Water We Cant Live Without It Water Water
































- Slides: 58

Water We Can’t Live Without It

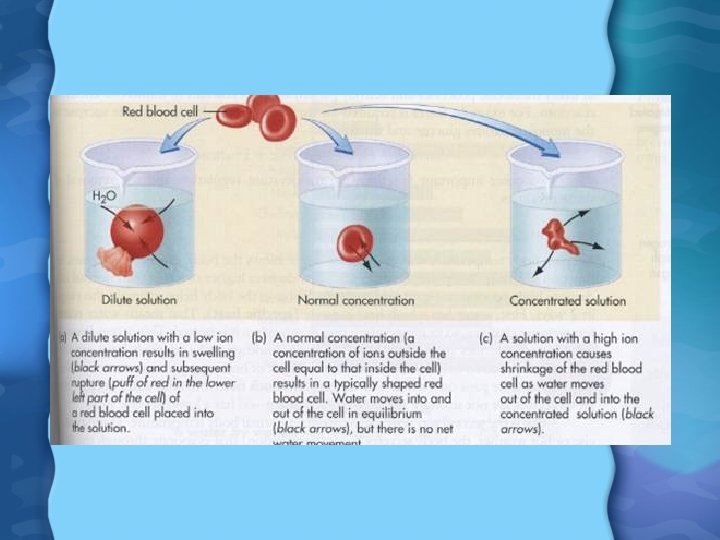
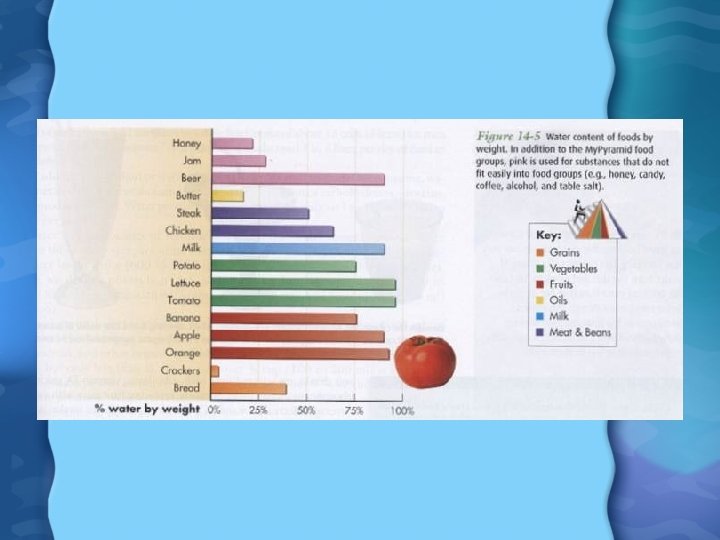
Water, Water Everywhere • Makes up 50 -70% of total body weight • Can survive for only a few days without water • It is found in: – Intracellular fluid – Extracellular fluid • Moves across cell membranes by osmosis


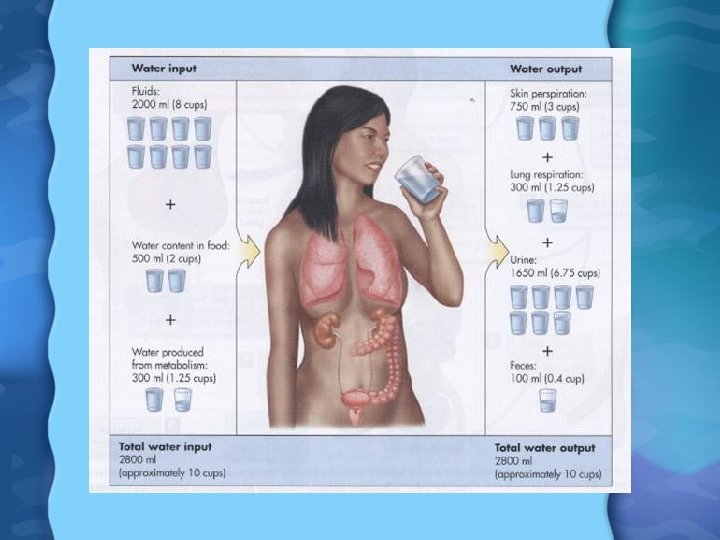
Functions of Water • Solvent for virtually all biochemical reactions • Helps maintain body temperature • Helps maintain proper osmotic pressure • Helps remove waste products • Serves as shock absorber surrounding joint and in production of amniotic fluid • Need roughly 8 cups of water a day



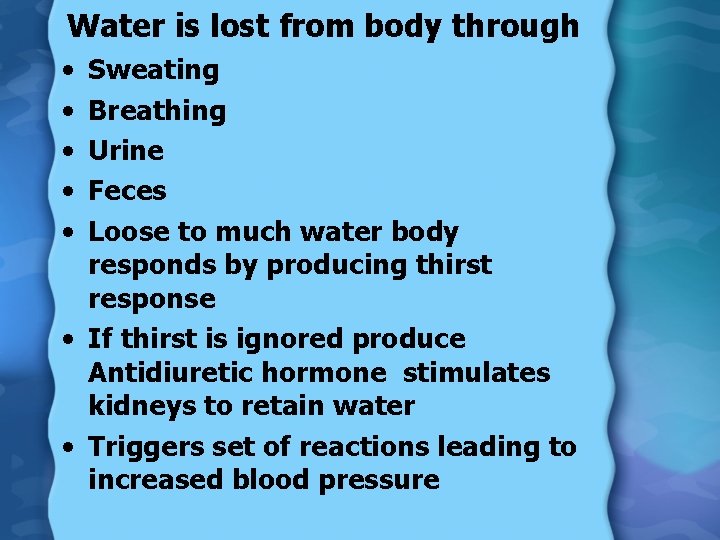
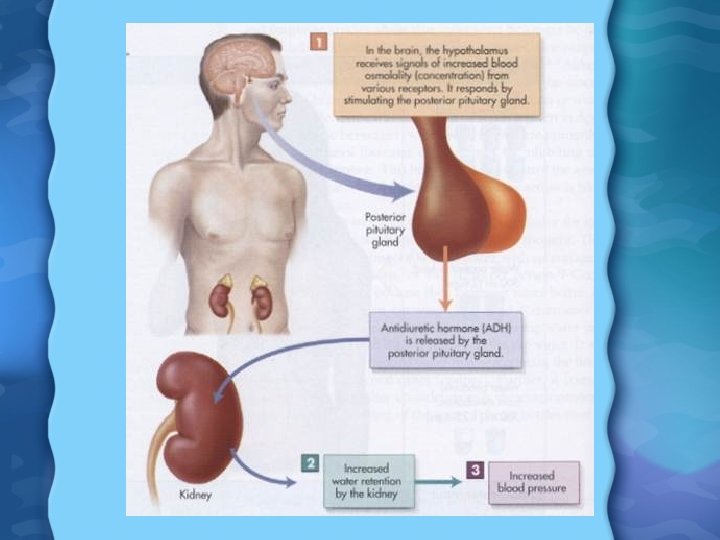
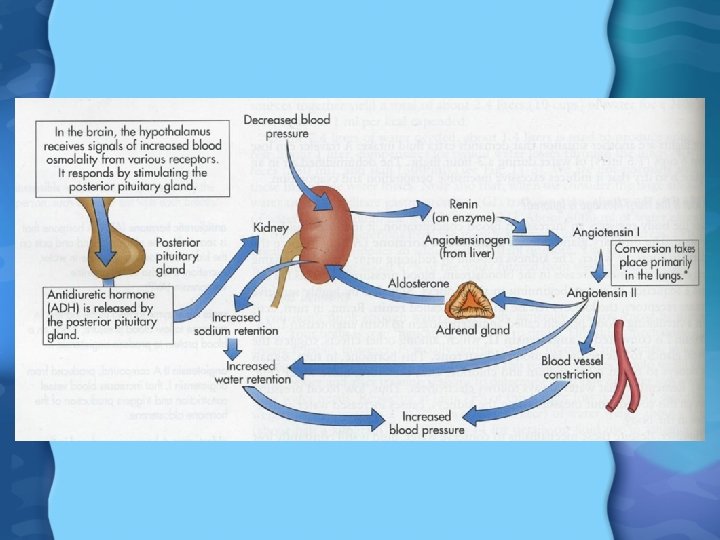
Water is lost from body through • • • Sweating Breathing Urine Feces Loose to much water body responds by producing thirst response • If thirst is ignored produce Antidiuretic hormone stimulates kidneys to retain water • Triggers set of reactions leading to increased blood pressure



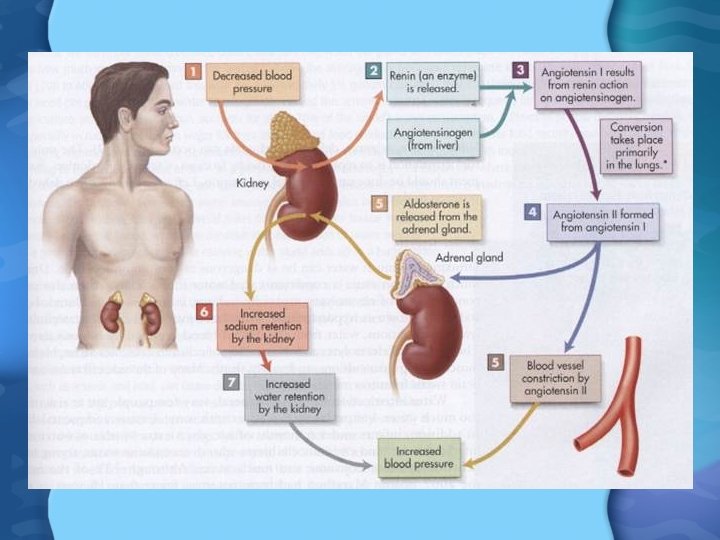
Water Regulation • Pituitary gland releases antidiuretic hormone (ADH) • ADH causes kidneys to reduce urine flow and increase water reuptake • Fall in BP causes kidney to release renin • Renin activates angiotensin II in the heart & lungs • Angio II causes adrenal glands to release aldosterone • Aldosterone causes kidney to retain salt, and therefore water as well




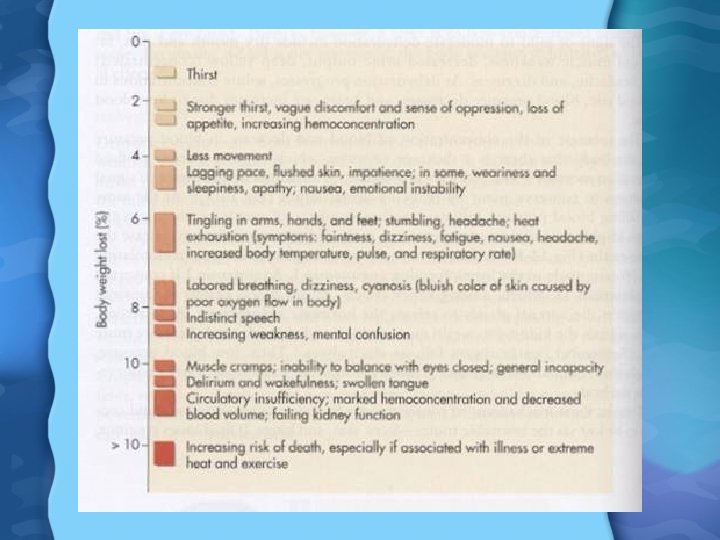
Water Toxicity • Drinking too much water can cause water intoxication – Concentration of electrolytes in blood falls (esp Na) causing hyponatremia – Affects osmotic pressure, action potential generation – Symptoms: headache, blurred vision, muscle cramps, convulsions, and death (brain swells from too much water)

Minerals What good are they?

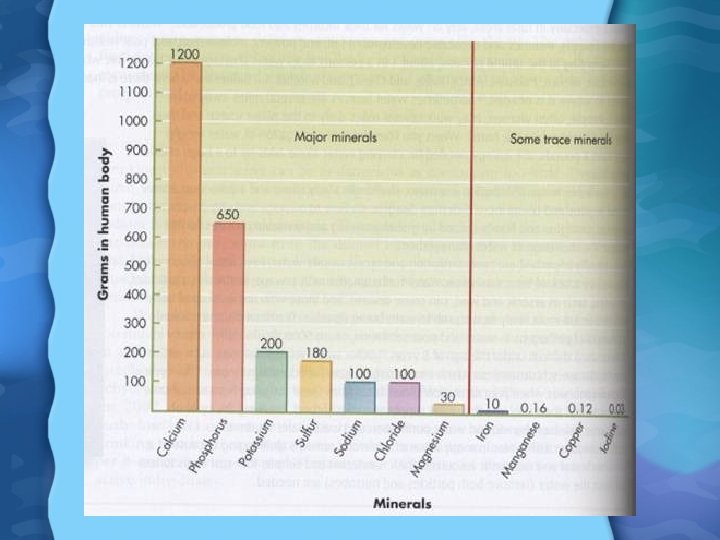
Minerals • Major minerals are required in relatively large quantities • Trace minerals are also required but in much smaller amounts • One factor in the absorption of minerals is their Bioavailability • All of the divalent cations are absorbed by the same mechanisms • If too many are present at the same time they can interfere with each others absorption • Some types of fiber may also tie up minerals so they are not available for absorption



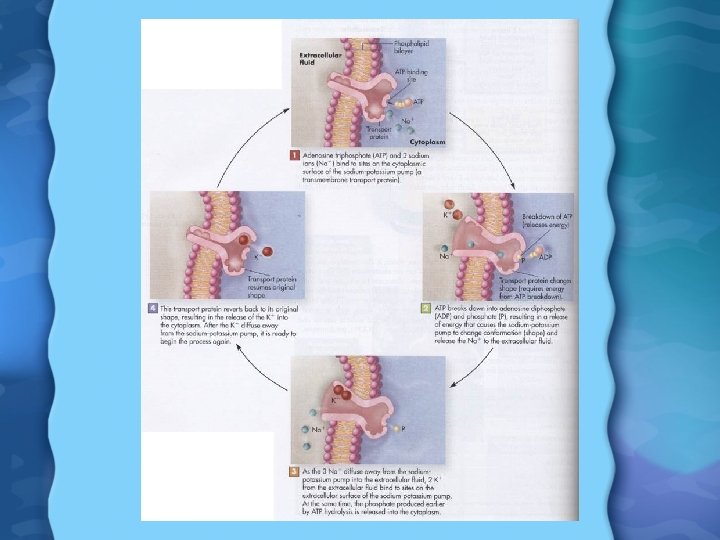
Sodium (Na) • • 95% of ingested salt is absorbed Easily absorbed from stomach, Sm. Int, Lg. Int Na is excreted via urine, sweat and GI tract Only 10% of Na consumed is actually needed - rest is excreted Na+ is major positive ion in ECF and for maintaining electrical gradients across membrane Na+ also used for glucose and AA transporters in intestine As Na+ and K+ move across membranes, they create an electrical potential charge Na+ also high in blood, used to maintain osmolarity


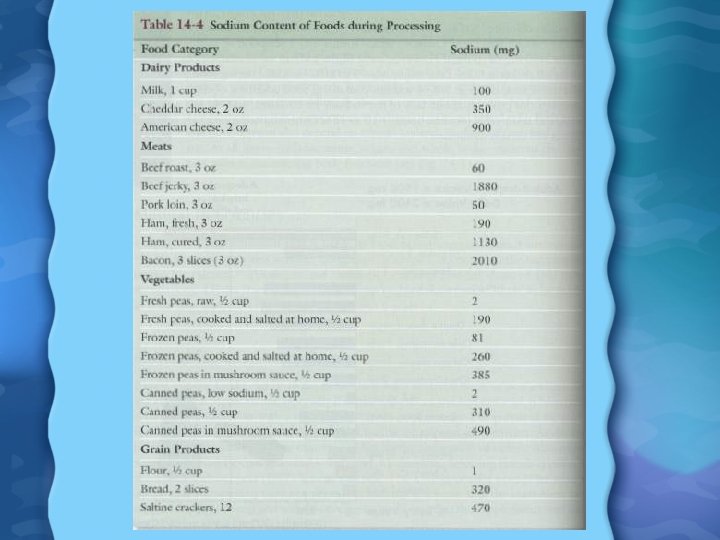
Sodium in Foods • About half the Na we eat is added at the time of eating • Other portion of Na is added to food during production • Major Na foods are: white breads, hot dogs, lunch meats, cheese, soups, tomato sauce, most sauces, and fried foods


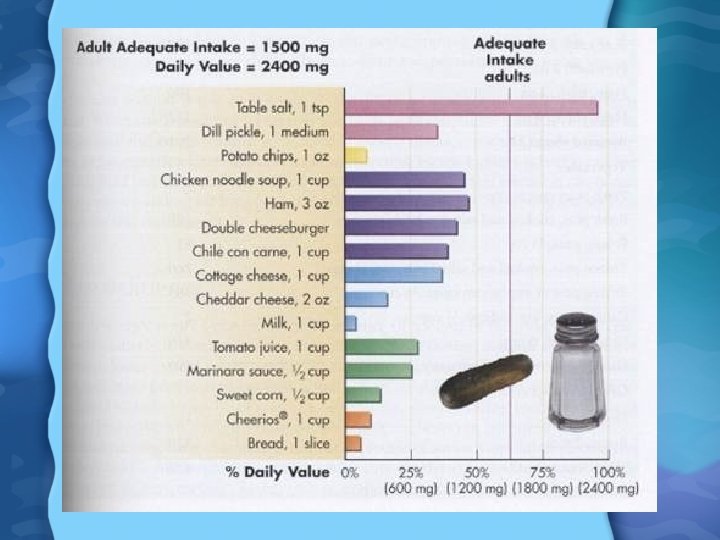
Sodium Needs • Only about 200 mg of Na is needed per day for normal physiological functions • AI is 51 -1500 mg/day • UL is around 2400 mg/day • Under FDA labeling rules, 2400 mg per day is assumed in the diet • Most people’s Na intake is around 4700 mg per day • Very high levels of Na cause water retention, and thus high BP • When going to low Na diet you can substitute oregano, lemon juice, garlic, and other herbs and spices

Sodium Deficiency • Low Na in diet, along with dehydration, sweating, vomiting, depletes Na • Causes muscle cramps, nausea, vomiting, dizziness, shock, and coma • Very unlikely due to kidney regulation of Na and aldosterone

Sodium Deficiency • When water loss is 2 or 3% (5 or 6 lbs) Na loss becomes noticable • Further loss causes fatigue, muscle cramps, dizziness, and confusion Sodium Toxicity • Only toxic when high Na is taken in without much water- overloads the kidney and causes thick urine • No UL for Na yet

Potassium (K) • We absorb about 90% of K consumed • K balance achieved primarily through kidneys, aldosterone is regulatory hormone • K highest inside cells • K is also involved in maintaining osmolarity, much like Na • K also involved in creating an electrical potential across membrane

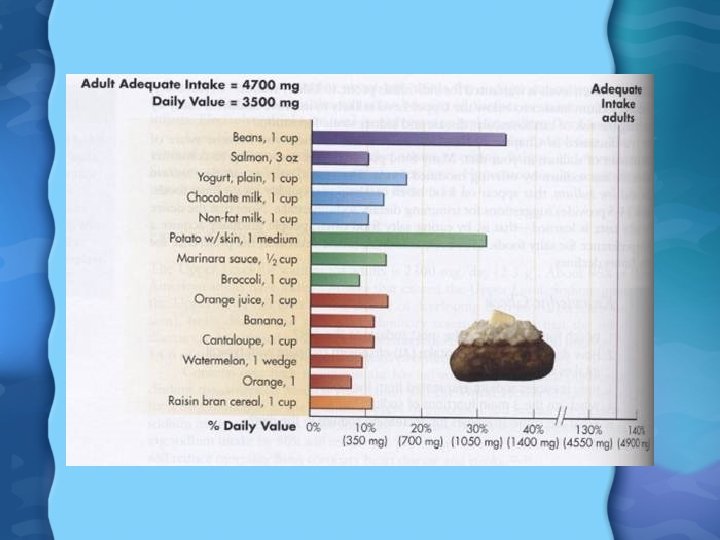
Potassium in Foods • Not generally added to foods • Fresh fruits and vegetables are highest in K, as well as milk, whole grains, potatos, and meats Potassium Needs • AI is 4700 mg/day • DV is 3500 mg/day • Average intake for most Americans is 2100 -3300 mg/day


Potassium Deficiency • Hypokalemia • People in most danger are one who take diuretics, as well as alcoholics due to inhibition of ADH • Anorexia nervosa or bulemia can cause K drop due to vomiting • Symptoms are loss of apetite, weakness, fatigue, cramps, confusion, constipation, irregular heartbeat, increased BP, stroke Potassium Toxicity • Excess K is harmless with good kidneys • Hyperkalemia- excess K in blood causes slowed heartbeat and/or cardiac arrest

Chloride (Cl) • Absorbed in Sm. Int and colon • Excreted through kidneys, skin, and GI tract • Cl- is important negative ion in ECF • Key in maintaining electrolyte balance • Used to make HCl, and in white blood cell antigen recognition • Helps in acid-base balance and disposes of CO 2 via exhaled air

Chloride in Foods • Most Cl consumed as salt added to foods (Na. Cl) • Seaweed, olives, rye, lettuce, some fruite and vegetables also have Cl Chloride Needs • AI for Cl is 2300 mg/day • DV is 3400 mg/day • Since most adults consume about 7. 5 g of Na. Cl per day, we get around 4. 5 g of Cl

Chloride Deficiency • Very unlikely due to high Na. Cl intake • Only seen with frequent vomiting in addition to nutrient poor diet Chloride Toxicity • UL set at 3. 6 g/day • Cl implicated along with Na in HBP • Cl intake over 15 g per day cause fluid retention

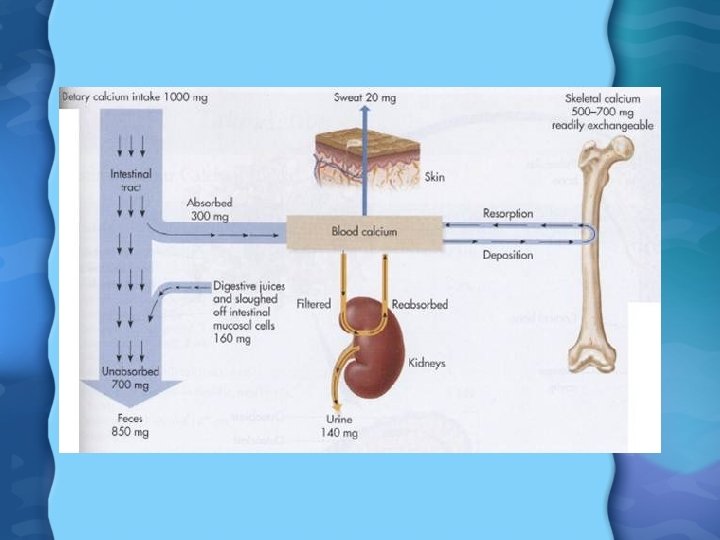
Calcium (Ca) • Ca absorbed mostly in duodenum, since it needs slightly acidic (p. H 6) to be absorbed by 1, 25(OH)2 D 3. • We absorb about 25% of Ca ingested, but during infancy and pregnancy, can be as high as 60% • Young people absorb Ca much better than older people, due to decreasing Vit D levels with age • Every cell in our bodies has vital need for Ca • Normal blood Ca can be maintained for quite a while despite inadequate Ca intake

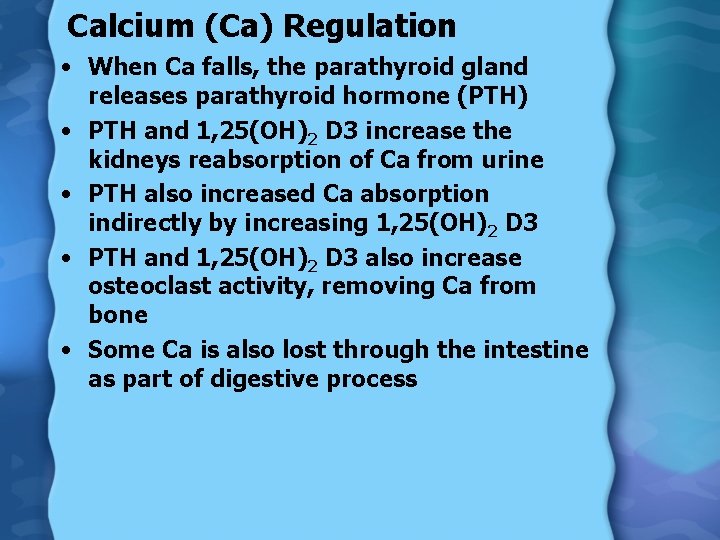
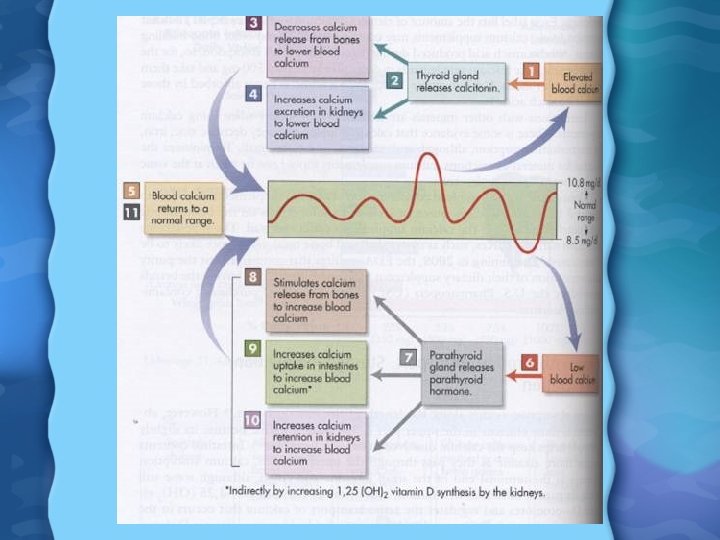
Calcium (Ca) Regulation • When Ca falls, the parathyroid gland releases parathyroid hormone (PTH) • PTH and 1, 25(OH)2 D 3 increase the kidneys reabsorption of Ca from urine • PTH also increased Ca absorption indirectly by increasing 1, 25(OH)2 D 3 • PTH and 1, 25(OH)2 D 3 also increase osteoclast activity, removing Ca from bone • Some Ca is also lost through the intestine as part of digestive process



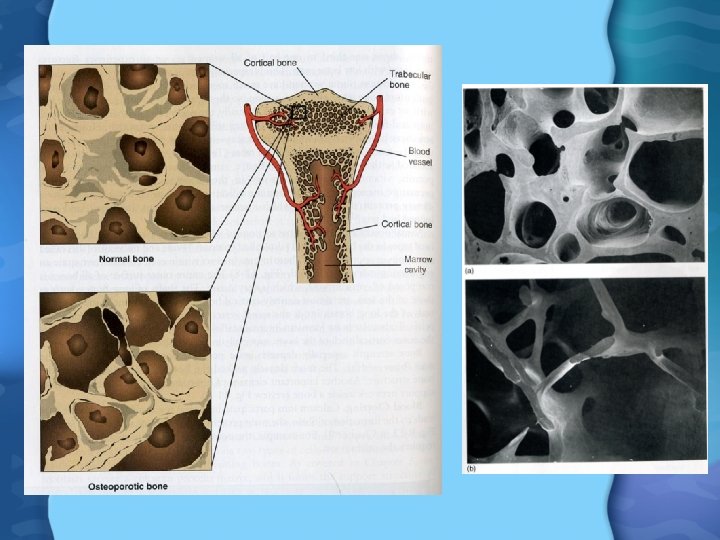
Calcium Functions • Bone development and maintainence – Osteoblasts lay down new bone matrix – Osteoclasts break down bone matrix – Bone matrix composed of hydroxyapatite (Ca 10(PO 4)6 OH 2) – Bone is constantly made and broken down, according to Ca needs of body – During growth and through adulthood, osteoblast activity exceeds osteoclast activity • Only past age 35 -40 does osteoclast activity exceed osteoclast

Calcium Functions – Bone mass and Ca deposition also increased by exercise and weightresistance activities – Risks for lowered bone mass include: genetics, slim build, irregular menstruation, use or corticosteroids, caffiene, alcohol, and prolonged bed rest



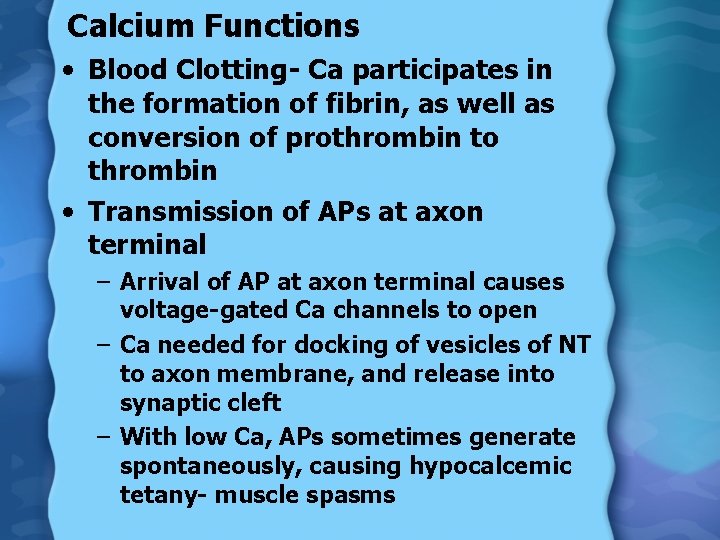
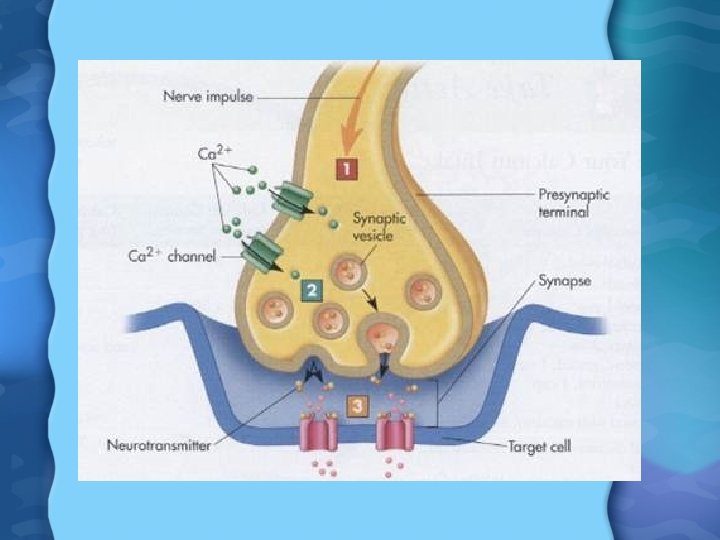
Calcium Functions • Blood Clotting- Ca participates in the formation of fibrin, as well as conversion of prothrombin to thrombin • Transmission of APs at axon terminal – Arrival of AP at axon terminal causes voltage-gated Ca channels to open – Ca needed for docking of vesicles of NT to axon membrane, and release into synaptic cleft – With low Ca, APs sometimes generate spontaneously, causing hypocalcemic tetany- muscle spasms



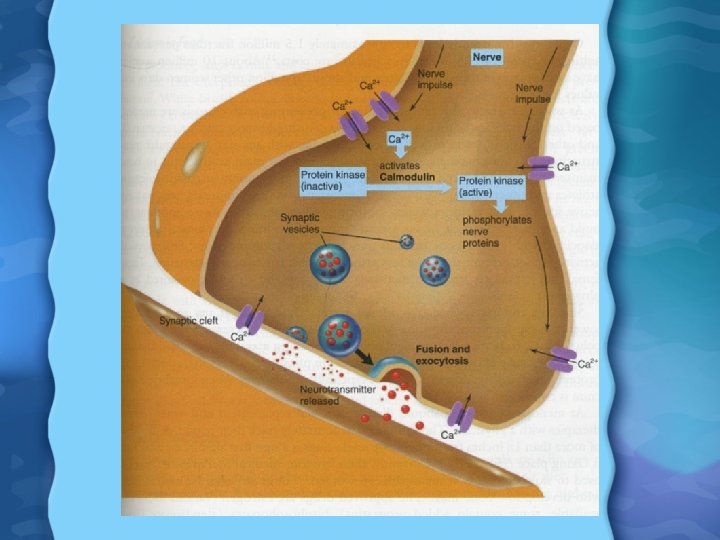
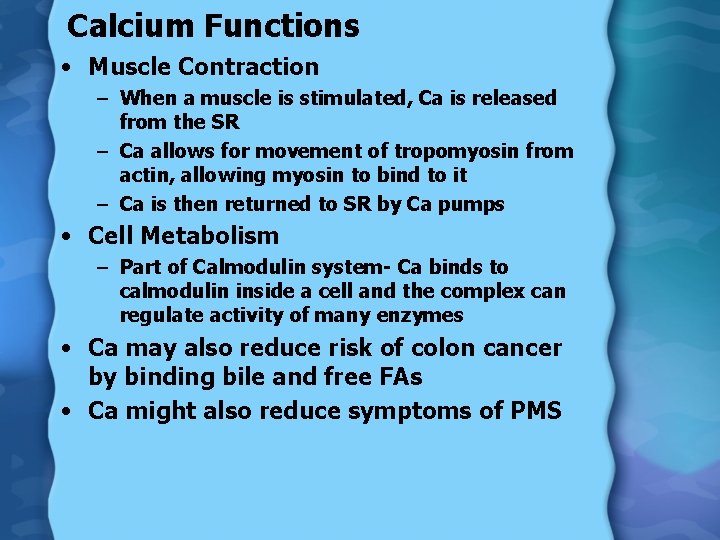
Calcium Functions • Muscle Contraction – When a muscle is stimulated, Ca is released from the SR – Ca allows for movement of tropomyosin from actin, allowing myosin to bind to it – Ca is then returned to SR by Ca pumps • Cell Metabolism – Part of Calmodulin system- Ca binds to calmodulin inside a cell and the complex can regulate activity of many enzymes • Ca may also reduce risk of colon cancer by binding bile and free FAs • Ca might also reduce symptoms of PMS


Calcium in Foods • Dairy products most abundant in Ca (except for cottage cheese) • White bread, crackers and other foods made with milk products are also high • Highest nutrient density Ca foods are broccoli, leafy greens, nonfat milk, salmon, romano cheese, swiss cheese, sardines • Calcium supplements can also be used, but must be monitored carefully or Ca toxicity can result – No more than 1000 mg/day of Ca in supplement for in divided doses of 500 mg each


Calcium Needs • AI for Ca is 1000 -1200 mg per day • For people 9 -18, it is 1300 mg/day • In the US, avg Ca intake is – 600 -800 mg/day for women – 800 -1000 mg/day for men Calcium Toxicity • Normally Sm. Int prevents excess Ca from being taken in • Ca supplements can cause calcification of body tissues, kidney stones, kidney failure, headaches, prostate cancer, and decreased absorption of other minerals • UL for Ca is 2500 mg/day

Calcium Deficiency • Osteoperosis is greatest risk • Women especially at risk due to lower production of estrogen, and hence vitamin D after menopause • Physical activity and weight resistance help to reduce rate of bone loss • Estrogen Replacement is another option – Estrogen- bind to receptors on bone and reduce bone loss – Bisphonates- stop bone resorption by binding to hydroxyapatite – Selective estrogen receptor modulators- same as estrogen – Calcitonin- inhibits osteoclast activity

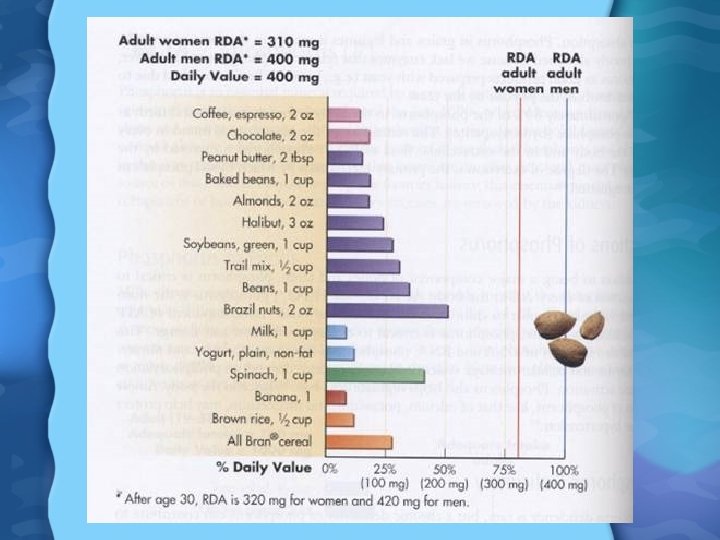
Phosphorus (P) • About 70% absorbed in adults, 90% in children • 1, 25(OH)2 D 3 plays major role in P absorption • P is excreted by the kidneys • P found in all body tissues and is vital for survival – 80% of P is in bones and teeth – Is also a component of many enzyme systems, ATP, DNA, RNA, and phospholipids in cell membranes

Phosphorus in Foods • Milk, cheese, yogurt, and meat are high in P • Cereals, eggs, bran, nuts and fish are good secondary sources • Around 30% of P comes from food additives, either as preservatives or phosphoric acid Phosphorus Needs • RDA is 700 mg/day for adults • Most adults in US consume 1000 to 1600 mg per day


Phosphorus Deficiency • Usually found in preterm infants, vegans, alcoholics, elderly with poor diets, and people using Al containing antacids daily • Usually show bone loss, decreased growth, anorexia, weight loss, weakness, irritability, stiff joints and bone pain Phosphorus Toxicity • Not harmful unless already renal stress • Reduced kidney excretion leads to high blood P and Ca causes P/Ca precipitates to form in body tissues, and contributes to bone loss by increasing PTH levels

Magnesium (Mg) • Usually absorb 40 to 60% of Mg ingested • 1, 25(OH)2 D 3 somewhat enhances Mg absorption • More than 300 enzyme catalyzed reactions in the body need Mg to function properly – Mg binds to ATP to form active ATP – Mg contributes to DNA and RNA during cell proliferation – Also involved in Ca and P metabolism, and therefore bone production – Important in nerve and heart function – Used in release of insulin from pancreas

Magnesium in Foods • Plant products high in Mg such as whole grains, broccoli, squash, green leafy vegetables, beans, nuts, seeds, and chocolate • Most tap water is fortified with Mg too (as well as Ca and fluoride) Magnesium Needs • RDA is 400 mg/day for men and 310 mg/day for women • In the US, men consume 325 mg/day and women 225 mg/day


Magnesium Deficiency • Usually only seen in users of some diuretics which causes excess Mg excretion in urine • Heavy perspiration for weeks or vomiting and diarrhea can cause Mg loss too • Alcoholics often low in Mg • Symptoms are irritibility, rapid heartbeat, weakness, muscle spasms, disorientation, nausea, vomiting, seizures, and eventually death Magnesium Toxicity • UL is 350 mg/day, but only dangerous with certain laxatives and antacids • Symptoms are diarrhea, renal failure, malaise, nausea, slowed breathing, and death

Sulfur (S) • S usually found in nonionic form as part of organic compounds – Found in biotin and thiamin – AAs methionine and cysteine contain S too, and present in proteins – Found in collagen as disulfide bonds, and in keratin – Also found as sulfate (SO 42 -) which is used in maintaining acid-base balance of the body