Vaccines Vaccines Our defense mechanism Production of white

































- Slides: 39

Vaccines

Vaccines • Our defense mechanism→ Production of white blood cells and antibodies • What does it mean to have immunity? • It is the capacity to resist a disease that we have been exposed to by being able to fight off the infectious agent that causes the disease

White Blood Cells • 2 ways of Defense 1. Destroy infectious agents through PHAGOCYTOSIS 2. Produce antibodies→ neutralize infectious agent and antigens they produce

White Blood Cells • When exposed to infectious agent, our bodies produce antibodies to defeat it. • Can take a few days or weeks to get the right antibodies (trial + error) • If infectious agent is really dangerous, could have enough time to do some damage to body • If infectious agent is re-introduced, will be defeated!

White Blood Cells • Our immune system “REMEMBERS” • Copies of antibodies will forever remain in our bodies

Vaccination • Introduce “weakened” infectious agent into the body • Cannot hurt us • Just strong enough to teach body how to defeat it • Not strong enough to take over • Allows our bodies to defeat the disease if exposed to it

Vaccine Manufacturing • Cell culture of infectious agent (growing cells) • The cells are harmless • Result is : LIVE VACCINE INACTIVE VACCINE

Live vaccine • Contains “live” infectious agent • Infectious agent is chemically treated to make it impossible for it to cause the illness • In order for the cells to live longer, they are mixed with other substances • It is still alive! • Live vaccines usually cause a more aggressive immune response

Live Vaccine • Very rare: infectious agent can become “virulent”, meaning it can become strong enough to cause the disease instead of immunizing it! • Examples: polio vaccine, mumps, measles, chicken pox vaccine, H 1 N 1, yellow fever, tuberculosis, seasonal flu… • 2 methods of creating a live vaccine

Live Vaccine • Traditional method: 1. Culture of infectious agent(growing the cells) 2. Chemical treatment of infectious agent to make it harmless 3. Addition of chemicals (to allow cells to live longer)

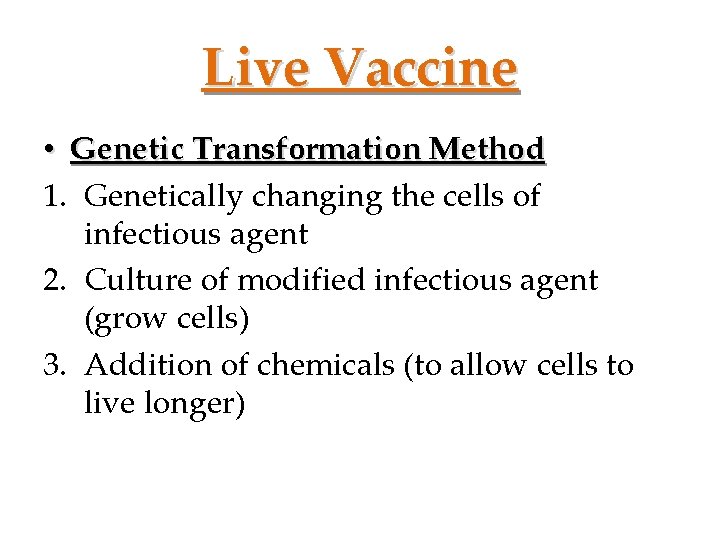
Live Vaccine • Genetic Transformation Method 1. Genetically changing the cells of infectious agent 2. Culture of modified infectious agent (grow cells) 3. Addition of chemicals (to allow cells to live longer)

Inactive Vaccine • Does not contain any live infectious agent • Made by using only a part or parts of the infectious agent • These parts can still be recognized by the body’s antibodies • Parts are called = ANTIGENS • Find which antigens are causing the disease

Inactive Vaccine • Isolate them and then treat them so that they become harmless • Examples: Meningitis, hepatitis A & B, tetanus… • 2 ways of producing inactive vaccines

Inactive Vaccine • Traditional Method 1. Culture infectious agent 2. Isolate antigen 3. Addition of antigen to other pharmaceutical products for increased “shelf life”

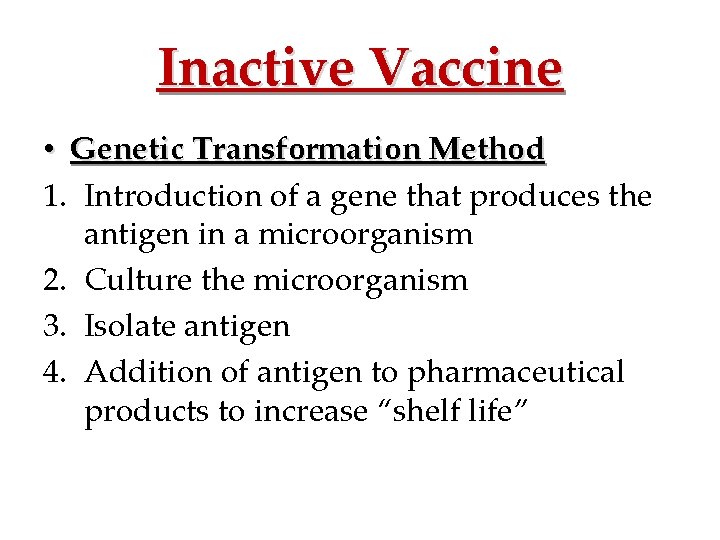
Inactive Vaccine • Genetic Transformation Method 1. Introduction of a gene that produces the antigen in a microorganism 2. Culture the microorganism 3. Isolate antigen 4. Addition of antigen to pharmaceutical products to increase “shelf life”

Mixtures • 2 types • Heterogeneous • Homogeneous

Heterogeneous Mixtures • Made up of at least 2 substances that can be seen with the “naked eye” • Examples: Vegetable soup Rocks Salt+ pepper mix Salad Blizzard at DQ

Homogeneous Mixtures • Made up of at least 2 substances that cannot be seen by the “naked eye” Colloid: is a homogeneous mixture in which substances can be seen under a microscopic instrument

Solutions • Are homogenous mixtures that are impossible to see its different parts even with a microscope Ex: Sugar and water→ water mix together, looks like you just have water • The substance that seems to “disappear” into the other is called: the solute

Solutions • The substance into which the “solute” dissolves is called: the solvent • Examples of solution in the body: Saliva, sweat, tears, urine etc. . They all share a common solvent→ water!!!

Properties of Solutions • There are 2 properties of solutions: 1. Concentration→ Concentration How much solute is dissolved in a certain amount of solvent. Ex: Making “Kool-Aid” depends on how much powder (solute) you mix with the water (solvent)

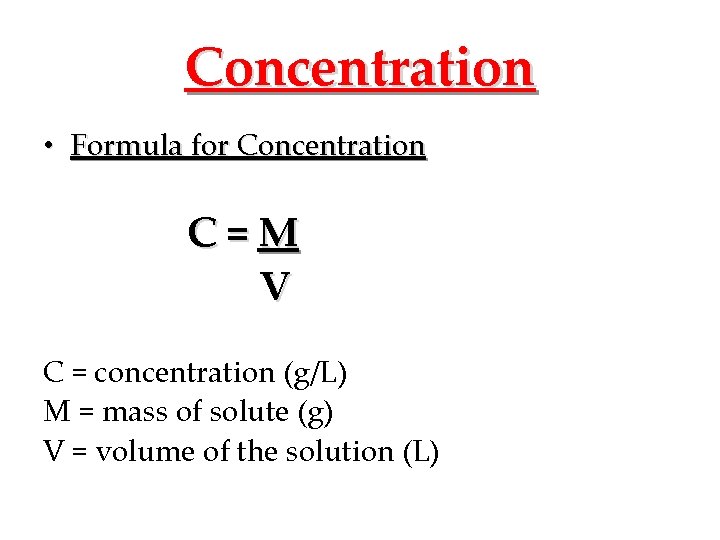
Concentration • Formula for Concentration C=M V C = concentration (g/L) M = mass of solute (g) V = volume of the solution (L)

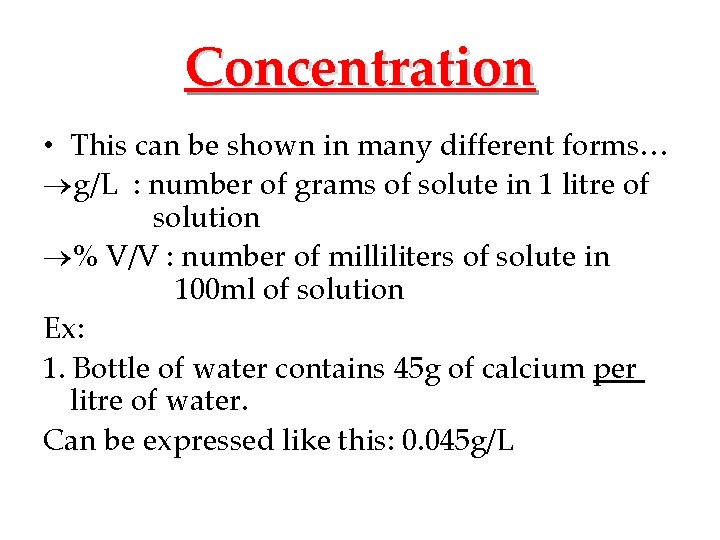
Concentration • This can be shown in many different forms… g/L : number of grams of solute in 1 litre of solution % V/V : number of milliliters of solute in 100 ml of solution Ex: 1. Bottle of water contains 45 g of calcium per litre of water. Can be expressed like this: 0. 045 g/L

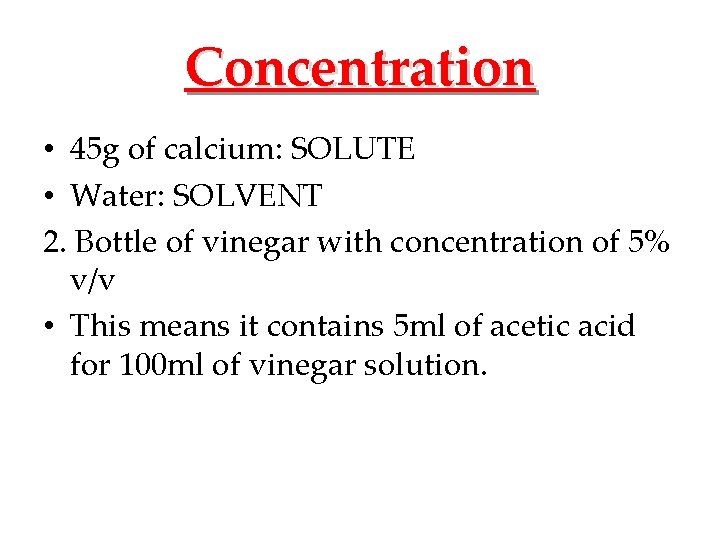
Concentration • 45 g of calcium: SOLUTE • Water: SOLVENT 2. Bottle of vinegar with concentration of 5% v/v • This means it contains 5 ml of acetic acid for 100 ml of vinegar solution.

Concentration • Let’s try these simple problems…. 1. 2 L of a salt water solution containing 5 g of salt. What is the concentration of the solution?

Concentration Answer: C=M V C= 5 g = 2 L 2. 5 g 1 L Ans: The concentration of the solution is 2. 5 g/L

Concentration 2. What mass of sugar do you need to make 300 ml of a 5 g/L sugar solution? Ans: You need to convert the 300 ml into Litres first!!!! * You must divide the milliliters by 1000.

Concentration 300 ml 1000 = 0. 3 L C=M V 5 g/L = mass of solute 0. 3 L Cross multiply: 5 g/L x 0. 3 L = 1. 50 g Ans: You will need 1. 50 g of sugar.

Concentration 3. What mass of solute do you need to make 50 ml of a 20 g/L solution? Ans: Change 50 ml to litres… 50 ml 1000 = 0. 05 L C= M V 20 g/L = mass of solute 0. 05 L

Concentration Cross multiply: 20 g/L x 0. 05 L = 1 g Ans: 1 g of solute is needed

Concentration How do you know if a solution is more concentrated than the other? By how dark the solution is when comparing it to another… This is an observation made by the naked eye. When comparing, the darker the solution, the more concentrated…

Concentration Also, by calculating the concentration… Concentration with a bigger number is always The stronger solution… Ex: 0. 1 g/L, 100 g/L Least Most Concentrated concentrated

Dilution What is DILUTION? • This involves decreasing the concentration of a solution by adding more SOLVENT!!! How does it change the concentration of a solution? • Let’s look at a solution with a concentration of 1 g/L

Dilution How will the concentration change if we add 3 L of water? • After the dilution, the quantity of solute does not change. • There is still 1 g of solute in the solution ! • But the quantity of solution has changed, changed there is now 4 L instead of 1 L!!! 1 L

Dilution • We can write this out like this… • The concentration is now 1 g/4 L!!! • This means there is 1 g of solute for every 4 L of solution… OR Divide 1 g into 4 L = 0. 25 g/L • So, you diluted a 1 g/L solution with 3 L of water to make a 0. 25 g/L solution!!!!

Dilution • Let’s us a formula for this!!!! We already know…. C=M concentration= mass of solute V volume of solution The mass always stays the same in a dilution!!!So, we can take it out of the equation! And use this….

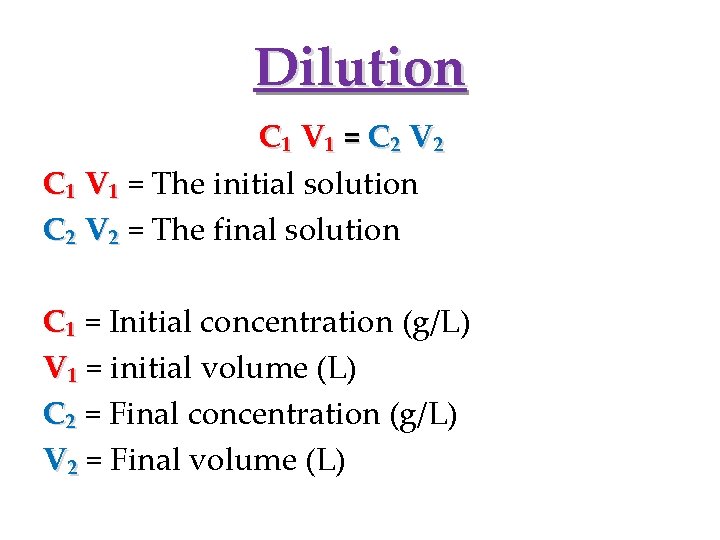
Dilution C₁ V₁ = C₂ V₂ C₁ V₁ = The initial solution C₂ V₂ = The final solution C₁ = Initial concentration (g/L) V₁ = initial volume (L) C₂ = Final concentration (g/L) V₂ = Final volume (L)

Dilution • Let’s look at our example mathematically… C₁ V₁ = C₂ V₂ (plug in what you know) C₁ = 1 g/L V₁ = 1 L C₂ = ? V₂ = 4 L Do the calculations…

Dilution C₁ V₁ = C₂ V₂ (1)x(1) = (C₂)x(4) C₂ 1 = (C₂)4 C₂ 4 4 C₂ = 0. 25 g/L There’s your answer!!!!
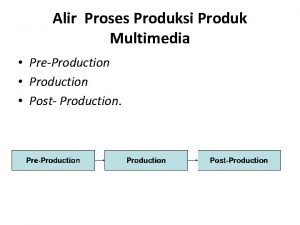
 Tahapan pre production
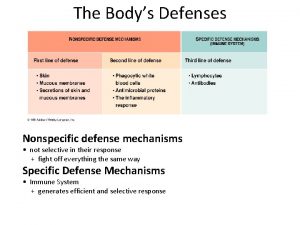
Tahapan pre production Specific defense vs nonspecific defense
Specific defense vs nonspecific defense What are the ego defense mechanisms
What are the ego defense mechanisms Bandura's reciprocal determinism
Bandura's reciprocal determinism Reaction formation
Reaction formation Sponge defense mechanisms
Sponge defense mechanisms Defense mechanism mature
Defense mechanism mature Regression psychology
Regression psychology Splitting defense mechanism example
Splitting defense mechanism example Oral fixation examples
Oral fixation examples Structure of personality
Structure of personality Three aspects of personality
Three aspects of personality Psychoanalysis psychology example
Psychoanalysis psychology example L
L Freud projection
Freud projection Sublimation freud example
Sublimation freud example Projective identification example
Projective identification example Example of regression defense mechanism
Example of regression defense mechanism Autistic fantasy defense mechanism example
Autistic fantasy defense mechanism example Rationalization defense mechanism examples
Rationalization defense mechanism examples Example for rationalization
Example for rationalization Coping mechanism
Coping mechanism Fixation defense mechanism
Fixation defense mechanism Hildegard peplau levels of anxiety
Hildegard peplau levels of anxiety Gingival sulcus
Gingival sulcus Nonspecific host defense mechanism
Nonspecific host defense mechanism Superegooo
Superegooo Erogenous zones psychology definition
Erogenous zones psychology definition Defense mechanism
Defense mechanism Regression psychology
Regression psychology Recurring pattern meaning
Recurring pattern meaning Sublimation defense mechanism
Sublimation defense mechanism Social cognitive theory of personality examples
Social cognitive theory of personality examples Sublimation psychology
Sublimation psychology Compensation defense mechanism example
Compensation defense mechanism example Airstream mechanism in sound production
Airstream mechanism in sound production Dna vaccines pros and cons
Dna vaccines pros and cons Glyphosate in vaccines
Glyphosate in vaccines Edible vaccines in pharmacognosy
Edible vaccines in pharmacognosy Glyphosate vaccines
Glyphosate vaccines