Iron Cant live without enough of it Cant





- Slides: 17

Iron: Can’t live without enough of it Can’t live with too much of it Camp Sunshine, July 15 th 2015 Adapted from DBA Day Iron Overload by Dr. Lawrence Wolfe

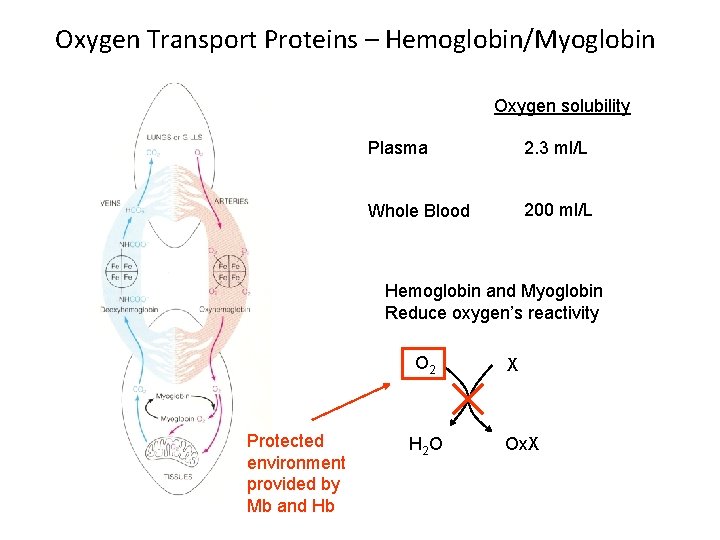
Oxygen Transport Proteins – Hemoglobin/Myoglobin Oxygen solubility Plasma 2. 3 ml/L Whole Blood 200 ml/L Hemoglobin and Myoglobin Reduce oxygen’s reactivity O 2 Protected environment provided by Mb and Hb H 2 O X Ox. X

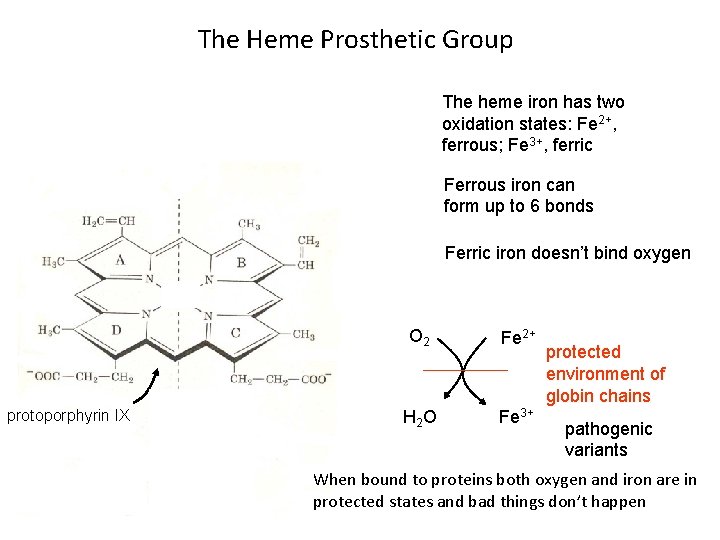
The Heme Prosthetic Group The heme iron has two oxidation states: Fe 2+, ferrous; Fe 3+, ferric Ferrous iron can form up to 6 bonds Ferric iron doesn’t bind oxygen protoporphyrin IX O 2 Fe 2+ H 2 O Fe 3+ protected environment of globin chains pathogenic variants When bound to proteins both oxygen and iron are in protected states and bad things don’t happen

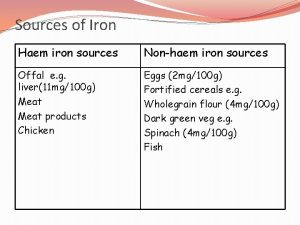
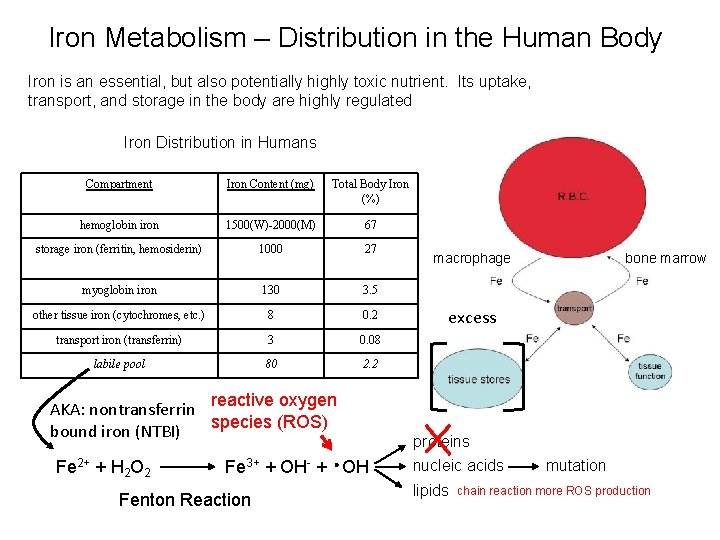
Iron Metabolism – Distribution in the Human Body Iron is an essential, but also potentially highly toxic nutrient. Its uptake, transport, and storage in the body are highly regulated Iron Distribution in Humans Compartment Iron Content (mg) Total Body Iron (%) hemoglobin iron 1500(W)-2000(M) 67 storage iron (ferritin, hemosiderin) 1000 27 myoglobin iron 130 3. 5 other tissue iron (cytochromes, etc. ) 8 0. 2 transport iron (transferrin) 3 0. 08 labile pool 80 2. 2 reactive oxygen AKA: nontransferrin species (ROS) bound iron (NTBI) Fe 2+ + H 2 O 2 Fe 3+ + OH- + OH Fenton Reaction macrophage bone marrow excess proteins nucleic acids lipids mutation chain reaction more ROS production

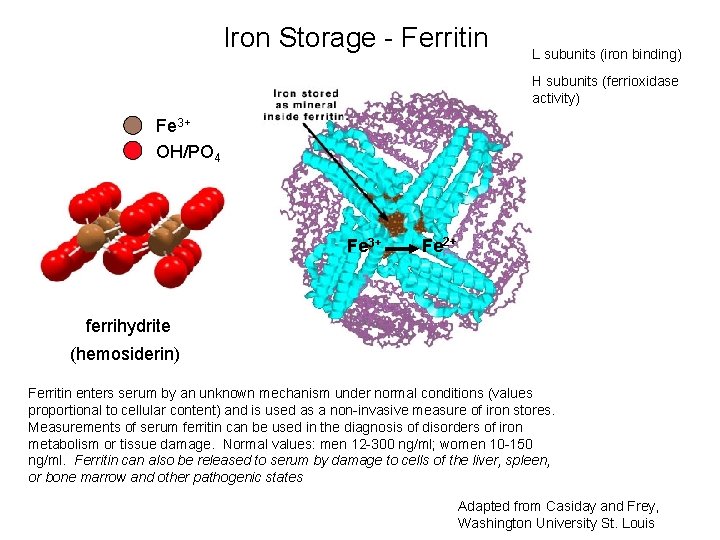
Iron Storage - Ferritin L subunits (iron binding) H subunits (ferrioxidase activity) Fe 3+ OH/PO 4 Fe 3+ Fe 2+ ferrihydrite (hemosiderin) Ferritin enters serum by an unknown mechanism under normal conditions (values proportional to cellular content) and is used as a non-invasive measure of iron stores. Measurements of serum ferritin can be used in the diagnosis of disorders of iron metabolism or tissue damage. Normal values: men 12 -300 ng/ml; women 10 -150 ng/ml. Ferritin can also be released to serum by damage to cells of the liver, spleen, or bone marrow and other pathogenic states Adapted from Casiday and Frey, Washington University St. Louis

Iron Transport - Transferrin enterocytes liver macrophages transferrin saturation Fe 2+ ceruloplasmin Fe 3+ + 33% Fe 3+-transferrin-Fe 3+ transferrin 67% transferrin receptor internalization

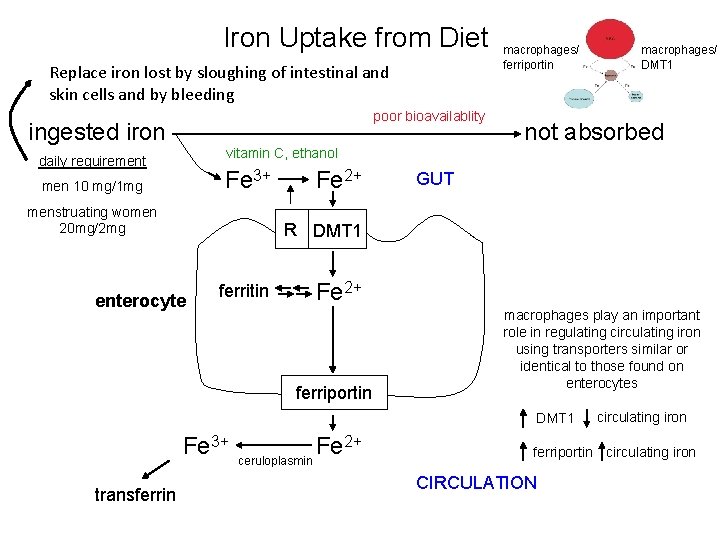
Iron Uptake from Diet Replace iron lost by sloughing of intestinal and skin cells and by bleeding poor bioavailablity ingested iron macrophages/ ferriportin not absorbed vitamin C, ethanol daily requirement Fe 3+ men 10 mg/1 mg menstruating women 20 mg/2 mg Fe 2+ GUT R DMT 1 enterocyte ferritin Fe 2+ ferriportin macrophages play an important role in regulating circulating iron using transporters similar or identical to those found on enterocytes DMT 1 Fe 3+ transferrin macrophages/ DMT 1 2+ Fe ceruloplasmin circulating iron ferriportin circulating iron CIRCULATION

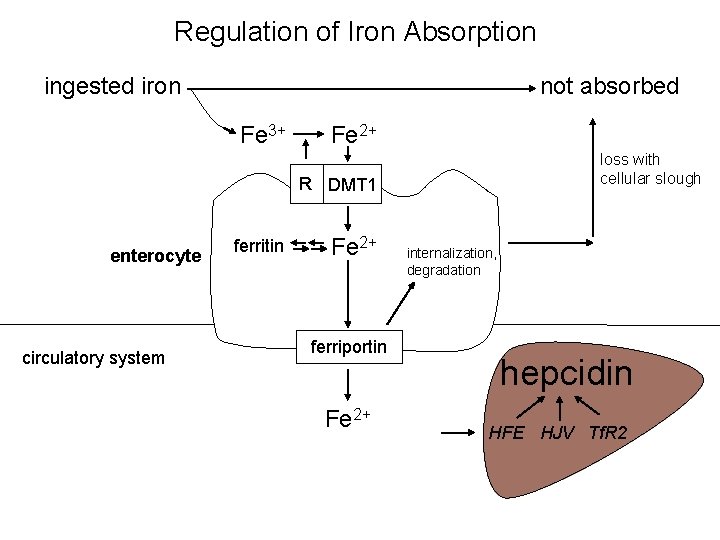
Regulation of Iron Absorption ingested iron not absorbed Fe 3+ Fe 2+ loss with cellular slough R DMT 1 enterocyte circulatory system ferritin Fe 2+ ferriportin Fe 2+ internalization, degradation hepcidin HFE HJV Tf. R 2

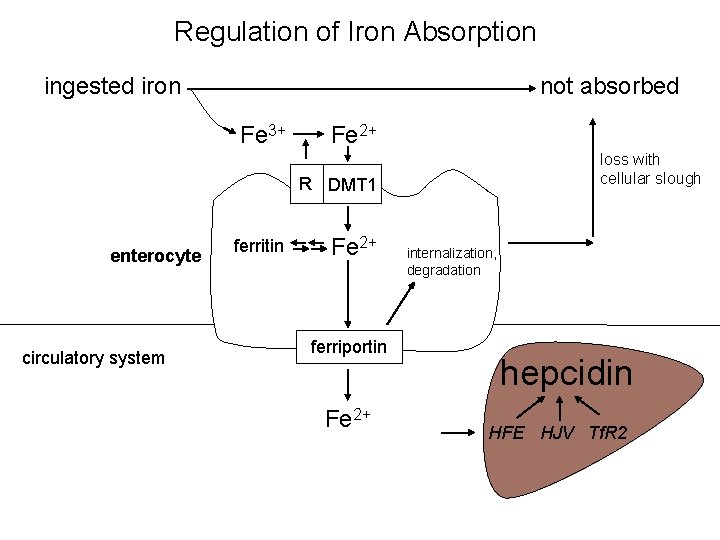
Regulation of Iron Absorption ingested iron not absorbed Fe 3+ Fe 2+ loss with cellular slough R DMT 1 enterocyte circulatory system ferritin Fe 2+ ferriportin Fe 2+ internalization, degradation hepcidin HFE HJV Tf. R 2

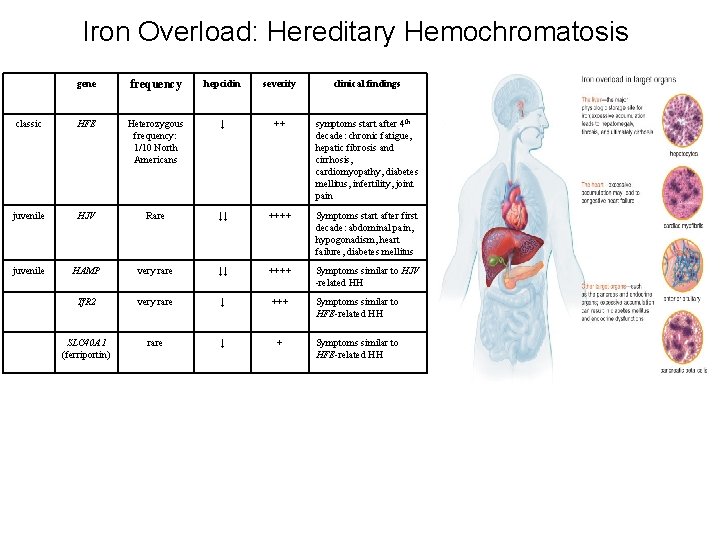
Iron Overload: Hereditary Hemochromatosis gene frequency hepcidin severity clinical findings classic HFE Heterozygous frequency: 1/10 North Americans ↓ ++ symptoms start after 4 th decade: chronic fatigue, hepatic fibrosis and cirrhosis, cardiomyopathy, diabetes mellitus, infertility, joint pain juvenile HJV Rare ↓↓ ++++ Symptoms start after first decade: abdominal pain, hypogonadism, heart failure, diabetes mellitus juvenile HAMP very rare ↓↓ ++++ Symptoms similar to HJV -related HH Tf. R 2 very rare ↓ +++ Symptoms similar to HFE-related HH SLC 40 A 1 (ferriportin) rare ↓ + Symptoms similar to HFE-related HH

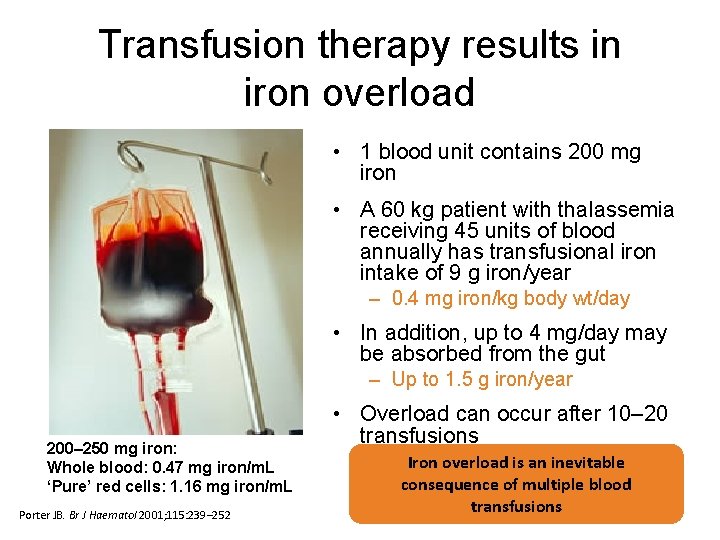
Transfusion therapy results in iron overload • 1 blood unit contains 200 mg iron • A 60 kg patient with thalassemia receiving 45 units of blood annually has transfusional iron intake of 9 g iron/year – 0. 4 mg iron/kg body wt/day • In addition, up to 4 mg/day may be absorbed from the gut – Up to 1. 5 g iron/year 200– 250 mg iron: Whole blood: 0. 47 mg iron/m. L ‘Pure’ red cells: 1. 16 mg iron/m. L Porter JB. Br J Haematol 2001; 115: 239– 252 • Overload can occur after 10– 20 transfusions Iron overload is an inevitable consequence of multiple blood transfusions

Normal distribution and turnover of body iron Erythron 2 g 20– 30 mg/day Parenchyma 0. 3 g Liver 1 g Transferrin 2– 3 mg/day 20– 30 mg/day 1– 2 mg/day Gut Iron balance is achieved in the normal state Hershko C et al. Ann NY Acad Sci 1998; 850: 191– 201 Reticuloendothelial macrophages 0. 6 g

Imbalance of distribution and turnover of body iron with transfusion therapy Erythron 20– 40 mg/day NTBI Parenchyma Transfusions Transferrin Reticuloendothelial macrophages Gut Iron balance is disturbed by blood transfusion because the body cannot remove the excess iron NTBI, non-transferrin-bound iron Hershko C et al. Ann NY Acad Sci 1998; 850: 191– 201

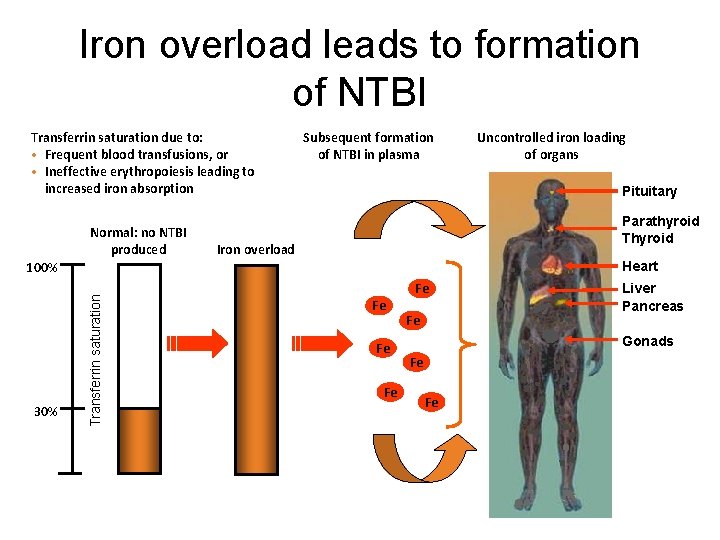
Iron overload leads to formation of NTBI Transferrin saturation due to: • Frequent blood transfusions, or • Ineffective erythropoiesis leading to increased iron absorption Normal: no NTBI produced Subsequent formation of NTBI in plasma Pituitary Parathyroid Thyroid Iron overload Heart Transferrin saturation 100% 30% Uncontrolled iron loading of organs Fe Fe Fe Liver Pancreas Gonads Fe Fe

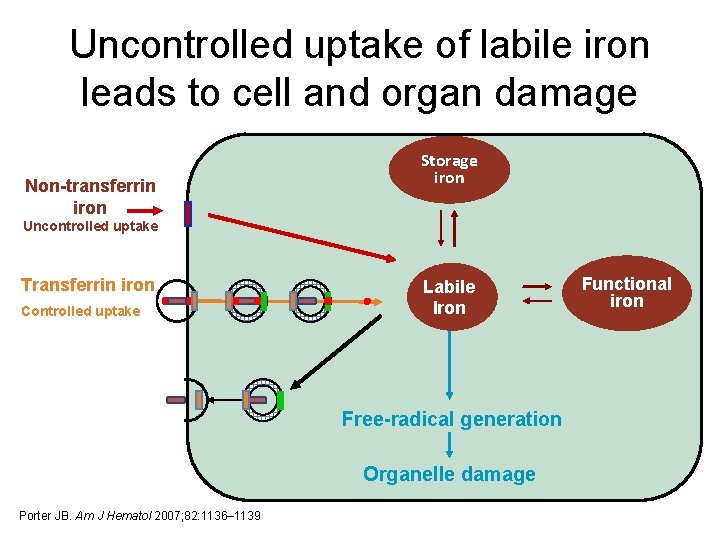
Uncontrolled uptake of labile iron leads to cell and organ damage Non-transferrin iron Storage iron Uncontrolled uptake Transferrin iron Controlled uptake Labile Iron Free-radical generation Organelle damage Porter JB. Am J Hematol 2007; 82: 1136– 1139 Functional iron

Iron overload negatively affects organ function Labile iron Free radical generation Lipid peroxidation Organelle damage Lysosomal fragility TGF-β 1 Collagen synthesis Enzyme leakage Cell death Fibrosis TGF, transforming growth factor Cohen AR and Porter JB. In Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management, Steinberg MH et al. (Eds); 2001: 979– 1027

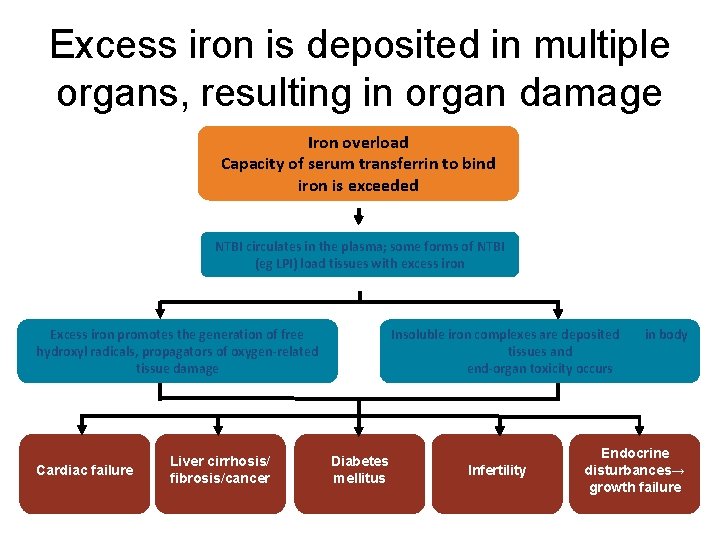
Excess iron is deposited in multiple organs, resulting in organ damage Iron overload Capacity of serum transferrin to bind iron is exceeded NTBI circulates in the plasma; some forms of NTBI (eg LPI) load tissues with excess iron Excess iron promotes the generation of free hydroxyl radicals, propagators of oxygen-related tissue damage Cardiac failure Liver cirrhosis/ fibrosis/cancer Insoluble iron complexes are deposited tissues and end-organ toxicity occurs Diabetes mellitus Infertility in body Endocrine disturbances→ growth failure
 If you can't measure it quote
If you can't measure it quote Can't manage what you don't measure
Can't manage what you don't measure How much vs how many
How much vs how many And doggone it i'm good enough
And doggone it i'm good enough Our failing schools enough is enough summary
Our failing schools enough is enough summary Our failing schools enough is enough summary
Our failing schools enough is enough summary You can't have one without the other examples
You can't have one without the other examples Abigail williams character chart
Abigail williams character chart Mass of iron in an iron tablet
Mass of iron in an iron tablet Iron sharpens iron friendship
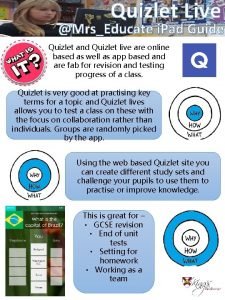
Iron sharpens iron friendship Quizlet ,live
Quizlet ,live Live healthy be happy
Live healthy be happy Can we live without minerals
Can we live without minerals Without title poem analyzing the text answers
Without title poem analyzing the text answers Justify the title of keeping quiet
Justify the title of keeping quiet Without title by diane glancy
Without title by diane glancy Close your eyes imagine that you are the protagonist
Close your eyes imagine that you are the protagonist Rail cant
Rail cant