WHY CANT WE SEE ATOMS l l l






- Slides: 11

WHY CAN'T WE SEE ATOMS? l l l l “seeing an object” n = detecting light that has been reflected off the object's surface light = electromagnetic wave; “visible light”= those electromagnetic waves that our eyes can detect “wavelength” of e. m. wave (distance between two successive crests) determines “color” of light wave hardly influenced by object if size of object is much smaller than wavelength of visible light: between 4 10 -7 m (violet) and 7 10 -7 m (red); diameter of atoms: 10 -10 m generalize meaning of seeing: n l l l seeing is to detect effect due to the presence of an object quantum theory “particle waves”, with wavelength 1/(m v) use accelerated (charged) particles as probe, can “tune” wavelength by choosing mass m and changing velocity v this method is used in electron microscope, as well as in “scattering experiments” in nuclear and particle physics

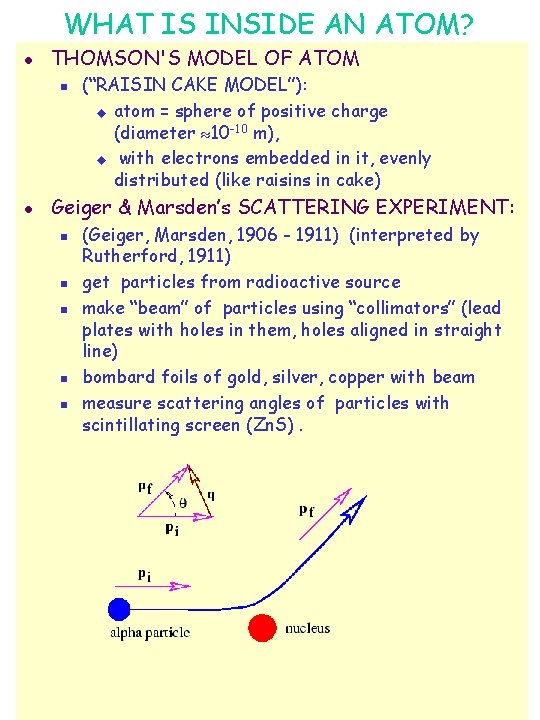
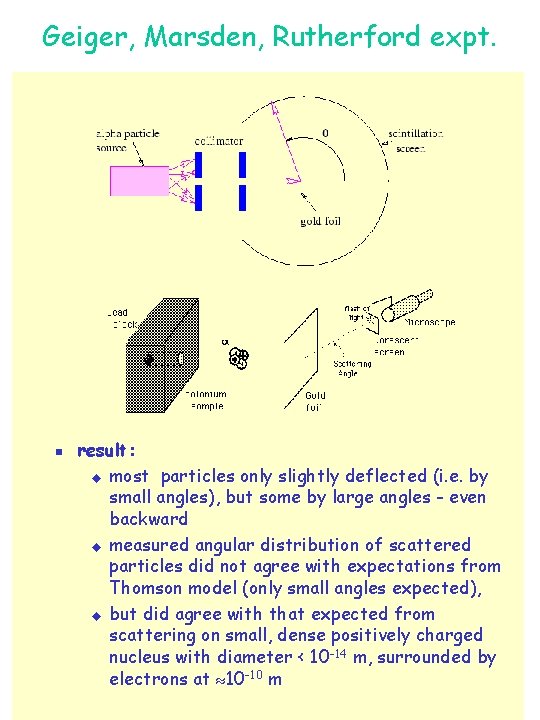
WHAT IS INSIDE AN ATOM? l THOMSON'S MODEL OF ATOM n l (“RAISIN CAKE MODEL”): u atom = sphere of positive charge (diameter 10 -10 m), u with electrons embedded in it, evenly distributed (like raisins in cake) Geiger & Marsden’s SCATTERING EXPERIMENT: n n n (Geiger, Marsden, 1906 - 1911) (interpreted by Rutherford, 1911) get particles from radioactive source make “beam” of particles using “collimators” (lead plates with holes in them, holes aligned in straight line) bombard foils of gold, silver, copper with beam measure scattering angles of particles with scintillating screen (Zn. S).

Geiger, Marsden, Rutherford expt. n result: u most particles only slightly deflected (i. e. by small angles), but some by large angles - even backward u measured angular distribution of scattered particles did not agree with expectations from Thomson model (only small angles expected), u but did agree with that expected from scattering on small, dense positively charged nucleus with diameter < 10 -14 m, surrounded by electrons at 10 -10 m

Rutherford model l RUTHERFORD MODEL OF ATOM: (“planetary model of atom”) n n n l problem with Rutherford atom: n n l positive charge concentrated in nucleus (<10 -14 m); negative electrons in orbit around nucleus at distance 10 -10 m; electrons bound to nucleus by Coulomb force. electron in orbit around nucleus is accelerated (centripetal acceleration to change direction of velocity); according to theory of electromagnetism (Maxwell's equations), accelerated electron emits electromagnetic radiation (frequency = revolution frequency); electron loses energy by radiation orbit decays, changing revolution frequency continuous emission spectrum (no line spectra), and atoms would be unstable (lifetime 10 -10 s ) we would not exist to think about this!!

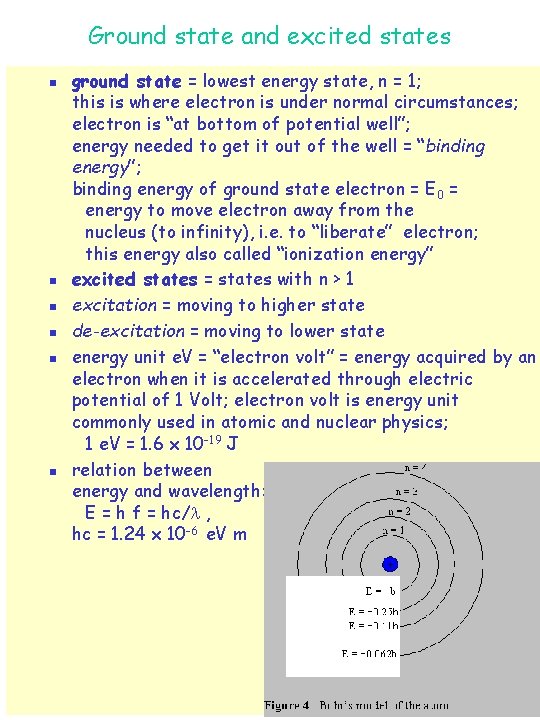
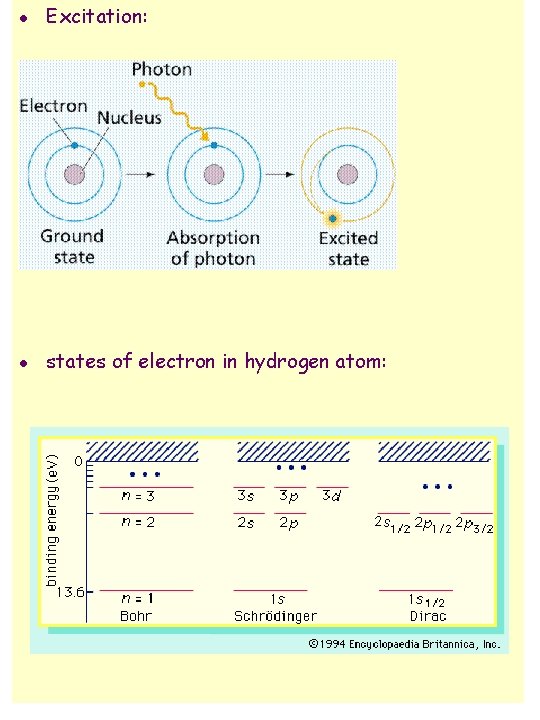
Bohr model of hydrogen (Niels Bohr, 1913) n n n n Bohr model is radical modification of Rutherford model; discrete line spectrum attributed to “quantum effect”; electron in orbit around nucleus, but not all orbits allowed; three basic assumptions: u 1. angular momentum is quantized L = n·(h/2 ) = n ·ħ, n = 1, 2, 3, . . . electron can only be in discrete specific orbits with particular radii discrete energy levels u 2. electron does not radiate when in one of the allowed levels, or “states” u 3. radiation is only emitted when electron makes “transition” between states, transition also called “quantum jump” or “quantum leap” from these assumptions, can calculate radii of allowed orbits and corresponding energy levels: radii of allowed orbits: rn = a 0 n 2 n = 1, 2, 3, …. , a 0 = 0. 53 x 10 -10 m = “Bohr radius” n = “principal quantum number” allowed energy levels: En = - E 0 /n 2 , E 0 = “Rydberg energy” note: energy is negative, indicating that electron is in a “potential well”; energy is = 0 at top of well, i. e. for n = , at infinite distance from the nucleus.

Ground state and excited states n n n ground state = lowest energy state, n = 1; this is where electron is under normal circumstances; electron is “at bottom of potential well”; energy needed to get it out of the well = “binding energy”; binding energy of ground state electron = E 0 = energy to move electron away from the nucleus (to infinity), i. e. to “liberate” electron; this energy also called “ionization energy” excited states = states with n > 1 excitation = moving to higher state de-excitation = moving to lower state energy unit e. V = “electron volt” = energy acquired by an electron when it is accelerated through electric potential of 1 Volt; electron volt is energy unit commonly used in atomic and nuclear physics; 1 e. V = 1. 6 x 10 -19 J relation between energy and wavelength: E = h f = hc/ , hc = 1. 24 x 10 -6 e. V m

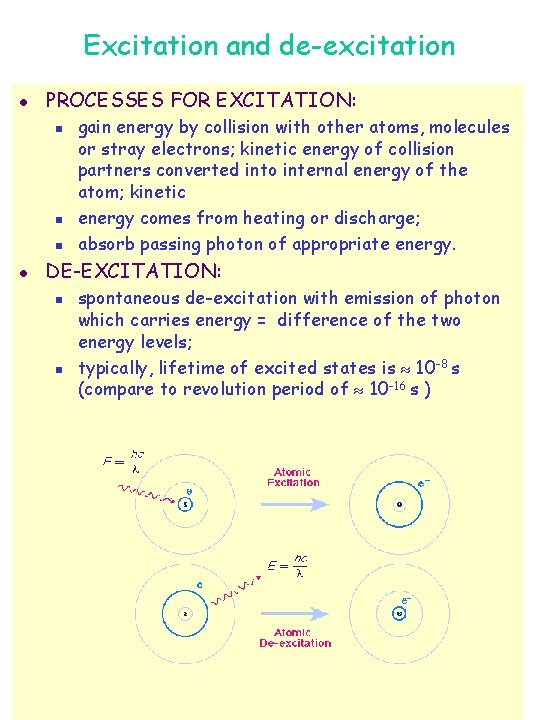
Excitation and de-excitation l PROCESSES FOR EXCITATION: n n n l gain energy by collision with other atoms, molecules or stray electrons; kinetic energy of collision partners converted into internal energy of the atom; kinetic energy comes from heating or discharge; absorb passing photon of appropriate energy. DE-EXCITATION: n n spontaneous de-excitation with emission of photon which carries energy = difference of the two energy levels; typically, lifetime of excited states is 10 -8 s (compare to revolution period of 10 -16 s )

l Excitation: l states of electron in hydrogen atom:

l MICROWAVE COOKING n n n l water molecule has rotational energy levels close together small energy difference can absorb microwaves; microwaves: wavelength 3 cm, frequency 10 GHz = 1010 Hz; energy of photon = h f 4. 13 x 10 -5 e. V it is water content that is critical in microwave cooking; most dishes and containers do not absorb microwaves are not heated by them, but get hot from hot food. IONIZATION: n n if energy given to electron > binding energy, the atom is ionized, i. e. electron leaves atom; surplus energy becomes kinetic energy of freed electron. this is what happens, e. g. in photoelectric effect ionizing effect of charged particles exploited in particle detectors (e. g. Geiger counter) aurora borealis, aurora australis: cosmic rays from sun captured in earth’s magnetic field, channeled towards poles; ionization/excitation of air caused by charged particles, followed by recombination/de-excitation;

Matter waves n n Louis de Broglie (1925): any moving particle has wavelength associated with it: = h/p = h/(mv) example: electron in atom has 10 -10 m; car (1000 kg) at 60 mph has 10 -38 m; wave effects manifest themselves only in interaction with things of size comparable to wavelength we do notice wave aspect of our cars. note: Bohr's quantization condition for angular momentum is identical to requirement that integer number of electron wavelengths fit into circumference of orbit. experimental verification of de Broglie's matter waves: u beam of electrons scattered by crystal lattice shows diffraction pattern (crystal lattice acts like array of slits); experiment done by Davisson and Germer (1927) u Electron microscope

QUANTUM MECHANICS l = new kind of physics based on synthesis of dual nature of waves and particles; developed in 1920's and 1930's. n n n Schrödinger equation: (Erwin Schrödinger, 1925) u is a differential equation for matter waves; basically a formulation of energy conservation. u its solution called “wave function”, usually denoted by ; u | (x)|2 gives the probability of finding the particle at x; u applied to the hydrogen atom, the Schrödinger equation gives the same energy levels as those obtained from the Bohr model; u the most probable orbits are those predicted by the Bohr model; u but probability instead of Newtonian certainty! Uncertainty principle: (Werner Heisenberg, 1925) It is impossible to simultaneously know a particle's exact position and momentum (or velocity) p x ħ = h/(2 ) (remember h is a very small quantity: h = 6. 63 x 10 -34 J s = 4. 14 x 10 -15 e. V) (note that here p means “uncertainty” in our knowledge of the momentum p) note that there are many such uncertainty relations in quantum mechanics, for any pair of “incompatible” observables.