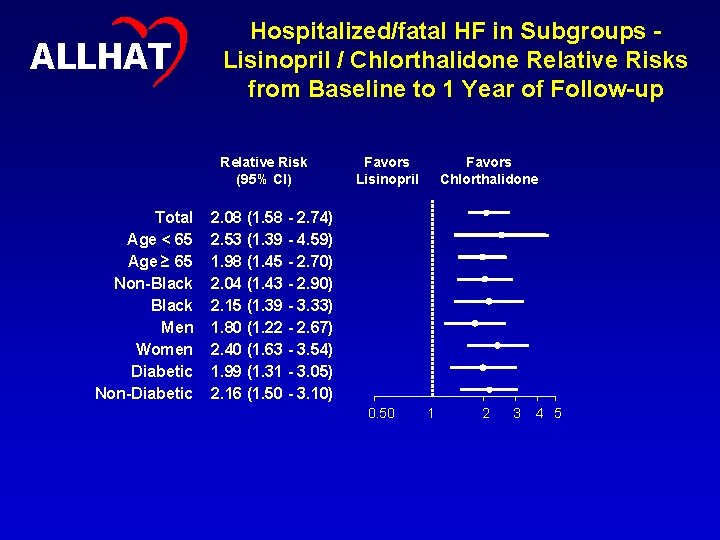
ALLHAT Hospitalized/fatal HF in Subgroups Lisinopril / Chlorthalidone Relative Risks from Baseline to 1 Year of Follow-up Relative Risk (95% CI) Total Age < 65 Age ≥ 65 Non-Black Men Women Diabetic Non-Diabetic Favors Lisinopril Favors Chlorthalidone 2. 08 (1. 58 - 2. 74) 2. 53 (1. 39 - 4. 59) 1. 98 (1. 45 - 2. 70) 2. 04 (1. 43 - 2. 90) 2. 15 (1. 39 - 3. 33) 1. 80 (1. 22 - 2. 67) 2. 40 (1. 63 - 3. 54) 1. 99 (1. 31 - 3. 05) 2. 16 (1. 50 - 3. 10) 0. 50 1 2 3 4 5 20

ALLHAT Hospitalized/fatal HF in Subgroups Lisinopril / Chlorthalidone Relative Risks After 1 Year of Follow-up Relative Risk (95% CI) Total Age < 65 Age ≥ 65 Non-Black Men Women Diabetic Non-Diabetic Favors Lisinopril Favors Chlorthalidone 0. 96 (0. 85 - 1. 10) 0. 95 (0. 74 - 1. 23) 0. 97 (0. 84 - 1. 13) 0. 90 (0. 77 - 1. 06) 1. 10 (0. 88 - 1. 37) 1. 02 (0. 86 - 1. 21) 0. 89 (0. 73 - 1. 09) 1. 01 (0. 84 - 1. 22) 0. 93 (0. 77 - 1. 12) 0. 50 1 2 21

22 ALLHAT HF Development and Relation to Other Outcomes • HF development associated with: – 6. 6 -fold increase in death rate – 11. 7 -fold increase in CV death rate • Previous MI → 5. 7 -fold increased HF risk • Of participants with hospitalized HF: – 72% hospitalized once – 23. 3% hospitalized 2 -3 times – 4. 7% hospitalized 4+ times

23 ALLHAT Why are hazard ratios not constant throughout? Hypotheses? • Withdrawal from BP meds used prior to enrollment • Time course for effect of first-step (primary) drug – Diuretic – immediate? – ACEI – delayed? • Addition of step-up meds (esp. anti-HF meds) • Differences in BP

24 ALLHAT Prior Use of Antihypertensive Agents • Prior medication use associated with HF risk, especially during first year – RR 1. 42 (1. 18 – 1. 71) • Relative benefits of chlorthalidone consistent with or without prior antihypertensive medication use

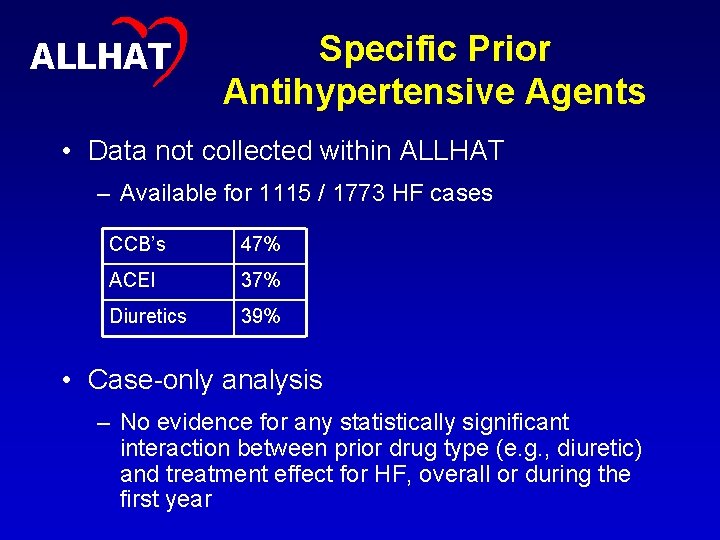
25 ALLHAT Specific Prior Antihypertensive Agents • Data not collected within ALLHAT – Available for 1115 / 1773 HF cases CCB’s 47% ACEI 37% Diuretics 39% • Case-only analysis – No evidence for any statistically significant interaction between prior drug type (e. g. , diuretic) and treatment effect for HF, overall or during the first year

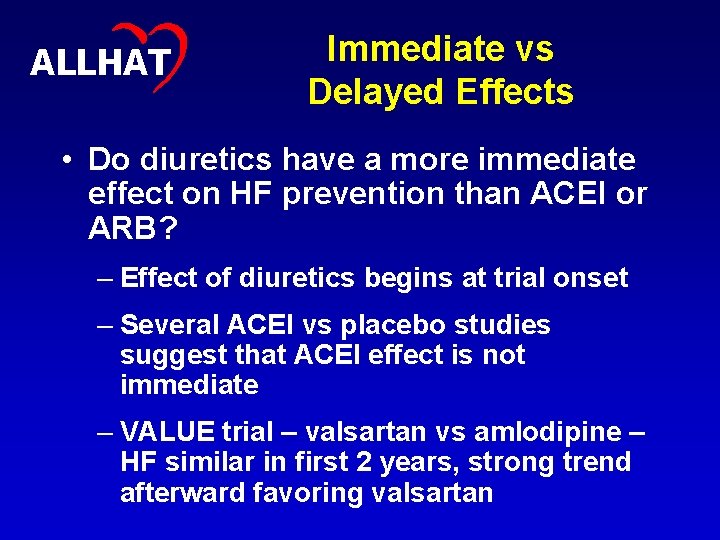
26 ALLHAT Immediate vs Delayed Effects • Do diuretics have a more immediate effect on HF prevention than ACEI or ARB? – Effect of diuretics begins at trial onset – Several ACEI vs placebo studies suggest that ACEI effect is not immediate – VALUE trial – valsartan vs amlodipine – HF similar in first 2 years, strong trend afterward favoring valsartan

27 ALLHAT Use of Step-up BP Meds Addition of Step 2 and Step 3 meds could have contributed to lessening or cessation of divergence of HF curves after 1 year.

ALLHAT Open-Label ACEI and Atenolol Use 28

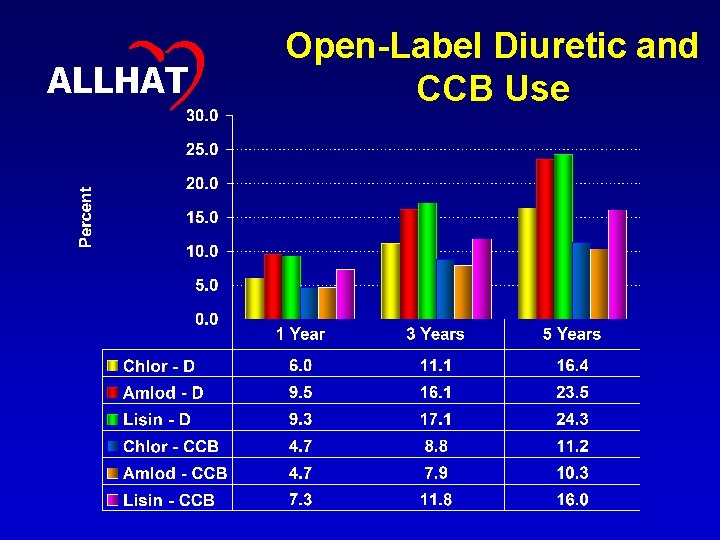
29 ALLHAT Open-Label Diuretic and CCB Use

ALLHAT Diuretic, ACEI, or Atenol Use 30

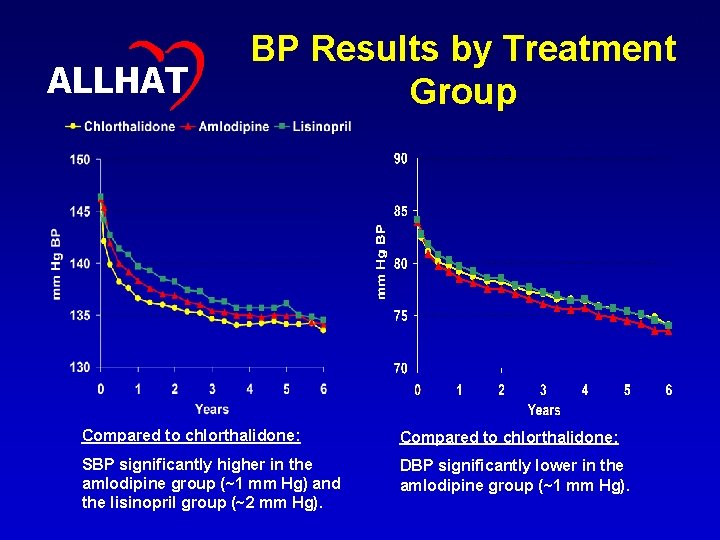
31 ALLHAT BP Results by Treatment Group Compared to chlorthalidone: SBP significantly higher in the amlodipine group (~1 mm Hg) and the lisinopril group (~2 mm Hg). DBP significantly lower in the amlodipine group (~1 mm Hg).

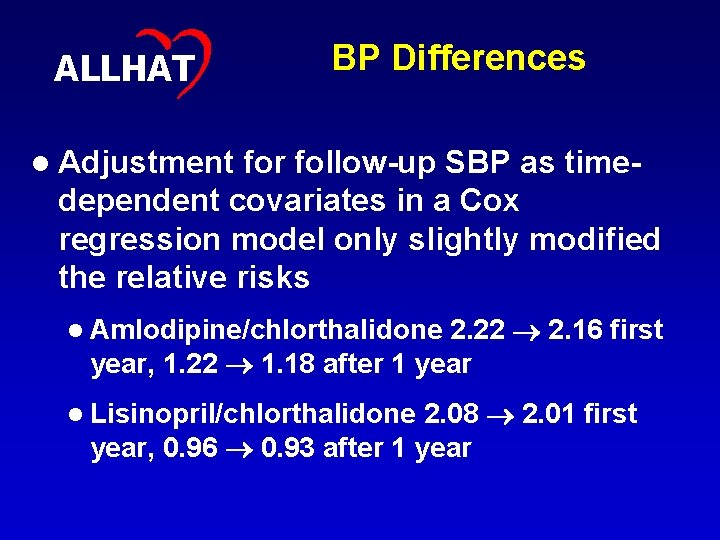
32 ALLHAT BP Differences l Adjustment for follow-up SBP as timedependent covariates in a Cox regression model only slightly modified the relative risks 2. 22 2. 16 first year, 1. 22 1. 18 after 1 year l Amlodipine/chlorthalidone 2. 08 2. 01 first year, 0. 96 0. 93 after 1 year l Lisinopril/chlorthalidone

33 Cumulative Event Rate ALLHAT All-Cause Mortality Chlorthalidone Amlodipine Lisinopril . 6. 5. 4. 3. 2. 1 0 0 1 2 3 4 5 Years from Hospitalized HF to Death 6 7

34 ALLHAT Post-HF Mortality • Mortality rates after hospitalized HF high relative to those seen in ALLHAT overall – 25% vs 5% at 2. 5 years, respectively • No significant treatment group differences for post-HF mortality • The reason that the treatment difference for hospitalized HF did not translate into an effect on total mortality is that only 5. 6% of all deaths were attributed to HF.

ALLHAT Heart Failure and Total Mortality • Lisinopril-chlorthalidone absolute difference in hospitalized HF over 6 years was 0. 4%. – The excess of cases in the lisinopril group = 36 patients. – Case-fatality rate over average follow-up of 2. 5 years = 25%. – Thus, 9 excess cases of fatal HF would be expected in the lisinopril group. This is fewer than 1% of all deaths in the lisinopril group (n=1314). • Similar calculations for the amlodipine group: – 154 excess cases of hospitalized HF – Estimated number of fatal HF cases was 39, 3% of the amlodipine deaths (n=1256). 35

36 ALLHAT Effect on Total Mortality • HF differences in the trial would not have affected differences in total mortality – Also noted in the BPLTTC analyses – Absolute HF risk low – Increase in RR outweighed by even small reduction in higher absolute risks for stroke and CHD – Differences in # of HF events during trial result in only very small differences in # of deaths – ALLHAT post-trial mortality surveillance to examine this further

37 ALLHAT Conclusions 1 • Chlorthalidone superior to amlodipine in both time periods • Chlorthalidone superior to lisinopril during the first year • True for subgroups – age, race, sex, diabetes history • Other factors could not individually account for all of the observed treatment differences – Prior antihypertensive meds – Other open-label BP meds – Follow-up BP differences

38 ALLHAT Conclusions 2 • Developing HF is associated with a high mortality rate (~50% at 5 years) • It may take time for HF differences to translate into detectable mortality differences between treatments • Diuretics are clearly preferred over CCBs overall and over ACE inhibitors, at least in the short term, in preventing HF.

39 Extra Slides

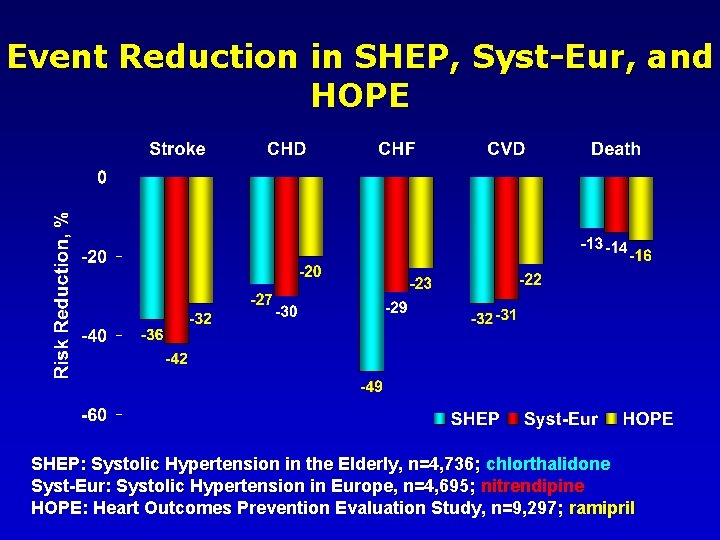
40 ALLHAT Placebo-Controlled Trials • Most placebo-controlled trial have used diuretics and/or β-blockers as active regimens • Diuretics & ACEI shown to prevent HF in patients with hypertension – SHEP, HOPE • CCB vs placebo trials less conclusive – Syst-Eur • Meta-analyses – active therapy of hypertension can prevent >40% of HF events – Psaty, Smith, Siscovick, et al.

41 ALLHAT Active-Controlled Trials • VALUE • STOP Hypertension-2 • ANBP 2 • INVEST • CONVINCE – CCB or diuretic/β-blocker – BP reduced similarly, HF 30% more with CCB

42 ALLHAT BPLTTC Meta-Analyses • CCB-based therapies – NS 20% increase in HF incidence compared with placebo – 33% higher risk of HF compared with diuretic/β-blocker • ACEI-based therapies – 18% fewer HF events than with CCB or placebo – 7% NS higher risk than with diuretic/ β-blocker • CCBs less effective in preventing HF than other regimens • ACEI no more effective in preventing HF than diuretic/ β-blocker

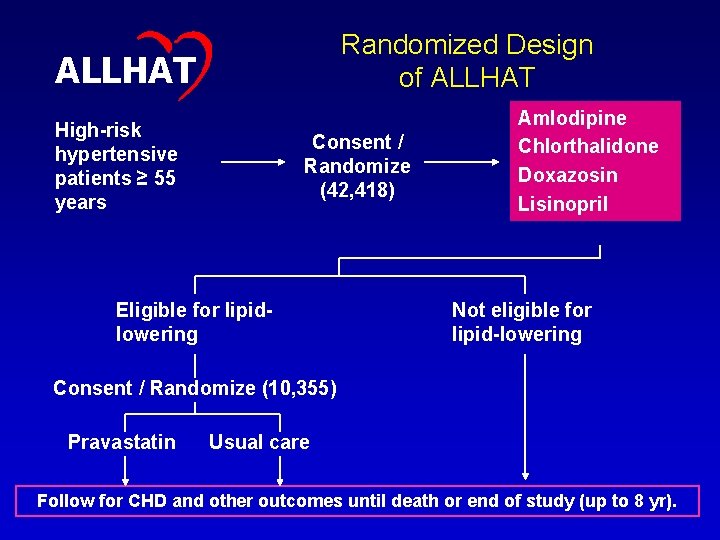
43 Randomized Design of ALLHAT High-risk hypertensive patients ≥ 55 years Consent / Randomize (42, 418) Eligible for lipidlowering Amlodipine Chlorthalidone Doxazosin Lisinopril Not eligible for lipid-lowering Consent / Randomize (10, 355) Pravastatin Usual care Follow for CHD and other outcomes until death or end of study (up to 8 yr).

44 Event Reduction in SHEP, Syst-Eur, and HOPE SHEP: Systolic Hypertension in the Elderly, n=4, 736; chlorthalidone Syst-Eur: Systolic Hypertension in Europe, n=4, 695; nitrendipine HOPE: Heart Outcomes Prevention Evaluation Study, n=9, 297; ramipril
 Primary prevention secondary prevention tertiary prevention
Primary prevention secondary prevention tertiary prevention Diuretics classification
Diuretics classification Harley nadler
Harley nadler Anti diuretics drugs name
Anti diuretics drugs name Site:slidetodoc.com
Site:slidetodoc.com Side effects of diuretics
Side effects of diuretics Potassium sparing diuretics mechanism of action
Potassium sparing diuretics mechanism of action Diuretic side effects
Diuretic side effects Isothenuria
Isothenuria Diuretic location of action
Diuretic location of action Loop diuretics adverse effects
Loop diuretics adverse effects Potassium sparing diuretics mechanism of action
Potassium sparing diuretics mechanism of action Diuretic classification
Diuretic classification Dr mukhopadhyay deb
Dr mukhopadhyay deb Mannitol uses
Mannitol uses Urinary acidifiers examples
Urinary acidifiers examples Loop diuretics
Loop diuretics Diuretics classification
Diuretics classification Diuretics classification
Diuretics classification Krappmann symbolischer interaktionismus
Krappmann symbolischer interaktionismus Role conflict occurs when fulfilling the role expectations
Role conflict occurs when fulfilling the role expectations Web role in azure
Web role in azure Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Lp html
Lp html Số nguyên tố là
Số nguyên tố là Tia chieu sa te
Tia chieu sa te đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Chụp tư thế worms-breton
Chụp tư thế worms-breton Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Hệ hô hấp
Hệ hô hấp ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Bổ thể
Bổ thể Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Thể thơ truyền thống
Thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Diễn thế sinh thái là
Diễn thế sinh thái là