Zinc cadmium lead tin gallium indium thallium Zinc






















- Slides: 29

Zinc, cadmium, lead, tin, gallium, indium, thallium


Zinc (Zn) Universe: 0. 3 ppm (by weight) Sun: 2 ppm (by weight) Carbonaceous meteorite: 180 ppm Earth's Crust: 75 ppm Seawater: Atlantic surface: 5 x 10 -5 ppm Atlantic deep: 1 x 10 -4 ppm

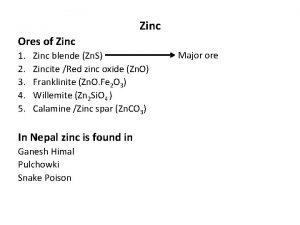
Zinc in magmatic processes Zinc abundance in different minerals is a function of the zinc concentration in the magma and the ability of the crystal structure to incorporate this element. It is a major constituent of more than 80 minerals, but there are only a few important commercial ores. The principal Zn sulphide minerals are sphalerite (cubic Zn. S) and wurtzite (hexagonal Zn. S). The occurrence of zinc in some rock-forming ferrous iron and magnesium silicates and oxides (magnetite, pyroxenes, amphiboles, micas, spinel (gahnite, franklinite) and staurolite) is far more important for the crustal abundance of this element than zinc in ore deposits. Most of the zinc deposits occur as fillings and replacements formed by low-temperature hydrothermal solutions.

Zinc in magmatic processes There are many substitution in sphalerite structure. The most important is Fe and Cd. The concentration of other elements in sphalerite depends on the temperature: Co, Mn, Fe, In, Ga, Ge, Tl (order in decreasing temperature). The unique zinc deposit is Franklin Furnace in New Jersey. The ore minerals there are zincite (Zn. O), willemite (Zn 2 Si. O 4 ) and franklinite (Fe, Zn, Mn)(Fe, Mn)2 O 4 occurring as grains in calcite and high temperature and high pressure of their origin is suggested.

Zinc in weathering and sedimentary processes The concentration of zinc in weathering solutions is controlled rather by adsorption (on clay minerals, Fe, Mn, AI hydroxides and organic matter) than by solubility of zinc carbonates, hydroxides and phosphates. The Zn content in soils depends on the nature of parent rocks, texture, organic matter and p. H and ranges from 10 to 300 ppm. Several soil profile studies show that extractable zinc content generally decreases with depth, while total Zn is uniformly distributed throughout the profile. Higher content of zinc is present in soils in the vicinity of deposits and smelters. Addition of phosphate fertilizers and atmospheric deposition increases zinc concentration in the soils.

Zinc in weathering and sedimentary processes In aqueous solutions it exists as Zn 2+ and some complex ions. Zn chloride, sulfate and nitrate are readily soluble in water, whereas Zn oxide, carbonate, phosphate, silicate and sulfide are practically insoluble in water. Common Zn minerals in the oxidation zone of Zn ore deposits are: smithsonite (Zn. CO 3), hemimorphite (Zn 4 Si 2 O 7(OH)2 • H 2 O), aurichalcite (Zn, Cu)5(CO 3)2(OH)6 and hydrozincite Zn 5(CO 3)2(OH)6.

Zinc in weathering and sedimentary processes In surface waters zinc occurs mainly bound to suspended matter (clays, AI, Fe, Mn, Si hydrous oxides). High concentrations of zinc are found in sludge. Freshwaters, especially rivers, are frequently contaminated by sewage and waste water and contain considerable zinc levels. Acid mine water can locally accumulate zinc up to a high concentration. Zinc occurs in the atmosphere mainly as fine particles (<21 -1 m). Atmospheric zinc results from the production and processing of zinc, non-ferrous smelters, fossil fuel combustion and car emissions.

Zinc in the biosphere Zinc plays an important role as an essential trace metal in all living systems from bacteria to humans. Zinc is found in all human tissues and all body fluids. The metal is essential for growth, development and reproduction in man. Compared to other elements such as cadmium, mercury, lead, zinc has a low toxicity.

Cadmium (Cd) Universe: 0. 002 ppm (by weight) Sun: 0. 006 ppm (by weight) Carbonaceous meteorite: 0. 45 ppm Earth's Crust: 0. 11 ppm Seawater: Atlantic surface: 1. 1 x 10 -6 ppm Atlantic deep: 3. 8 x 10 -5 ppm

Cadmium in magmatic processes Cadmium concentration in igneous rocks is generally low (0. 07 -0. 25 ppm). It is a chalcophile element, favoring an association with sulfur, and is closely associated with zinc. The bulk of cadmium in nature is dispersed as isomorphic impurities in various other minerals, usually sulfide minerals. The principal carrier is in sphalerite. Common Cd-containing minerals are: greenockite, hexagonal Cd. S, hawleyite, cubic Cd. S, otavite, trigonal Cd. CO 3. It always substitutes Zn in different minerals (sphalerite, smithsonite, hemimorphite etc. ).

Cadmium in weathering and sedimentary processes It is relatively mobile in the surficial environment. Cadmium often forms complexes with natural organic matter. In many natural environments, aqueous cadmium concentrations are controlled primarily by sorption reactions. It to be enriched in shales, oceanic and lacustrine sediments, and phosphorites, and depleted in red shales, sandstones, and limestones. Carbonaceous shales, formed under reducing conditions, tend to contain the most cadmium. In oxidized zones of ore deposits, it is found in smithsonite, hemimorphite, manganese oxides, and hydrous iron oxides. During weathering, cadmium forms complexes with sulfate and chloride in solution.

Lead (Pb) Universe: 0. 01 ppm (by weight) Sun: 0. 01 ppm (by weight) Carbonaceous meteorite: 1. 4 ppm Earth's Crust: 14 ppm Seawater: Atlantic surface: 3 x 10 -5 ppm Atlantic deep: 4 x 10 -6 ppm

Lead in magmatic processes It is widely distributed throughout the Earth and can be found in all environmental media (air, soil, rocks, sediments, waters). The average crustal abundance of lead is 16 ppm. In the Earth's crust, Pb is the most abundant of the heavy elements with atomic number > 60. Lead occurs in rocks as a discrete mineral, or the major portion of the metal in the Earth's crust replaces K, Sr, Ba and even Ca and Na in the mineral lattice of silicate minerals. Among silicates potassium feldspars and micas are notable accumulators of Pb, therefore granitic rocks tend to have higher levels than basaltic ones.

Lead in magmatic processes It can substitutes Ca in some phosphates (apatites), carbonates (aragonite). The largest accumulations connect to post-magmatic hydrothermal processes. The most important Pb-sulphide mineral is galena (cubic Pb. S), but it forms many Pb-bearing sulphosalts (e. g. boulangerite, bournonite, jamesonite). More than 200 other minerals are known.

Lead in weathering and sedimentary processes In the oxidation zone of Pb-bearing ore deposit found many secondary Pb-minerals, the most common are cerussite (orthorhombic Pb. CO 3 ) and anglesite (orthorhombic Pb. SO 4). However, we know many other compounds in this environments, oxides (minium, litarge, plattnerite), phosphates (pyromorfite), arsenates (beudantite, carminite), chromates (crocoite), molybdates (wulfenite), and vanadates (vanadinite). It can concentrates in sedimentary rocks, which contain organic matters.

Lead in weathering and sedimentary processes Lead in surface run-off comes from chemical weathering, municipal and industrial water discharges and largely from atmospheric deposition. The concentration of lead in natural waters is much lower than would be expected from the inputs because of adsorption of the element onto particulate matter (clay minerals, oxides and hydroxides of aluminum, iron and manganese). The adsorption decreases with lowering p. H of the water. Under reducing conditions lead precipitates as highly insoluble sulfide. Lead occurs in atmosphere as fine particulates ( < 1 1 -1 m), generated mainly by anthropogenic high temperature sources.

Tin (Sn) Universe: 0. 004 ppm (by weight) Sun: 0. 009 ppm (by weight) Carbonaceous meteorite: 1. 2 ppm Earth's Crust: 2. 2 ppm Seawater: Atlantic surface: 2. 3 x 10 -6 ppm Atlantic deep: 5. 8 x 10 -6 ppm

Tin in magmatic processes The main tin carriers in granitic rocks are hornblende, biotite, muscovite, garnet, ilmenite and magnetite. Common substitution are in complex oxides, as niobates, tantalates (Sn 2+ Ca 2+, Sn 4+ Ti 4+ or Fe 2+) in high temperature processes. Cassiterite (tetragonal Sn. O 2, the most common tin mineral) occurs in pegmatites, high temperature quartz veins and metasomatic deposits (greisens and tin skarns), generally genetically associated with granitic rocks. Postmagmatic hydrothermal interaction and chemical alteration of granitic rocks produce greisen enriched in tin. It also occurs in a few volcanogenic massive sulfide deposits related to felsic rocks. At relatively low temperatures the affinity of tin for sulfur increases (e. g. stannite, cylindrite).

Tin in weathering and sedimentary processes Because of the cassiterite chemical stability, it concentrates in clastic sediments. Most tin is produced from secondary alluvial placers, which were eroded from cassiterite deposits. Except for alluvial placers, the abundance of tin is very low in the sediments.

Gallium (Ga) Universe: 0. 01 ppm (by weight) Sun: 0. 04 ppm (by weight) Earth's Crust: 18 ppm Seawater: 3 x 10 -5 ppm

Gallium in magmatic processes Concentration of Ga in most of the igneous rocks varies between 1 -40 ppm. The Al/Ga ratio decreases only slightly from ultramafic to mafic and felsic rocks. Volatile components and fluoride complexing cause the enrichment of Ga in the late stages of magmatic processes. Together with rare alkali elements it is enriched in pegmatites, and sometimes in minerals of greisens and skarns. The chalcophile character is emphasized especially under hydrothermal, sulfur-rich conditions. Gallium is enriched mainly in sphalerite (up to 0. 16%). The gallium concentration is temperature dependent and typical for mesothermal ore associations. (1. 88% Ga).

Gallium in weathering and sedimentary processes Gallium is dispersed in the oxidation zone of sulfide mineralizations. The average concentration of Ga in shales and the Al/Ga ratio of the latter remain similar to igneous rocks. Ga, like AI, is enriched in weathering. It is more mobile and the Al/Ga ratio tends to decrease in residual materials. Coal may be a collector for Ga. The content of Ga in bauxites (20 -200 ppm) is of economic importance. The concentration of Ga depends on the weathered rock materials. The highest values are reported from bauxites originating from alkali rocks. The carbonatederived bauxites display average contents around 50 ppm Ga.

Indium (In) Universe: 0. 0003 ppm (by weight) Sun: 0. 004 ppm (by weight) Earth's Crust: 0. 049 ppm Seawater: 1 x 10 -7 ppm

Indium in magmatic processes Indium is a rare elements, it occurs mostly as a trace constituent of other minerals. Indium minerals are very rare. Indium prefers tin minerals, especially cassiterite, cylindrite and teallite, as well as minerals with tetrahedral covalent bonds, such as sphalerite, chalcopyrite and stannite. Concentrations in silicates are low, frequently in the range of ppb. Significant concentration of In takes place only in the late fluid-rich stages of magmatic processes, notably in tin-rich associations. Indium is enriched during the formation of greisens, skams and high temperature hydrothermal sulfide mineralizations. In addition to tin minerals, dark iron-rich sphalerites are the most common host mineral.

Indium in weathering and sedimentary processes In indium-rich ore deposits, secondary In minerals may be expected to occur in the oxidation zone; however, only the hydroxide dzahlindite has been described so far. Most of the indium is dispersed in minerals of the oxidation zone. Iron hydroxides have a high sorption capacity for the negatively charged ln(OH)4 anion complex which might help indium to be enriched like germanium or gallium. In fossil organic matter like coal, the carbonate sedimenst, and shales have very low concentration of In.

Thallium (Tl) Universe: 0. 0005 ppm (by weight) Sun: 0. 001 ppm (by weight) Carbonaceous meteorite: 0. 08 ppm Earth's Crust: 0. 6 ppm Seawater: 1. 4 x 10 -5 ppm

Thallium in magmatic processes Thallium is both a chalcophile and a lithophile element. Its chalcophile character is expressed in the formation of a number of independent sulfides, sulfosalts and selenides with As, Sb, Cu, Pb, Fe, Hg, and Ag (e. g. lorándite Tl. As. S 2, vrbaite, hutchinsonite), and in trace amounts in sulfides (galena, sphalerite, pyrite, etc. ). These chalcophile minerals are formed by hydrothermal (epithermal stage) or by supergene processes. Thallium shows lithophile character in K-minerals in igneous and metamorphic rocks. It is concentrated in K-minerals because of their similar size.

Thallium in weathering and sedimentary processes Thallium may be easily released during weathering, but because of its large ionic radius it will be fixed by clay minerals and the oxides of Fe and Mn in the weathering products. It is very mobile in oxidized conditions, and in the oxidizing zone of sulfide deposits it is often enriched in jarosite and manganese oxides. Because of the higher concentrations of Th in hydrothermal altered rocks, it can be used as an indicator element for hydrothermal deposits, especially for epithermal gold deposits.
 Thallium
Thallium Indium bump
Indium bump Indium heat of fusion
Indium heat of fusion Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Example of a chemical change
Example of a chemical change Selenium unpaired electrons
Selenium unpaired electrons Gallium lewis dot structure
Gallium lewis dot structure Cadmium linux
Cadmium linux Source of cadmium
Source of cadmium Cadmium
Cadmium References
References Source of cadmium
Source of cadmium Lead magnesium niobate/lead titanate
Lead magnesium niobate/lead titanate Zinc carbonate
Zinc carbonate Zinc finger structure
Zinc finger structure Rock salt structure
Rock salt structure Cscl structure
Cscl structure Dr junaid mughal
Dr junaid mughal Water fluid
Water fluid Occurrence of zinc
Occurrence of zinc Oxido de zinc y eugenol zoe
Oxido de zinc y eugenol zoe Electrolyse iodure de zinc
Electrolyse iodure de zinc Zinc dissolves in hydrochloric acid to yield hydrogen gas
Zinc dissolves in hydrochloric acid to yield hydrogen gas Zinc lewis dot structure
Zinc lewis dot structure Mini zinc
Mini zinc Zinc ethyl silicate primer
Zinc ethyl silicate primer Saginata
Saginata No3 oxidation number
No3 oxidation number Deficiencia de calcio en café
Deficiencia de calcio en café Alphahelix
Alphahelix